Oligodendrocytes in central nervous system diseases:the effect of cytokine regulation
2024-01-24ChengfuZhangMengshengQiuHuiFu
Chengfu Zhang ,Mengsheng Qiu ,Hui Fu
Abstract Cytokines including tumor necrosis factor,interleukins,interferons,and chemokines are abundantly produced in various diseases.As pleiotropic factors,cytokines are involved in nearly every aspect of cellular functions such as migration,survival,proliferation,and differentiation.Oligodendrocytes are the myelin-forming cells in the central nervous system and play critical roles in the conduction of action potentials,supply of metabolic components for axons,and other functions.Emerging evidence suggests that both oligodendrocytes and oligodendrocyte precursor cells are vulnerable to cytokines released under pathological conditions.This review mainly summarizes the effects of cytokines on oligodendrocyte lineage cells in central nervous system diseases.A comprehensive understanding of the effects of cytokines on oligodendrocyte lineage cells contributes to our understanding of central nervous system diseases and offers insights into treatment strategies.
Key Words:astrocyte;central nervous system disease;CXC chemokine;cytokine;interferon γ;interleukin;microglia;oligodendrocyte;oligodendrocyte precursor cell;tumor necrosis factor α
Introduction
Oligodendrocytes (OLs) are the sole myelinating glial cells of the central nervous system (CNS).They expedite action potential conduction and preserve axon integrity through a compact myelin sheath (Duncan et al.,2021).In diseases such as multiple sclerosis (MS),OL precursor cells (OPCs) migrate rapidly to the lesion area and differentiate into mature OLs to repair the demyelinated axons (Klotz et al.,2023).During this process,many factors,especially cytokines,are elevated and have a great impact on the OL lineage cells.
Cytokines contain more than six large subfamilies,among which tumor necrosis factors (TNFs),interleukins (ILs),interferons (IFNs) and chemokines are principal members(Dinarello,2007).The TNF superfamily,whose 19 members are trimeric transmembrane proteins,function as both proinflammatory and anti-inflammatory cytokines implicated in a variety of biological processes that control inflammatory reactions,anti-tumor responses,and immune system homeostasis (Croft and Siegel,2017;Gonzalez Caldito,2023).ILs are a group of lymphokines that are associated with innate immunity,hemopoiesis,and cancer (Mantovani et al.,2019;Saraiva et al.,2020).IFN,designated as types-I,-II,and -III according to their amino acid sequences and crystal structures,play pivotal roles in antiviral infection,anti-tumor activity,and adaptive immune response (Walter,2020).Chemokines,which have a low molecular weight (8–13 kDa),are well-known chemotactic factors that can recruit inflammatory cells to the injury site (Charo and Ransohoff,2006).In the peripheral immune system,cytokines are mostly secreted by macrophages,lymphocytes,monocytes,and vascular endothelial cells.In the CNS,cytokines are primarily secreted by microglia,astrocytes,neurons,or infiltrating immune cells (Galic et al.,2012;Wu et al.,2023).The effects of cytokines are pleiotropic and different cytokines may have synergistic or opposite effects (Kany et al.,2019).For example,TNFα,IL-1α,and IFNγ synergistically induce neuronal cell injury in primary cultures (Jeohn et al.,1998),while IL-6 opposes their effects and protects neurons from damage in many pathological conditions (Huang et al.,2009).
Both OLs and OPCs are very susceptible to death caused by inflammatory events,oxidative stress,and excitotoxicity in CNS diseases such as MS,stroke,leukodystrophy,and Alzheimer’s disease (AD) (van der Knaap and Bugiani,2017;Wang et al.,2023).The failure of OPC proliferation and differentiation in CNS diseases impairs the generation of enough new myelin for demyelinated areas.To date,various cytokines have been discovered to play roles in the survival of OLs and OPCs(Chamling et al.,2021),and show diverse effects on OPC proliferation and differentiation in pathological conditions.In this review,we will discuss the cytokine regulation of OL lineage cells in CNS diseases and highlight potential therapeutic strategies to target cytokines for OL-associated CNS diseases.
Retrieval Strategy
In this narrative review,we searched PubMed for relevant articles published before July 31,2023.The following keywords were used for the literature search: oligodendrocyte,tumor necrosis factor α,interleukin,interferon gamma,chemokine,multiple sclerosis,Alzheimer’s disease,stroke,microglia,astrocyte,and neuron.Various combinations of keywords were applied during the literature retrieval stages.All the papers were initially screened by their titles and abstracts.Those relevant to the topic of this review were carefully studied and discussed.Only English articles are cited in this review.
Oligodendrocyte Lineage Cells in Central Nervous System Diseases
Initially arising from the ventricular zone,OPCs migrate to the nearby subventricular zone and undergo continuous proliferation,differentiation,and maturation,eventually forming myelin around the axons (Elbaz and Popko,2019).Myelin is a specific proteolipid structure that comprises multiple cellular plasma membranes.This delicate structure facilitates the rapid and efficient transmission of signals through axons.Besides,some reports also show that OLs can secrete many trophic signals to axons to support their survival and growth.OLs also provide metabolic support,such as lactate,to axons (Narine and Colognato,2022).
Death of OLs and collapse of myelin are hallmarks of demyelinating diseases caused by auto-immune disorders,trauma,and infections.Demyelination destroys the precise organization of ion channels along axons resulting in disturbed ionic equilibrium and more energy consumption for signal transmission (Saab et al.,2013).Together with the growing energy consumption,the deficiency of trophic signals from OLs in CNS diseases can exacerbate the vulnerability of demyelinated axons to oxidative stress and may eventually cause axon degeneration (Alizadeh et al.,2015).The injury of OL lineage cells can be regulated by cell-cell contact or via the soluble mediators secreted by inflammatory cells.The expression of major histocompatibility complex class I molecules makes OL lineage cells susceptible to be recognized by immune cells such as myelin-reactive CD8-positive T lymphocytes (Jurewicz et al.,1998).In addition,CNS diseases also cause metabolic stress to OL lineage cells,which can make them more susceptible to cell death.The excitatory neurotransmitter glutamate,produced by activated microglia and macrophages,is elevated in the cerebrospinal fluid of MS patients (Stover et al.,1997).The increased level of glutamate exerts excitotoxicity on OLs by overactivating ionotropic receptors that are expressed by OLs (Alberdi et al.,2002).
