Connecting cellular mechanisms and extracellular vesicle cargo in traumatic brain injury
2024-01-24NikitaOllenBittleAustynRoseboroughWenxuanWangJengliangWuShawnWhitehead
Nikita Ollen-Bittle ,Austyn D.Roseborough ,Wenxuan Wang ,Jeng-liang D.WuShawn N.Whitehead,
Abstract Traumatic brain injury is followed by a cascade of dynamic and complex events occurring at the cellular level.These events include: diffuse axonal injury,neuronal cell death,blood-brain barrier break down,glial activation and neuroinflammation,edema,ischemia,vascular injury,energy failure,and peripheral immune cell infiltration.The timing of these events post injury has been linked to injury severity and functional outcome.Extracellular vesicles are membrane bound secretory vesicles that contain markers and cargo pertaining to their cell of origin and can cross the blood-brain barrier.These qualities make extracellular vesicles intriguing candidates for a liquid biopsy into the pathophysiologic changes occurring at the cellular level post traumatic brain injury.Herein,we review the most commonly reported cargo changes in extracellular vesicles from clinical traumatic brain injury samples.We then use knowledge from animal and in vitro models to help infer what these changes may indicate regrading cellular responses post traumatic brain injury.Future research should prioritize labeling extracellular vesicles with markers for distinct cell types across a range of timepoints post traumatic brain injury.
Key Words:axonal injury;biomarkers;blood-brain barrier;chronic traumatic encephalopathy;extracellular vesicles;glial activation;neuroinflammation;traumatic brain injury
Introduction
Traumatic brain injury
Traumatic brain injury (TBI) is characterized as any injury to the brain resulting from an external force to the head that causes acute and/or chronic neural dysfunction.TBI can present across a wide spectrum of severity and can arise from several different mechanisms including blunt nonpenetrating trauma,penetrating trauma,and blast injury(National Academies of Sciences,Engineering,and Medicine,2019).Blunt non-penetrating trauma results when an external force causes the brain to contact the cranial vault,whereas penetrating trauma occurs when an external object penetrates the brain.Blast injury is commonly seen in military veterans where shockwaves can transfer through blood vessels and cerebrospinal fluid within the cranium leading to blood-brain barrier (BBB) dysfunction (National Academies of Sciences,Engineering,and Medicine,2019).In addition to the mechanism of injury,TBI can be classified as focal or diffuse.Focal TBI produces damage in a localized area and often precipitates skull fractures and/or hematomas,whereas diffuse TBI,also referred to as diffuse axonal injury is primarily associated with widespread axonal injury,swelling of the brain,and hypoxia (Gupta and Sen,2016).
TBI is a heterogeneous and clinically challenging condition due to varying degrees of severity at presentation,high symptom variability,history of polytrauma,vast secondary mechanisms of injury,and duration of time elapsed prior to seeking medical attention.Mild TBI (mTBI) is most prevalent;with headache,sleep disturbances,light sensitivity and fatigue among the most common symptoms (de Kruijk et al.,2002;Mollayeva et al.,2014).The majority of patients with mTBI and no intracranial bleeding (concussion),recover clinically in 7–14 days while about 15% will experience symptoms beyond the two week mark (McCrory et al.,2017).Acute moderate to severe TBI is characterized by a disruption in consciousness and post-traumatic amnesia (Nakase-Richardson et al.,2007).TBI can also cause chronic deficits.Chronic deficits are most prevalent in patients with a history of polytrauma/multiple mTBIs or moderate to severe TBI (Washington et al.,2012;Aungst et al.,2014).The most common chronic cognitive deficits include memory,attention,declined processing speed and executive functioning (Aungst et al.,2014;Wilson et al.,2021).
Emergent management of severe TBI focuses on maintaining cerebral perfusion pressure,circumventing hypotension and hypoxia,and surgical interventions when indicated (Vella et al.,2017).One of the greatest challenges associated with treating TBI is the secondary mechanisms of injury that follow the primary injury.Figure 1outlines secondary mechanisms including neuroinflammation,edema,ischemia,vascular injury,energy failure,increased BBB permeability,and diffuse axonal injury that can perpetuate pathophysiologic changes in the brain (Lazaridis et al.,2019).Although acute TBI is evaluated using neuroimaging and clinical evaluation,increasing evidence suggests microstructural damage and chronic functional impairment do not correlate well with symptom scores.This highlights the need to develop better indicators/biomarkers of underlying TBI pathophysiology that can serve as prognosticators of long-term outcomes (De Beaumont et al.,2007;Bazarian et al.,2014;Johnson et al.,2015).