In general,following demyelination,spontaneous remyelination will occur to repair the injured axons.The main contribution for remyelination is attributed to the new OPCs recruited from undamaged areas,but not the OLs which have survived the demyelinating insults.The activation of OPCs in undamaged areas,migration of OPCs to lesion sites,differentiation of OPCs to mature OLs,and the formation of myelin around axons are regarded as the key elements for remyelination following the onset of CNS diseases.During the activation phase,OPCs alter their gene expression to enter the cell cycle and increase in number for remyelination (Moyon et al.,2015).Later,OPCs can be recruited to the lesion site according to the guiding cues (Chu et al.,2017).In the setting of CNS diseases in adults,ciliary neurotrophic factor and the CXC chemokines act as chemoattractants that guide the migration of OPCs (Omari et al.,2005;Vernerey et al.,2013).In physiological conditions,with the stimulation of intracellular and extracellular cues,including various cytokines,most OPCs differentiate into mature OLs that eventually reform the myelin to envelop demyelinated axons (Uyeda and Muramatsu,2020).
The OL lineage cells are involved in different CNS diseases.MS is considered an autoimmune disease,which often appears among young adults.In the initial stages of MS,CNS antigens such as myelin debris can leak to the periphery and subsequently trigger autoimmune responses by immune cells.Inflammation reaction might cause oxidative stress and destroy OLs in MS (Lassmann,2018).A study also showed apoptosis of OLs induced by activated CD8-positive T lymphocytes,aggravating the loss of OLs in MS(Jäkel et al.,2019).Following MS injury,OPCs could revert from a quiescent state and undergo migration,proliferation,differentiation,and reformation of myelin sheaths (Rajendran et al.,2021).Other than acting as passive targets in MS,OL lineage cells also actively participate in other processes such as antigen processing and presentation,phagocytosis,and immunoprotection.Furthermore,OL lineage cells from MS could indirectly increase the production of cytokines such as TNFα and IFNγ when co-cultured with memory CD4-positive T cells (Falcão et al.,2018).Thus,it would be interesting to investigate the novel roles of OL lineage cells with respect to the origin and progression of MS.
Unlike MS,AD mainly occurs in older patients and causes progressive dementia.Amyloid-β (Aβ),an important etiological factor in AD,can disrupt myelin,which can lead to demyelination at the sites where Aβ extensively deposits(Chen et al.,2023).Aβ also restrains OPC maturation by decreasing the levels of growth factors such as transforming growth factor-β and fibroblast growth factor 2 (Facchinetti et al.,2022).Expressions of mutant presenilin-1 and exposure to Aβ1–42in OLs results in myelin defect by disturbing the distribution of myelin basic protein,a key structural protein for myelin (Desai et al.,2011).Recently,OL lineage cells have been demonstrated to play more active roles in AD.A subpopulation of plexin-B3-positive OPCs in adult rat brain were discovered to release massive Aβ1–42,which is consistent with the report that Aβ1–42accumulation appeared around plexin-B3-positive OPCs.These findings suggested that OL lineage cells might play essential roles in the pathogenesis of AD (Nihonmatsu-Kikuchi et al.,2021).Using single-cell transcriptomics,Kenigsbuch et al.(2022) identified a subpopulation of OL lineage cells,termed as disease-associated OLs (DOLs) that could respond to multiple brain pathological conditions,such as AD and experimental autoimmune encephalomyelitis (EAE,a murine model for MS).These DOLs were abundantly present in areas enriched with Aβ plaques.Anin vitrostudy showed that Aβ alone was insufficient to induce the appearance of DOLs,suggesting that other factors may also participate in this progress (Kenigsbuch et al.,2022;Chen et al.,2023).Disease-associated OLs in AD are cytokine-producing cells,upregulating the expression of IL-33 (Kenigsbuch et al.,2022).However,much is still unknown about DOLs for now.
Stroke is an acute cerebrovascular disease,which usually occurs in the middle-aged and elderly population.About 75–80% of all strokes are ischemic,while the remaining are hemorrhagic.At the early stages of stroke,the OLs become swollen and later die because of the generation of abundant lipid peroxidation,iron oxidation,and superoxide radicals (Hu et al.,2022).The dying OLs and/or myelin debris can trigger inflammation that has a secondary impact on surviving OLs and OPCs,as well as other neural cells.Even as stroke has been shown to induce the proliferation of OPCs,less than 10%of OPCs in stroke can differentiate into mature OLs with even 4–10% of OPCs differentiating into astrocytes,which leads to insufficient remyelination (Chen et al.,2020a).However,OPCs are very vulnerable to stroke insult and show major cell death through ferroptosis in hemorrhagic stroke (Shen et al.,2022).OL lineage cells seem to be the first injured cells from stroke insult.Therefore,it would be interesting to investigate whether OL lineage cells are cytokine-producing cells mediating the pathological progression of stroke.
The Overview of Cytokines in Central Nervous System Diseases
Cytokines are small soluble polypeptide factors (<40 kDa) that play profound roles in inflammatory responses to diseases.Cytokines comprise a variety of small signaling proteins that participate in intercellular communication.They have pleiotropic effects on the survival,migration,proliferation,and differentiation of neural cells involved in CNS diseases.In the context of immune response,cytokines can be classified into pro-inflammatory or anti-inflammatory categories (Turner et al.,2014).The well-known pro-inflammatory cytokines are TNFα,IL-1,IL-6,IL-12,IL-23,and IFNγ (Marrocco and Ortiz,2022).Their expressions are often elevated in CNS diseases such as AD and MS,and collectively trigger deleterious inflammatory reactions aggravating the pathological condition.Owing to their overzealous cytotoxicity,pro-inflammatory cytokines are selectively inhibited to be produced or released to avoid further tissue injury in the therapy of these diseases.The anti-inflammatory cytokines contain IL-4 and IL-10,which play similar functions in MS,stroke,and AD (Porro et al.,2020;Gärtner et al.,2023).They can control or eliminate inflammation to create an appropriate circumstance for healing by suppressing the antigen presentation function of dendritic cells or macrophage activation and infiltration into the lesion site (O’Garra et al.,2008).The source of cytokines in the CNS depends on the integrity of the BBB.In ischemic disease such as stroke,cytokines are predominantly secreted by microglia and astrocytes.However,the major source of cytokines in infectious encephalitis and MS are leukocytes that infiltrate into the CNS (Becher et al.,2017).Although the exact roles of specific cytokines are relatively clear,the synergistic and/or antagonistic interactions between different cytokines remain to be explored in pathological conditions.
The Involvement of Microglia and Astrocytes in Central Nervous System Diseases
A report has shown that the other two resident glial cells–microglia and astrocytes– played multiple roles in the regulation of OL lineage cells in CNS diseases (de Waard and Bugiani,2020).Astrocytes,comprising nearly one-third of cells in the CNS,are critical mediators that maintain the integrity of the BBB,provide metabolic support for neurons,and repair the damaged areas in the CNS (Segura-Aguilar et al.,2022;Chambel et al.,2023).Microglia,accounting for 10–20% of all glial cells,are the major resident inflammatory cells that can phagocytose myelin debris or apoptotic cells,modulate the migration of infiltrated immune cells,and trigger intense inflammations in lesion areas (Yu et al.,2022).It is now well recognized that after being activated in demyelinating situations,astrocytes undergo morphological and functional changes.They move to lesion sites and play multiple functions.Multifaceted astrocytes can show both beneficial and detrimental actions on OL lineage cells by releasing various cytokines such as TNFα,IL-11,CXC chemokine ligand (CXCL)1,CXCL8,and CXCL10 (Omari et al.,2005;Zhang et al.,2006;Su et al.,2011).Similar to astrocytes,microglia could also be rapidly activated and migrate to the damaged sites in MS and EAE.Similarly,they can release various cytokines and exert complicated effects on OL lineage cells (Patel et al.,2010;Steelman and Li,2011).Although multiple studies have been published on the functions of microglia and astrocytes in pathological conditions,the details about the specific involvement of these cells in CNS diseases need much more investigation.
The Major Cytokines Mediating Oligodendrocyte Lineage Cells in Central Nervous System Diseases
TNFα/TNF receptor signaling shows both harmful and protective effects on OL lineage cells
TNFα is a strong pro-inflammatory cytokine,which is produced in two biologically active forms– a 26 kDa membranebound TNFa (tmTNFa) and a 17 kDa soluble TNFα (sTNFα)by the matrix metalloproteinase TNFα-converting enzyme(Kriegler et al.,1988;Luettig et al.,1989).In vitroandin vivostudies have revealed that TNFα in CNS is mainly released by reactive astrocytes,microglia,subpopulations of neurons,or infiltrating macrophages (Tuttolomondo et al.,2008;Su et al.,2011;Valentin-Torres et al.,2016;Wang et al.,2022).
There are two receptors for TNFα,namely TNF receptor(TNFR)1 (p55,CD120a) and TNFR2 (p75,CD120b),which belong to two different subgroups of the TNFR superfamily.These two receptors show relatively high homology in the extracellular TNFα-binding domains,with no sequence homology in the intracellular domains which results in different cellular signal pathways (Ihnatko and Kubes,2007;Zelová and Hošek,2013).TNFR1 can be activated by either sTNFα or tmTNFα,with a priority for sTNFα,which induces strong pro-inflammatory effects;whereas,TNFR2 is preferentially activated by tmTNFα and triggers anti-inflammatory activities(McCoy and Tansey,2008).In adult rodent brains,TNFR2 but not TNFR1 is expressed in OLs.However,in MS lesions,both TNFR1 and TNFR2 are expressed in OLs,especially evident at the lesion edge (Bonetti and Raine,1997).In a demyelination model caused by the toxin cuprizone,the expression of TNFR2 was substantially increased during demyelination and remyelination stages,but the expression of TNFR1 showed no change (Arnett et al.,2001).In the EAE demyelination model,both TNFR1 and TNFR2 are very likely to be expressed in OLs(Eugster et al.,1999;Suvannavejh et al.,2000).However,till now,the mechanistic regulation of TNR1 and TNFR2 expression remains to be explored (Holbrook et al.,2019).
Several studies underline the importance of TNFα/TNFR signaling in mediating the effect of inflammation on OL lineage cells.As a pleiotropic cytokine,TNFα shows distinct functions on OLs according to the type of receptor and the cellular source of this cytokine.TNFα predominantly exerts detrimental effects on OL lineage cells in pathological conditions.In one study,60 minutes after traumatic spinal cord crush injury in rats,OLs in the lesion area showed an intense immunoreactivity to TNFα.These cells then underwent apoptosis within 48 hours after injury,while the neutralizing antibody against TNFα dramatically impeded this process (Lee et al.,2000).In transgenic mice with CNS-specific TNFα overexpression,OL apoptosis and myelin vacuolation were potently and selectively induced,further proving the cytotoxic effects of TNFα (Akassoglou et al.,1998).TNFαinduced OL death was also observed in other diseases such as MS and glaucoma (Bitsch et al.,2000;Nakazawa et al.,2006).Furthermore,TNFα was also strongly implicated in the death of OPCs and OL precursors (preOLs) (Kim et al.,2011;Su et al.,2011).