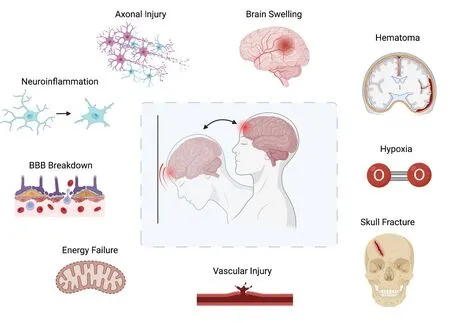
Figure 1 |Sequalae of traumatic brain injury.
The prediction of long-term outcomes post TBI is even more important in the context of repetitive injury.Repetitive TBI is associated with progressive neurodegeneration,recently termed chronic traumatic encephalopathy (CTE).CTE is predominantly associated with repeated injury through contact sports (Goldstein et al.,2012) and is characterized by the progressive accumulation of phosphorylated tau (p-tau)neurofibrillary tangles,amyloid beta,astrocytic tangles and neurite clusters around small blood vessels in the cortex(Grundke-Iqbal et al.,1986;Alonso et al.,1996;Omalu et al.,2011;Ameen-Ali et al.,2022).The mechanism by which repetitive TBI drives neurodegenerative changes remains unclear but is thought to be associated with dysregulated neuroinflammation (Simon et al.,2017).
Cellular responses in TBI and the need for a blood-based biomarker
Following TBI a dynamic cascade of cellular events occurs to respond to the injury.Neuroinflammation has been implicated in both the early and chronic components of TBIinduced neuropathology and is characterized by distinct cellular responses throughout the time course of injury resolution.The initial mechanical impact at the trauma site causes neuronal injury at dendrites,cell body,and axons.The damaged neuronal tissue release damage-associated molecular patterns (DAMPs),cytokines,chemokines into the local environment immediately following injury that peaks within minutes to hours (Davalos et al.,2005).This is followed by activation of microglia and astrocytes and the recruitment of circulating peripheral monocytes within hours to modulate injury resolution (Alam et al.,2020).Monocyte infiltration into the brain parenchyma leads to the production of chemokines that promote further astrocyte and immune cell activation and recruitment (Alam et al.,2020).Activated microglia and astrocytes accumulate at the injury site and are the main cellular players driving the inflammatory response that plays a key role for debris clearance,repair,and regeneration post TBI,but may also mediate secondary injury (Karve et al.,2016).Interestingly,microglia and astrocyte activation phenotypes are highly dynamic.A shift from neuroprotective into a dominant neurotoxic activation phenotype seemingly occurs in the first week post injury (Pischiutta et al.,2018),while more chronic stages post injury may include a complex mix of both pro-and anti-inflammatory profiles depending ontiming and injury severity (Villapol et al.,2017).The severity of brain damage is also dependent upon secondary injury mediated by sustained microglia activation and inflammatory cytokine release that exacerbates neuronal death.Chronic microglia activation is considered to be one of the hallmarks of unresolved inflammation that may promote long-term clinical sequalae post-neural injury (Block and Hong,2007).Improved biomarkers capable of serving as a peripheral window into the pathophysiologic changes occurring in the brain post TBI would not only greatly increase our understanding of TBI at the cellular level but would also allow for the identification of individuals at greatest risk for long-term functional impairment.
Extracellular vesicles
Extracellular vesicles (EVs) are secretory vesicles enclosed by a lipid bilayer membrane (Zaborowski et al.,2015;Doyle and Wang,2019).EVs are secreted by all cell types and have been isolated from a vast number of biological fluids (Pulliero et al.,2019).While previously thought to be a means of cellular waste disposal,EVs are emerging as important regulators of cell-to-cell communication and immune modulation(Raposo et al.,1996;Parolini et al.,2009;Mittelbrunn et al.,2011;Tkach et al.,2017;Torralba et al.,2018).EVs can be characterized based on their size,cargo,and surface markers as well as their route of formation.EVs carry surface markers aligned with their parent cells and can contain a vast variety of cargo including proteins,lipids,and nucleic acids (Zaborowski et al.,2015;Doyle and Wang,2019).
EVs include a variety of vesicle types that are often broadly characterized as exosomes or microvesicles (MVs)/ectosomes based on their route of formation (Cocucci and Meldolesi,2015;Doyle and Wang,2019).Briefly,MVs are 50–1000 nm diameter irregularly shaped vesicles formed by direct outward budding of the cell’s plasma membrane (Heijnen et al.,1999).Alternatively,exosomes are the smallest subclass of EVs ranging from 20–150 nm in diameter (Pan et al.,1985;Heijnen et al.,1999).Unlike MVs,exosomes are formed by an endosomal route by which early endosomes bud inwards to become multivesicular bodies (MVBs).MVBs are then either targeted to the lysosome for degradation or to the plasma membrane where they can fuse and release their membrane enclosed content (Cocucci and Meldolesi,2015;Doyle and Wang,2019).Figure 2depicts the formation of both exosomes and microvesicles.It is important to note that MVs and exosomes can be produced by the same cell types,found in the same biological fluids and can be similar in size due to their heterogenous nature (Cocucci and Meldolesi,2015).In keeping with the Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines created by the International Society for Extracellular Vesicles,we use the term EVs as the umbrella term for lipid bilayer-delimited particles released from cells (Théry et al.,2018).
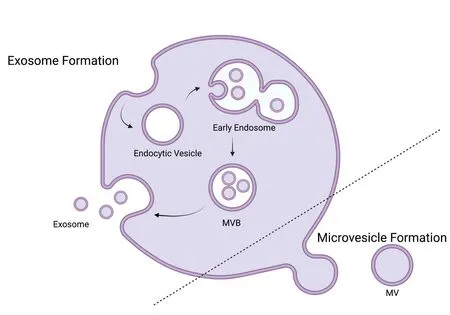
Figure 2 |Extracellular vesicle formation.
Extracellular vesicles as indicators of cell responses to traumatic brain injury
Due to the small size and lipid membrane of EVs,they are hypothesized to pass through the BBB via transcytosis (Chen et al.,2016).Additionally,since they display surface markers and contain cargo reflective of their parent cells,EVs are a strong candidate for a peripherally accessible biomarker of cellular changes within the central nervous system.Technologies to assess EVs are continually emerging and a more in depth review has been previously published by our group (Ollen-Bittle et al.,2022).In brief,emerging technologies include scanning,transmission and cryo-electron microscopy,dynamic light scattering,atomic force and super-resolution microscopy and nanoparticle tracking analysis.Each of these techniques possess unique advantages and limitations but collectively can be used for both quantification and characterization of EVs.
In the following sections,we discuss EV targets that have been reported post TBI and the cell specific responses they may reflect.Previous EV biomarker studies have predominantly centred around small molecules found within the brain associated with TBI pathology.The objective of this manuscript is to explore the potential use of EVs as a novel,non-invasive window into the pathophysiologic changes occurring at the cellular level post TBI.Based on a literature survey of EV studies from human TBI patients,outlined inTable 1,we identified the most commonly reported changes to EV cargo post TBI including proteins,cytokines and miRNA.Using combinations of cell-specific markers and cargo analysis,EVs may serve as a window into the pathophysiological changes occurring in the TBI brain making them appealing biomarkers and potential therapeutic targets.Knowledge fromin vitrostudies,animal models and other human neuropathological findings are then used to help infer what may be driving these clinical findings on a cellular level.
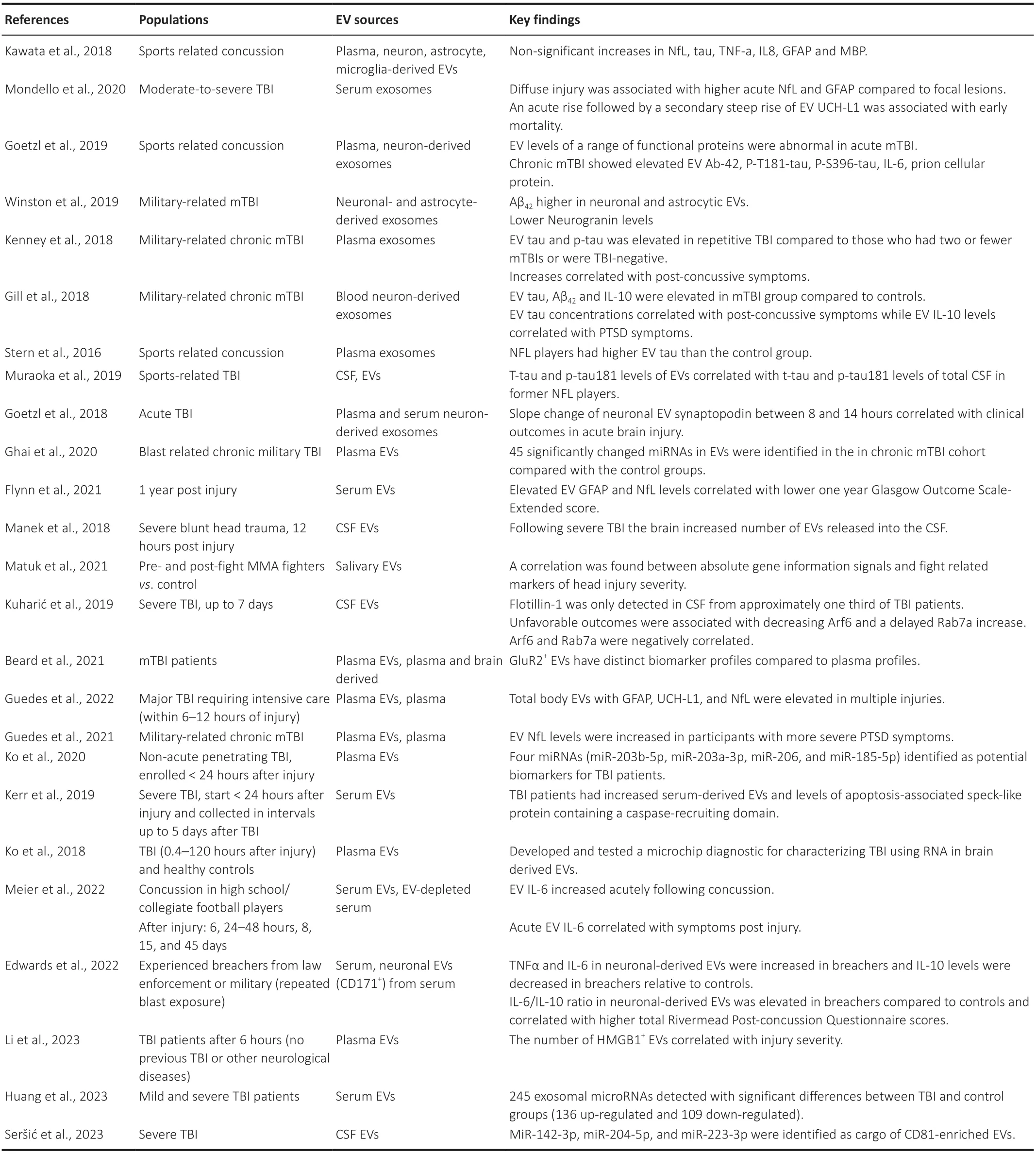
Table 1 |Literature survey of human TBI and EV studies
Search Strategy
In this narrative review,relevant literature published between 2016 and 2023 was acquired during 2023 via the PubMed database through searching the terms: (“Extracellular Vesicles”[Mesh] OR “extracellular vesicle*” OR “exosom*”OR “microvesicle” OR “ectosom*”) AND (“Brain Injuries,Traumatic”[Mesh] OR “traumatic brain injur*” OR “chronic traumatic encephalopath*” OR “concussion*”) AND(“Humans”[Mesh] OR “player*” OR “patient*” OR “militar*”).
Extracellular Vesicle Cargo Changes Following Traumatic Brain Injury and the Link to Glial Response
Proteins