The mechanism by which TNFα induces OL death is very sophisticated with multiple factors and cell types.It has been reported that nitric oxide synthase and IL-1β-converting enzyme have contributed to TNFα-initiated apoptosis.After the function of nitric oxide synthase in the lesion area or IL-1β-converting enzyme was inhibited in primary cultures of OLs,TNFα-induced OL apoptosis decreased significantly(Hisahara et al.,1997;Lee et al.,2000).The nuclear factor kappa B and bone morphogenic protein-7 have been shown to prevent TNFα-induced apoptosis (Hamanoue et al.,2004;Wang et al.,2016).Data suggest that the death of human mature OLs induced by TNFα did not involve the classical caspase activation,rather are dependent on the translocation of apoptosis inducing factors from the mitochondria to nuclei and the latter induction of large-scale DNA cleavage (Jurewicz et al.,2005).TNFα-induced OL apoptosis has been suggested to be closely related to the function of TNFR1,as it was abolished in mice genetically deficient for TNFR1 (Akassoglou et al.,1998).The deletion of TNFR1 specifically impaired the induction of EAE,further confirming the critical role of TNFR1 in TNFα-induced OL apoptosis (Hövelmeyer et al.,2005).
Astrocytes and microglia are actively involved in the process of TNFα-induced OL apoptosis.Reactive astrocytes secrete TNFα to inhibit OPC survival and prevent them from differentiating into mature OLs (Su et al.,2011).Reports have also shown that astrocytes promoted TNFα-mediated death of pre-OLs.This process requires direct contact with astrocytes,which may form gap junctions with pre-OLs and propagate cell injury(Kim et al.,2011).After activation,microglia also enhance pre-OLs death in a TNFα/TNFR1-dependent manner (Li et al.,2008;Steelman and Li,2011).A study on glial cell culture showed the cytotoxic effect of TNFα on human OPCs involved in microglia polarizing to the M1 phenotype but not the M2 phenotype (Moore et al.,2015).In addition,the activated microglia secreted TNFα and other cytokines to induce a subtype of reactive astrocytes,which in turn could promote the death of OLs (Liddelow et al.,2017).
Different from TNFR1,TNFR2 has been shown to mediate protective and reparative effects on OL lineage cells (Figure 1;Arnett et al.,2001).TNFR2 could protect OPCs against oxidative stress by increasing the gene expression of antiapoptotic protein BCL2 and detoxifying protein superoxide dismutase 2 (Maier et al.,2013).Ablation of TNFR2 in OLs increased the expression of hypoxia inducible factor-1α,which is considered to induce OL death in MS and progressive multifocal leukoencephalopathy (Aboul-Enein et al.,2003;Madsen et al.,2020).By mediating the activation of phosphatidylinositol-3-kinase/Akt pathway in astrocytes,TNFR2 can increase the expression of neurotrophic cytokine leukemia inhibitory factor,which is then secreted to the extracellular space and promotes the differentiation of OPCs(Fischer et al.,2014).TNFR2 also contributes to decreased detrimental inflammatory response,by inhibiting antigen presentation by downregulating the expression of major histocompatibility complex class I,which damages the cellular machinery for OPC proliferation and differentiation,thus enhancing their repair function (Madsen et al.,2016;Desu et al.,2021).
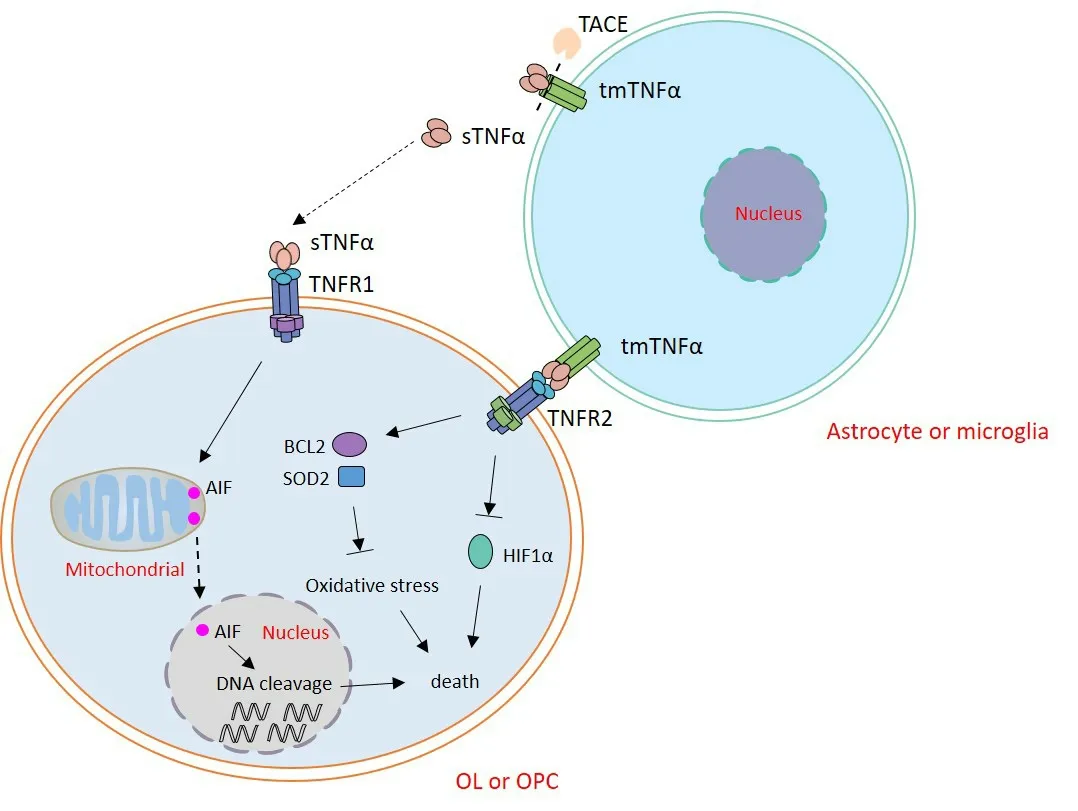
Figure 1 |The regulation of TNFα on OL lineage cells.