Phosphorylated tau protein
Tau proteins are microtubule-associated proteins that are highly prevalent within the central nervous system (Sato et al.,2018).They play a key role in stabilizing microtubules in the axons of neurons but are also known to be expressed to a lesser extent in microglia,astrocytes and oligodendrocytes(Fiock et al.,2020).In adults,the brain expresses six or more tau isoforms.These isoforms are produced via alternative splicing of the messenger RNA (mRNA) derived from the microtubule-associated protein tau gene (Goedert et al.,1989;Sündermann et al.,2016).Aggregates of p-tau have been closely linked to neurodegenerative tauopathies including Alzheimer’s disease (AD),corticobasal degeneration,progressive supranuclear palsy and some types of frontotemporal lobar dementia (Hutton et al.,1998;Gong et al.,2006;Falcon et al.,2019;Martínez-Maldonado et al.,2021).When tau becomes hyperphosphorylated in these conditions,it dissociates from microtubules and forms insoluble aggregates (Lindwall and Cole,1984;Alonso et al.,2004;Liu et al.,2007).Histopathologically,this appears as inclusions of insoluble and abnormally modified tau into neurofibrillary or gliofibrillary tangles (Alonso et al.,2004;Liu et al.,2007).As previously described accumulation of p-tau is also associated with CTE (Dale et al.,1991;Mufson et al.,2016).Interestingly however,tau filaments in CTE are distinct from those in AD (Falcon et al.,2019).Specifically,a different conformation of the -helix region creates a hydrophobic cavity not seen in the structure of tau within AD brains (Falcon et al.,2019).
Several studies have identified significantly increased levels of p-tau and/or total tau in EVs from patients with TBI relative to controls.In 2016,Stern et al.identified increased levels of tau in EVs from national football league (NFL) players relative to controls.Similar findings have also been made in other sportselated concussion studies (Kawata et al.,2018;Goetzl et al.,2019;Muraoka et al.,2019),as well as in military-related chronic mTBI (Gill et al.,2018;Kenney et al.,2018),general moderate-to-severe TBI (Mondello et al.,2020) and mTBI(Beard et al.,2021).Stern et al.(2016) found tau-positive EVs positively correlated with worse cognitive function within the group of NFL players.Kenney et al.(2018) found greater EV total tau and p-tau was correlated with post-concussive symptoms and post-traumatic stress disorder (PTSD)symptoms,however interestingly tau load was not associated with loss of consciousness or post-traumatic amnesia.These results imply that tau accumulation may be linked to history of mTBI polytrauma rather than the severity of TBI or loss of consciousness/post-traumatic amnesia.
It is not hard to reason that tau levels would spike immediately post injury due to the direct physical damage to neurons and their axons.This is precisely what Mondello et al.(2020) showed in moderate-to-severe cases of TBI.Total-tau levels were significantly increased immediately post injury and steadily dropped off with measurements taken in the first 24 hours being fourfold higher than measurements take between 72 to 120 hours (Mondello et al.,2020).However,as with CTE,tau pathology is known to occur beyond the scope of the initial trauma.In order to infer what is happening,we have to speculate which cell type is excreting the EVs with p-tau.
Following TBI the vast cellular damage causes a large increase in extracellular adenosine triphosphate (ATP) (Liu et al.,2017).ATP acts as a DAMP,activating microglia and driving neuroinflammatory responses (Inoue,2002).Microglia are also known to alter their EV production following TBI and other neurodegenerative conditions (Joshi et al.,2014;Kumar et al.,2017;Liu et al.,2017).ATP has been shown to drive EV production in both microglia and astrocytesin vitrothrough:the P2X7 receptor,the P38 MAPK cascade and lysosomal acid sphingomyelinase (A-SMase) (Bianco et al.,2005).In rats post TBI,the P2X7 receptor antagonist A804598 and the immune inhibitor FTY720 significantly reduced EVs in the injured and adjacent regions of the brain and in the cerebrospinal fluid (Liu et al.,2017).Additionally,inflammation and DAMP related microglial activation is known to alter EV composition(Kumar et al.,2017;Yang et al.,2018).Intriguingly,only under lipopolysaccharide and ATP (DAMP) stimulation,tau has been shown to be sorted into EVs (Asai et al.,2015).Moreover,this study demonstrated in an adeno-associated virus-based model of AD,both depleting microglia and inhibiting their EV synthesis significantly reduced tau propagation,suggesting microglial EVs may contribute to the progression of tauopathy(Asai et al.,2015).This is in combination with the knowledge that in a humanized mouse model of AD,plaque-associated microglia phagocytose plaque associated tau and hypersecrete tau containing EVs (Clayton et al.,2021).It is possible that we see a similar response in TBI.Microglia may respond both acutely respond to the physical damage associated with the increase in tau protein,and chronically in the case of repetitive injury or CTE.These chronic neuroinflammatory responses and subsequent EV release may then contribute to the progression of tauopathy.
Amyloid-beta proteins
Amyloid-beta (Aβ) is a protein produced by the proteolytic cleavage of the transmembrane protein amyloid precursor protein (APP).APP is an integral membrane protein that is enriched within the synapses of neurons and is also found in glia and throughout many other tissues and cell types of the body (Plummer et al.,2016).The amyloid hypothesis famously proposes Aβ accumulation in addition to misfolded tau protein,to be the driving source of cognitive decline in AD.Similar to the Aβ hypothesis in AD,it has been proposed that an increase in Aβ post TBI is detrimental and may lead to an increased risk of developing cognitive impairment (Fleminger,2003).However,this theory has been met with controversial results including an unclear correlation between TBI and AD (Plummer et al.,2016),and a decrease in Aβ density in the later stages post TBI (Chen et al.,2009).Contrarily,it has also been postulated that an increase in APP post TBI may be a neuroprotective response.As first hypothesized by Van den Heuvel et al.(1999),APP upregulation appears early in TBI and may serve as an early indicator for neuronal injury.Additionally,mice lacking APP have shown increased susceptibility to TBI related neuronal injury showing greater cognitive and motor deficits (Corrigan et al.,2012).Collectively,the role of Aβ in TBI is not fully understood.Understanding the cell types responsible for Aβ elevation post TBI may help understand the role of APP post TBI.
EV studies may play a useful role in this process.Studies in human samples post TBI have found an increase in Aβ in EVs.In a return from combat study,relative to age matched controls,Wintson and colleagues found an increase in Aβ42in both plasma derived neuronal and astrocytic EVs (Winston et al.,2019).Another study assessing plasma neuron derived EVs in acute mTBI (within 1 week) or chronic mTBI (after 3 months or greater from last 2–4 mTBIs) relative to controls with no recent history of TBI found elevated levels of Aβ42in chronic mTBI (Goetzl et al.,2019).In keeping with Winston and colleagues findings of increased Aβ in neuronal and astrocytic derived EVs post TBI,studies in rats have similarly shown an increase in APP in both neurons and astrocytes post TBI (JE Pierce et al.,1996;Bramlett et al.,1997).Cell work has shown Aβ42exposed glia consisting mainly of astrocytes release EVs that are toxic to neuronal cultures,causing synaptic loss,mitochondrial dysfunction and apoptosis (Beretta et al.,2020).This is in keeping with the hypothesis that Aβ accumulation exerts neurotoxic effects.