In addition to its direct effect,TNFα may regulate OL lineage cells through secondary effects resulting from its actions on other neural cells.The actions of TNFα on microglia and astrocytes have impacts on OL lineage cells.In general,microglia can be activated into M1 pro-inflammatory or M2 anti-inflammatory status,while astrocytes can switch to the A1 pro-inflammatory phenotypes or A2 anti-inflammatory phenotypes after activation.The M1 microglia and A1 astrocytes are considered harmful for the surrounding cells,including OL lineage cells,by secreting inflammatory mediators such as IL-1β and reactive oxygen species.The M2 microglia and A2 astrocytes exert beneficial effects on ambient cells by releasing anti-inflammatory regulators such as IL-6,IL-10,and transforming growth factor β (Liu et al.,2020).Some evidence also suggests that TNFα can induce reactive microglia into M1 phenotype and trigger astrocytes into A1 phenotype in pathological conditions (Brás et al.,2020;Liu et al.,2020).Therefore,TNFα can act through pro-inflammatory microglia and astrocytes,which produce various cytokines to impair the viability of OL lineage cells in neurological disorders.
TNFα is considered an intense pro-inflammatory factor that can induce neuronal death.The exposure of hippocampal neurons to glial-derived TNFα increases the number of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic-type glutamate receptor and N-methyl-D-aspartic acid receptor on their surface.The elevated expression of these receptors can cause the death of hippocampal neurons by glutamate excitotoxicity and excessive intracellular calcium (Leonoudakis et al.,2004;Zhu et al.,2010).In addition to apoptosis,TNFα can further induce the necroptosis of neuronal cells (Wang et al.,2022).The death of neurons caused by TNFα will deteriorate the pathological condition of OL lineage cells.Without communications with neurons,the proliferation and differentiation of OPCs will be disturbed,which is detrimental to remyelination after injury (Bradl and Lassmann,2010).Owing to the deficiency of neuronal stimulation,myelin can easily break down,followed by OL death in traumatic diseases(Totoiu and Keirstead,2005).
Given their powerful pro-inflammatory effects and essential protective functions on OL lineage cells,TNFα/TNFRs are extensively considered as potential therapeutic targets for OLassociated diseases such as MS.In some previous designs,researchers had not taken the beneficial aspect of TNFα/TNFR signaling into account,but planned to extinguish all effects of this signaling.Unselective inhibitors of TNFα,such as infliximab and lenercept,have shown unfavorable effects on MS patients,such as the enhancement of attack duration and exacerbation of MS (van Oosten et al.,1996;No authors listed,1999).The selective suppressors of sTNFα/TNFR1 signaling seemed more effective.XPro1595,a dominant negative blocker of sTNFα,increased the number of OPCs and improved the expression of myelin-specific genes in EAE mice (Brambilla et al.,2011).Atrosab,a novel humanized monovalent antibody against TNFR1,significantly reduced the demyelination and CNS immune cell infiltration in EAE mice(Williams et al.,2018).However,the outcome of this drug on MS patients remains to be determined in clinical trials (Zahid et al.,2021).The reported effects of TNFα/TNFR signaling modulators on MS patients or EAE mice are summarized inTable 1.
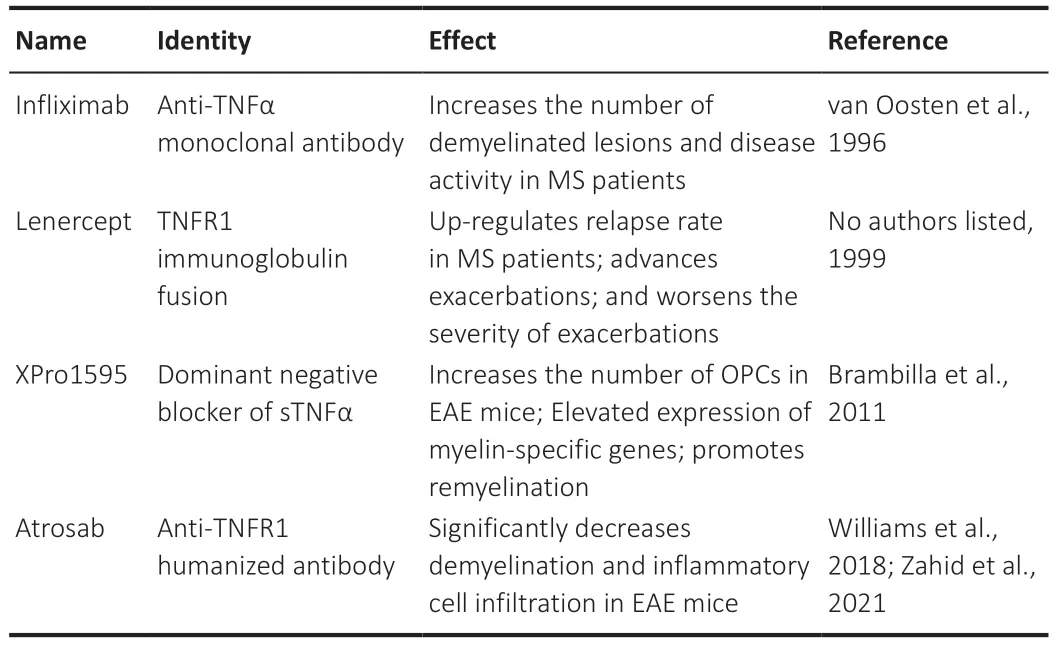
Table 1 |Summary of reported TNFα/TNFR signaling modulators
Regulation of IL family on OL lineage cells in CNS diseases
Interleukins are a series of lymphatic cytokines that play roles in multiple biological processes.This cytokine superfamily contains about 40 members,and their expressions are usually drastically altered in CNS diseases such as MS and stroke(Catalan-Dibene et al.,2018;Ye et al.,2018).In the CNS,ILs are mainly secreted by activated microglia and astrocytes,as well as by infiltrating lymphocytes.In the normal adult brain,the expression of ILs is low,and they are usually difficult to detect.However,in pathological conditions,they are highly expressed and have profound impacts on cell migration,survival,proliferation,and differentiation (Duffy et al.,2019;Bobbo et al.,2021;McAlpine et al.,2021).
Several members of IL family have been implicated in the survival of OL lineage cells.IL-4 could promote survival of differentiating OPCs by activating peroxisome proliferatoractivated receptor γ under neuroinflammatory conditions(Paintlia et al.,2006).IL-6 could prevent the degeneration of OLs induced by excitotoxic injury (Pizzi et al.,2004).Expressed by reactive astrocytes in MS tissue samples,IL-11 has been proven to promote the survival of OLs via the IL-11 receptor subunit α (Zhang et al.,2006).Produced by grafted mesenchymal stem cells in CNS,IL-13 could impede OL loss and demyelination in the cuprizone mouse model (Le Blon et al.,2016).