Microglia are likely to be significant producers of Aβ-containing EVs post TBI as well given their known role in the clearance of Aβ (Ries and Sastre,2016).Therefore,it is possible Aβ cargo in microglia may indicate a restorative process/response to the TBI.Controversially,EVs from microglia post TBI have also been suggested to propagate pathology as in the case of tau pathology (Asai et al.,2015).Future studies should consider the temporal pattern of microglia EV cargo,as it may provide vast insight into changes occurring in the brain and help us speculate if these changes are maladaptive or protective,or if their outcome is dependent on the time post injury.
Additionally,oligodendrocytes have also been shown to secrete Aβ40and Aβ42(Skaper et al.,2009).Therefore,it is possible Aβ40and Aβ42content post TBI may also reflect oligodendrocyte damage,or perhaps if in the later phases,oligodendrocyte proliferation which is known to occur within the initial weeks following TBI (Dent et al.,2015).Further research assessing Aβ content in EVs derived from distinct glial populations at targeted time points post TBI will better elucidate the role of APP and Aβ in TBI pathology.
GFAP and UCH-L1
The intermediate filament-III protein prevalent within astrocytes,glial fibrillary acidic protein (GFAP),has garnered significant attention as a biomarker for astrocyte activity and astrogliosis in a number of neurologic diseases including TBI.Additionally,ubiquitin C-terminal hydrolase L1 (UCH-L1),a prominent neuronal protein estimated to account for a remarkable 1–5% of neuronal proteins,has also gained increasing attention.Previous literature has found benefit in combining these biomarkers and so they are often explored together (Diaz-Arrastia et al.,2014).UCH-L1 and GFAP have shown to be detectable in serum within hours post TBI (Papa et al.,2012a,b).Moreover,GFAP and UCH-L1 are increased in patients with CT detectable intracranial lesions from TBI(Papa et al.,2012a,b;Diaz-Arrastia et al.,2014) and have a sensitivity for detecting said lesions ranging between 94% and 100% in both paediatric and adult populations (Papa et al.,2016).
More recently,GFAP and UCH-L1 have been explored in EVs post TBI.A study by Beard and colleagues assessing samples from patients with mTBI (<24 hours) found elevated GFAP in both measurements from plasma and plasma-based brain derived EVs (GLuR2+) relative to controls.Interestingly,this study also found that UCH-L1 protein dominated the GLuR2+EVs and suggested that this indicates these EVs may represent a protein clearance system utilized by damaged or distressed cells post TBI (Beard et al.,2021).Another study assessing cerebrospinal fluid (CSF) 12 hours post severe TBI found increased EV levels of GFAP and UCH-L1 relative to control samples (Manek et al.,2018).At a more chronic stage post TBI,Flynn et al.(2021) showed EV GFAP concentrations were increased in moderate and severe TBI plasma samples 1 year post injury compared to controls and could distinguish controls from both moderate and severe TBI.Again at 1 year post injury time point a separate study showed total body EVs containing GFAP (glial derived),and UCH-L1 and neurofilament light chain (NfL) (neuronal derived) were greater in patients with multiple injuries and found a correlation between GFAP and Head Abbreviated Injury Score (Guedes et al.,2022).As with NfL,another study found patients with diffuse injury displayed increased acute EV GFAP concentrations in serum compared with those with focal lesions (Mondello et al.,2020).They also report that an acute elevation of UCH-L1 positive EVs followed by a secondary rise was correlated with early mortality,although this study was limited to two patients (Mondello et al.,2020).Finally,Goetzl et al.(2019)demonstrated that neuronal EV levels of UCH-L1 are higher in acute mTBI subjects compared to chronic mTBI subjects and controls,further supporting an upregulation of a ubiquitindependent pathway for the clearance of damaged neuronal proteins.
GFAP is considered to be an astrocytic marker and UCH-L1 to be neuronal.However,it is known that EVs can possess double positive labeling for microglial markers and neuron specific proteins (Kawata et al.,2018),therefore it remains possible that microglia may be responsible for some of the UCH-L1 positive EVs post TBI.In terms of GFAP+EVs,microglial phagocytosis and internalization of astrocytes are known to play a critical role in astrocyte death (Puñal et al.,2019).Additionally,increased GFAP positivity in neurons post TBI has been observed,although this has largely been attributed to artifact (Zwirner et al.,2021).Further work confirming the specificity of GFAP and UCH-L1 as indicators of astrocyte and neuronal EVs,respectively,is required in the post TBI setting where protein expression levels are highly dynamic.
In 2018,the U.S.Food and Drug Administration (FDA)approved GFAP and UCH-L1 as clinical biomarkers in adult patients with mild-moderate TBI,to assist clinicians in determining the need for a CT scan within 12 hours of injury (U.S.Food and Drug Administration,2018).Although there are current limitations to using EVs clinically,including both access to high-throughput processing equipment and confidence in the specifics of EV markers across different neurological conditions,assessing EVs in the context of GFAP and UCH-L1 may enhance the usage of these biomarkers in TBI.One important consideration is the timing of biomarker sampling post TBI.This is critical in acute neurological injury and outside of the 12-hour window it is unclear the benefit of these biomarkers.Assessing the flux of GFAP and UCH-L1 in EVs from different cell populations over time post TBI would not only paint a picture of the cellular mechanisms occurring but could also alter the timing post injury from which they are able to be utilized.EV characterization may also further the usage of these biomarkers by limiting confounds such as patient age since concentration in EVs from distinct cell types may be more specific to injury.Endogenous levels of GFAP(among other potential biomarkers) are known to increase with age (Abdelhak et al.,2018).A 2018 study by Gardner et al.(2018) found that GFAP was less able to discriminate CT+and CT–mTBI subjects in the older age group.Additionally,another study assessing the sensitivity and specificity of the Banyan Brain Trauma Indicator (a GFAP and UCH-L1 assay)showed that while sensitivity was 100% for both age groups,specificity decreased significantly from 0.44 to 0.13 in elderly patients.As the levels of GFAP and UCH-L1 were significantly varied by age in patients without abnormal CT findings (CT–)but not those with a positive CT,this decrease in specificity was attributed to higher levels of GFAP and UCH-L1 in the CT–subjects (Ward et al.,2020).Therefore,delineating GFAP+and UCH-L1+EV populations that are injury-specific and age dependent may broaden their utility clinically.
Neurofilament light chain
Neurofilaments are proteins in neurons that provide support and shape to the neurons.Neurofilaments are found within the dendrites,soma and axon of the neuron (Yuan et al.,2017).Neurofilaments are known to be released into the extracellular environment upon axonal injury and as such have garnered attention as a possible biomarker for AD,multiple sclerosis,Parkinson’s disease,and TBI (Khalil et al.,2018).NfL is the most common subunit of neurofilaments and is also the most soluble in both cerebrospinal fluid and blood,making it an intriguing biomarker candidate (Narayanan et al.,2021).Unsurprisingly NfL has been found to increase in TBI patients,likely reflecting neuronal injury.
More recently NfL has been explored in EV cargo post TBI.One study looked at plasma vs brain derived EVs (characterized by GLuR2 expression) from mTBI patients from samples taken within 24 hours of hospital admittance.Intriguingly,differential results were seen between these samples with NfL,tau and UCHL1 showing elevation in the plasma but not in GLuR2+EVs (Beard et al.,2021).Another study reported increased NfL,and GFAP EV content in patients 5 days after diffuse injury relative to focal lesions (Mondello et al.,2020).
There is support for the significance of NfL content in EVs in the chronic phase post TBI and in the context of repetitive TBI as well.Flynn and colleagues showed an increased EV GFAP and NfL were associated with lower Glasgow Outcome Scale-Extended (GOS-E) scores one year after TBI (Flynn et al.,2021).Total EVs of GFAP,UCH-L1,and NfL were found to be higher in those with multiple TBI injuries (Guedes et al.,2022).Finally,elevated NfL EV cargo was associated with increased PTSD symptoms in a cohort of military service members and Veterans with a positive history for TBI (Guedes et al.,2021).These studies suggest that while NfL increases acutely in TBI,NfL in EVs in chronic stages of TBI may associated with worse patient outcome.
It is probable that NfL is being released by neurons reflecting damage post TBI.However,microglia must again be considered as a possible key player in the production of NfL+EVs,given their response to DAMPs both immediately and chronically post TBI.While one may assume an increase in NfL means neuronal injury,if the NfL+EVs are coming from microglia,does this reflect merely a clean-up event or is something greater going on? In patients at least six months post TBI,minocycline,a drug known to impede inflammatory microglial activation,has been shown to reduce chronic microglial activation as measured on PET MRI;however,this decrease in microglial activation was coupled with an increase in plasma NfL (Scott et al.,2018).Additionally,a separate study assessing patients with mild cognitive impairment/early AD with raised cortical Aβ negatively correlated increased microglial/brain inflammation with plasma NfL levels (Parbo et al.,2020).This suggested that promotion of neuroinflammation may be beneficial in the prodromal or early stages of AD.Studies assessing both microglial EV NfL content and plasma NfL levels at varying timepoints post TBI in the context of other neuroinflammation biomarkers would increase our insight into the role of neuroinflammation post TBI.