However,IL-1β showed detrimental effects on OLs.Secreted by the activated microglia in MS,IL-1β did not kill OLs directly,instead induced their apoptosis by destroying the glutamatebuffering ability of astrocytes in a mixed glia environment(Takahashi et al.,2003).IL-1β markedly suppressed the proliferation of OPCs through decreasing the expression of miR-202-3p and increasing the expression of β-catenin and glioma-associated oncogene homolog 1 (Li et al.,2020).The roles of IL-17 in OPC proliferation are somewhat controversial.One group demonstrated that IL-17 inhibited the proliferation of OPCs by increasing the expression of voltage-gated K+channel 1.3 and decreasing AKT activation in cell culture(Liu et al.,2021).Another group claimed that IL-17 activated the NOTCH1 signaling pathway in OPCs and induced the expression of several pro-proliferation genes (Wang et al.,2017).However,they did not provide direct evidence that IL-17 promoted OPC proliferation.
Some members of the IL family play inhibitory roles in the differentiation and maturation of OLs.Derived from activated microglia,IL-1β could inhibit OPC maturation via suppressing the Fyn proto-oncogene/mitogen-activated protein kinase NOTCH 1/extracellular signal-regulated kinase signaling pathway (Xie et al.,2016).Even though IL-6 showed no effects on OL apoptosis and the recruitment of OPCs to the lesion site in cuprizone-treated mice,it inhibited the early OL differentiation via the reduction in astroglial and microglial activation which contributed to the removal of myelin debris(Petković et al.,2016).Many members of the IL family also facilitate the differentiation and maturation of OLs.IL-1β could promote OPCs to differentiate via miR-202-3p/β-catenin/glioma-associated oncogene homolog 1 axis (Li et al.,2020).After stroke,IL-4 receptor appeared in OL lineage cells,and its activation by IL-4 promoted OPC differentiation via activating peroxisome proliferator-activated receptor γ (Zhang et al.,2019).IL-17 receptor was expressed in OPC during the peak phase of EAE,and its activation by IL-17 promoted OPC differentiation by activating extracellular signal-regulated kinase 1/2 (Rodgers et al.,2015).
Similar to TNFα,ILs may also produce some secondary effects on OL lineage cells through their actions on other neural cells in pathological events.Many studies have shown that ILs were involved in the activation of astrocytes and microglia.IL-1β was shown to induce astrocytes into pro-inflammatory A1 status during neuroinflammation caused by stroke (Liu et al.,2020).Apart from its direct action on OLs,IL-17 also mediated astrocytes into the A1 phenotype to cause OL damage and deteriorate demyelination (Zhou et al.,2022).Similarly,IL-18 was able to induce the activation of astrocytes to the A1 status via the nuclear factor kappa B signaling pathway (Hou et al.,2020).IL-4 could stimulate the transformation of microglia to anti-inflammatory M2 phenotype and exert protective effects in stroke,AD,and epilepsy (Chen et al.,2020b).Other ILs such as IL-10,IL-13,IL-25,and IL-33 have also been shown to participate in the activation of microglia toward the M2 phenotype (Maiorino et al.,2013;Jiang et al.,2018;Laffer et al.,2019;Lu et al.,2022).The pro-inflammatory or antiinflammatory status of reactive astrocytes and microglia activated by ILs would eventually affect the physiological functions of OL lineage cells in pathological conditions.
IL-1 can significantly trigger neuronal death in acute ischemic stroke and AD (Griffin,2006;McColl et al.,2009).Exposure to IL-2 has been reported to result in neuronal swelling,vacuolations,and neurite retractions,while other ILs did not provoke these effects (Araujo and Cotman,1995).However,the elevated expression of IL-10 facilitated the survival of neurons by inhibiting the proapoptotic effects and enhancing the survival signals in stroke,AD,and MS (Strle et al.,2001).IL-6 prevents hippocampal neurons from death by clearing up oxidative stress (Fujita et al.,2009).Given the importance of intact axons for OLs,the survival of neurons regulated by different kinds of ILs greatly affects the vitality of OLs in these above-mentioned diseases.
IFNγ plays a role in the biological activities of OL lineage cells
IFNγ is a soluble cytokine secreted by immune cells including macrophages,T lymphocytes,and natural killer cells (Ferrara et al.,2022).This cytokine forms a homodimer and binds to its ubiquitously expressed IFNγ receptor which is a heterodimer with IFNγ receptor 1 and IFNγ receptor 2 chains (Castro et al.,2018).IFNγ signaling induces the expression of interferonstimulated genes by activating the Janus kinase and signal transducer and activator of transcription (JAK-STAT) pathway(Ivashkiv,2018).Functionally,IFNγ has remarkable impacts on different cell processes such as apoptosis,proliferation,differentiation,and immune regulation (Bhat et al.,2018).
Previously published studies support the notion that IFNγ plays a deleterious role in OL lineage cells.In MS patients,owing to the infiltration of macrophages and T lymphocytes or the damaged BBB,IFNγ was detected at the margins of chronic MS plaques (Traugott and Lebon,1988).Moreover,there were several apoptotic mature OLs and IFNγ-positive cells in this area,suggesting that IFNγ could induce the apoptosis of OLs.This hypothesis was supported by experiments showing that IFNγ treatment killed OLs and the anti-IFNγ antibodies could diminish this effectin vitro(Vartanian et al.,1995).The cytotoxic effect of IFNγ signaling on OLs was mainly mediated by its downstream molecules,signal transducer and activator of transcription 1,and interferon regulatory factor 1,which could elevate the expression of Fas,FADD (Fas-associated death domain),and TNFR1.In turn,these three molecules formed a signaling complex and mediated CD8-positive cell cytotoxicity which led to OL death (Patel and Balabanov,2012).IFNγ could also exert a cytotoxic effect on OL via reducing aerobic glycolysis and mitochondrial respiration(Minchenberg and Massa,2019).In addition to apoptosis,OLs were also likely to succumb to necrosis when exposed to IFNγ (Baerwald and Popko,1998),which can worsen the demyelination condition in MS.
IFNγ also has certain harmful effects on OPCs similar to those on OLs.Treatment with IFNγ induces OPC apoptosis with the classical hallmark release of mitochondrial cytochrome c into the cytoplasm and cleavage of caspase 3 (Tirotta et al.,2012).IFNγ-induced OPC death depends on signal transducer and activator of transcription 1 and interferon regulatory factor 1,which upregulates the expression of two pro-apoptotic genes– double-stranded RNA-dependent protein kinase and caspase 1 (Wang et al.,2010).IFNγ also promotes antigen cross-presentation in OPCs to cytotoxic CD8-positive T cells by increasing the expression of major histocompatibility complex class I,leading to the death of OPCs (Kirby et al.,2019).In addition,the OPC recruitment and proliferation were also significantly suppressed by IFNγ in the demyelinated adult spinal cord.Further,the IFNγ signaling impaired OPC differentiation involving hyperactive Wnt/bone morphogenic protein genes (Saraswat et al.,2021).
Research evidence has suggested that IFNγ likely also has protective effects on OL in MS.Suppressing IFNγ signaling by a transgenic approach significantly increased OL apoptosis in an EAE mouse model (Balabanov et al.,2007).The low expression level of IFNγ specifically in OLs caused fewer oligodendroglial deaths in cuprizone-treated mice (Gao et al.,2000),providing evidence that IFNγ could protect OLs from cell death.IFNγ was shown to protect mature OL from oxidative stress and reduce proteasome activity (Balabanov et al.,2007).The different effects of IFNγ on OL lineage cells might be associated with the stage-and dosage-specific roles of IFNγ in MS (Zhang et al.,2005;Arellano et al.,2015).In cell culture,IFNγ was toxic to OPCs at concentrations of >200 mg/mL,but had no effects on OPCs at concentrations of <200 mg/mL (Zhang et al.,2005).At the primary autoimmune stage,IFNγ promoted the death of OL lineage cells by enhancing antigen presentation to cytotoxic CD8-positive T cells.However,at the second inflammatory stage caused by the OL lineage cell death,IFNγ showed protective effects by removal of extracellular myelin debris and restricting lipid peroxidation,both of which could generate neurotoxic products and induce cell death(Sosa et al.,2015).The results of early clinical trials with intravenous administration of IFNγ at different dosages for MS patients were not very encouraging (Panitch et al.,1987).The symptoms were exacerbated during these trials,suggesting that the effects of IFNγ on MS are quite complicated and need further studies for clarity.
Apart from the direct function,the secondary effects of IFNγ on OL lineage cells via its action on other neural cells may be very important in CNS diseases.It has been widely reported that IFNγ is involved in the polarization of astrocytes and microglia in pathological conditions.IFNγ signaling promotes the pro-inflammatory A1 polarization of astrocytes (Lu et al.,2020).Similarly,microglia were reported to be driven into pro-inflammatory M1 status by IFNγ (Chauhan et al.,2021).Thus,reactive microglia induced by IFNγ signaling could exert pathological effects,including triggering the production of TNFα to inhibit oligodendrogenesis in MS (Butovsky et al.,2006).Pro-inflammatory conditions caused by IFNγ-mediated astrocytes and microglia potentially attenuate the vitality of OL lineage cells in CNS diseases.
Many studies have documented that IFNγ held both protective and detrimental roles in neurons.In a non-cytolytic manner,IFNγ mediated the clearance of neurotropic virus to prevent neurons from death during viral infections in CNS (O’Donnell et al.,2012).Low IFNγ expression was reported to promote neurogenesis in the dentate gyrus of the AD mouse model(Baron et al.,2008).However,IFNγ was also discovered to aggravate neuronal death in response to Aβ peptides (Bate et al.,2006).This cytokine could directly induce neuronal excitotoxicity through phosphorylating alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic receptors to cause neuronal death (Mizuno et al.,2008).As mentioned above,neuronal survival or death regulated by IFNγ in different pathological events ultimately mediates the stabilization of myelin and OLs,along with the physiological function of OPCs.
The effects of CXC chemokines on OL lineage cells in CNS diseases
CXCLs,also termed as α-chemokines,are a subfamily of chemotactic cytokines with a CXC motif at their N-terminus.They are ligands for CXC motif chemokine receptors (CXCRs)(Hughes and Nibbs,2018).Until now,17 members and 8 receptors (Korbecki et al.,2021) have been identified in this subfamily.In normal CNS development,CXCLs and CXCRs are expressed by neural cells and are important for their migration,proliferation,and differentiation (Zou et al.,1998;Filipovic et al.,2003).
In some CNS diseases such as MS,CXCLs were found to be secreted by reactive astrocytes and microglia,while CXCRs expression was markedly increased in OLs (Omari et al.,2005;Lindhout et al.,2021),suggesting the important roles of CXCL/CXCR signaling in OLs during pathological conditions.CXCL1 and its receptor CXCR2 exert both harmful and protective functions on OL lineage cells under different experimental circumstances (Skinner and Lane,2020).On the one hand,sustained expression of CXCL1 induced in transgenic mice contributed to the elevated neutrophil infiltration,resulting in increased mortality of OLs and exacerbating demyelination induced by mouse hepatitis virus (Marro et al.,2016).Repression of CXCR2 by antagonist in spinal cord cultures augmented myelin basic protein positive cells,suggesting the inhibitory role of CXCR2 in OPC differentiation (Kerstetter et al.,2009).On the other hand,hypertrophic astrocytes within the damaged areas in MS could secrete CXCL1,which recruited proliferating OLs with the expression of CXCR2 to these areas and repaired the lesion (Omari et al.,2006).In addition,CXCL1/CXCR2 signaling also had a role in protecting OL lineage cells from apoptosis in a viral-induced demyelination model (Hosking et al.,2010).
Other CXCLs and CXCRs are also involved in regulating biological activities of OPCs.CXCL8 was found elevated in patients with MS.This chemokine recruited OPCs to the lesions in MS via engagement of CXCR1,with no induction of OPC death,suggesting its potential role in facilitating remyelination (Kelland et al.,2011).However,treatment of CXCL10 resulted in OPC death through receptor CXCR3 (Tirotta et al.,2011).In the murine model of demyelination induced by cuprizone or PLP139-151,CXCL12 was upregulated by activated astrocytes and microglia in the corpus callosum,whilst the expression of CXCL12 receptor CXCR4 in OPCs was also significantly increased (Patel et al.,2010;Zilkha-Falb et al.,2016).The CXCL12/CXCR4 signaling promoted the migration of OPCs through activating phosphatidylinositol-3-kinase/Akt and mitogen-activated protein kinase kinase 1/extracellular signal-regulated kinase pathways (Li et al.,2015;Tian et al.,2018).Later on,the activation of this signaling also enhanced the differentiation of OPCs,suggesting the critical role of this signaling for remyelination (Patel et al.,2010).
The interaction of different cytokines on OL lineage cells
During pathological conditions,cytokines are released by resident glial cells and infiltrated immune cells (Table 2).The cytokines mentioned above work together synergistically or antagonistically exerting their diversified effects on OL lineage cells (Figure 2).IFNγ could increase OL susceptibility to apoptosis,and TNFα could augment this effect (Pouly et al.,2000).In an EAE mouse model,IL-9 combined with IFNγ promoted OPC proliferation and differentiation,whereas IL-9 plus IL-17,TNFα,or IL-1β impaired OPC differentiation (Ding et al.,2015).IL-17 alone had no effects on the survival of OLs,but exacerbated OL apoptosis induced by TNFα (Paintlia et al.,2011).
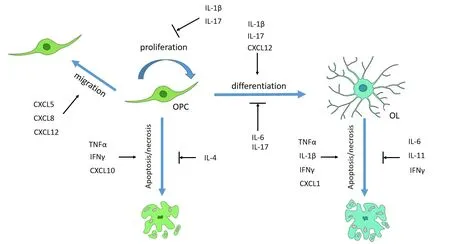
Figure 2 |The summary of cytokines involved in the regulation of OL lineage cells in CNS diseases.
CXC chemokines often act downstream of TNFα,IL,and IFNγ.The activation of TNFR2 in astrocytes induced CXCL12 expression,which promoted OPC proliferation and differentiation (Patel et al.,2012).IFNγ inhibited CXCL2 expression in the microglia of EAE mice (Stoolman et al.,2018).Astrocytes could produce CXCL8 and CXCL1 upon stimulation by IL-1β,but produce CXCL10 upon combined stimulation by IL-1β and IFNγ (Omari et al.,2005).Obesity triggered the expression of IL-17B,which in turn increased the expression of CXCL5,regulating OPC migration (Xiao et al.,2022).Although the treatment of IFNγ would result in the apoptosis of OPCs from wild-type mice,this effect was significantly diminished in OPCs from CXCL10 or CXCR3 knockout mice,demonstrating that IFNγ induced OPC apoptosis partially through CXCL10 (Tirotta et al.,2011).However,CXCL1/CXCR2 signaling attenuated OPC apoptosis induced by IFNγ or CXCL10 (Tirotta et al.,2011,2012).
Conclusion
Both OPCs and OLs are very reactive to cytokines secreted by microglia,astrocytes,or infiltrating immune cells,which exert complex effects in CNS diseases,including MS,stroke,and AD.As a pro-inflammatory mediator,TNFα plays a detrimental role in survival,proliferation,and differentiation of OL lineage cells through TNFR1.However,this cytokine also shows beneficial effects on OL lineage cells via TNFR2.The different roles of TNFα on OL lineage cells might be partially determined by the stages of CNS diseases.In the early stages of CNS diseases,owing to the intense pro-inflammatory condition,TNFα plays a detrimental role to destroy the damaged OL lineage cells.While in the later stages of CNS diseases,TNFα exerts reparative effects on OL lineage cells without the disturbance of inflammatory mediators.Many members of the IL family participate in the regulation of OL lineage cells.IL-1β,IL-4,IL-11,and IL-13 are involved in the survival of OPCs and OLs,while IL-1β,IL-4,IL-6,and IL-17 are implicated in the proliferation or differentiation of OPCs.IFNγ has both damaging and protective effects on OL lineage cells in a dosage-and stage-dependent manner.CXC chemokines also play an important role in OL lineage cells.CXCL1 and CXCL10 participate in the regulation of OPC or OL death.CXCL5,CXCL8,and CXCL12 promote the migration of OPCs.In addition,there are other cytokines such as transforming growth factor β,IFNβ,and granulocyte colony-stimulating factor implicated in the regulation of OL lineage cells.Studies on the mechanism of these cytokines mediating OL lineage cells in CNS diseases remain to be extended.
Apart from direct effects,cytokines also indirectly regulate OL lineage cells in CNS diseases through their actions on other neural cells.They can affect the activation of astrocytes and microglia.The pro-inflammatory and anti-inflammatory statuses of these two resident immune cells dramatically affect the vitality and other aspects of OL lineage cells.Upon cytokine stimulation,astrocytes and microglia could produce more cytokines and amplify the effects.Cytokines also greatly affect neurons.In most cases,they induce the death of neurons that can result in the instability of myelin and OLs,as well as the deficiency of OPCs stimulation.Given the importance of cytokines in OL lineage cells,therapies targeting specific cytokines may help to maintain the normal function of OL lineage cells and cure myelin-associated diseases such as MS and progressive multifocal leukoencephalopathy.Although the mechanisms by which cytokines regulate OL lineage cells in CNS disease models or cell cultures were extensively discussed in this review,the therapeutic effects of antagonists or agonists aimed at single or combined cytokines in CNS diseases remain to be determined in future papers.
Author contributions:Manuscript writing and figure preparation:CZ;manuscript revision:HF,MQ.All authors edited and approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:Not applicable.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