Cytokines
IL-6
In response to DAMPs released in the acute stages of TBI,marked neuroinflammatory changes occur in the brain including the release of pro-inflammatory cytokines.Interleukin-6 (IL-6) is one of the predominant proinflammatory cytokines released following neuronal injury although it is undetectable under physiological conditions(Gabay,2006).IL-6 is known to be released by microglia,astrocytes and neurons (Frei et al.,1989;Lau and Yu,2001).It has shown to increase in experimental models of TBI as early as one hour post injury (Williams et al.,2007) and peak between two and eight hours (Woodcock and Morganti-Kossmann,2013).Although neuroinflammatory,the release of IL-6 has been linked to neuroprotective effects.IL-6 deficient mice demonstrate worse functional outcome post TBI (Ley et al.,2011).IL-6 has also been found to exert beneficial effects on the cellular level including: promotion of neuronal differentiation,tumor necrosis factor (TNF) inhibition,excitotoxicity modulation and nerve growth factor synthesis(Woodcock and Morganti-Kossmann,2013).Systemically,IL-6 is also known to recruit immune cells,regulate lipid metabolism and contribute to insulin resistance (Lehrskov and Christensen,2019).Although nonspecific for TBI,the timing of release and the complex role IL-6 plays post-neuronal injury makes it an intriguing biomarker.
IL-6 has been investigated in EVs from patients post TBI.In a study assessing sports-related concussion,EVs from blood samples taken within 6 hours post-concussion showed elevated IL-6 both relative to the individuals baseline measurements and non-injured controls (Meier et al.,2022).IL-6 levels were also positively correlated with the number of days the patients reported symptoms (Meier et al.,2022).Another study assessed inflammatory cytokines in EV cargo from career breachers (military personnel exposed to hundreds to thousands of low-level blast exposures).Despite no difference in the concentration of IL-6 in the serum to that of controls,IL-6 in neuronal derived EVs was increased in breachers (Edwards et al.,2022).Additionally,the IL-6/IL-10 ratio in neuronal derived EVs was greater compared to controls and correlated with higher scores on the Rivermead Post-concussion Questionnaire (Edwards et al.,2022).A separate study assessing EV cargo within 24 hours of mTBI found increased IL-6 both in plasma and in the EV cargo measurements,while IL-10 and TNF-α were only found to be elevated in plasma (Beard et al.,2021).Collectively,more studies assessing IL-6 in EVs utilizing labeling for multiple cell types are needed to deduce the cell type responsible for the upregulation of IL-6 in EVs post TBI.When considering the role of glia,microglia stimulated with LPS release EVs with increased levels of IL-6 (Kumar et al.,2017;Yang et al.,2018).Additionally,microglia-derived EVs have been shown to upregulate IL-6a in addition to IL-10 in astrocytes (Drago et al.,2017).Proinflammatory cytokines such as IL-6 may be released from a variety of cell types while also mediating the response of other cells.Ultimately the timing of release from distinct cell types may explain the correlation between IL-6 and both neuroprotective and detrimental effects.
TNF-α
Tumor necrosis factor-α (TNF-α) is another pro-inflammatory cytokine that has been shown to be upregulated in the brain post TBI.TNF-α is produced by a variety of cell types including microglia,endothelial cells,astrocytes and neurons (McCoy and Tansey,2008).TNF-α has been shown to posses a range of effects from inducing cell death to stimulating cellular growth and differentiation (McCoy and Tansey,2008).Similar to IL-6,in central nervous system injury it is likely a combination of cellular environment factors,timing of release,timing of receptor activation,concentration and cell types responding that dictate if TNF-α will exert neuroprotective or neurotoxic events (Longhi et al.,2013).
In animal models,levels of TNF-α have been shown to increase early post TBI and peak within a few hours (Schmidt et al.,2005).Some studies have shown TNF-α is released before other pro-inflammatory cytokines and acts as an initial mediator for the recruitment of immune cells and the activation of other pro-inflammatory cytokines and growth factors (Longhi et al.,2013).The effects of TNF-α antagonization remain unclear.Acute antagonization has been associated with improved outcome post TBI (Shohami et al.,1996) while TNF-α (-/-) mice exhibit worse outcome more chronically (Scherbel et al.,1999).This implies that TNF-α may exert neurotoxic properties acutely but may play a role in neuroprotective or regenerative mechanism chronically post TBI (Longhi et al.,2013).
Interestingly,a study assessing TNF-α in plasma and EVs within 24 hours post TBI found although TNF-α was increased in the plasma samples,there was no difference in the brain derived EVs relative to controls (Beard et al.,2021).Another study assessing sports related concussion,showed no increase in TNF-α in EVs from samples taken 6 hours post-injury,although significant changes were observed in the overall pro-inflammatory profile (Meier et al.,2022).In the study assessing career military breachers chronically exposed to low level blasts,serum TNF-α was decreased in breachers,while neuronal derived EVs had higher levels (Edwards et al.,2022).The information derived from recent human TBI samples and from experimental models demonstrates that while plasma levels of TNF-α may increase acutely post TBI,TNF-α levels in EVs may peak at more chronic time points.It is possible this may reflect the initial pro-inflammatory response by microglia,and then their subsequent anti-inflammatory clean-up response in more chronic stages of TBI,that has been noted in pre-clinical models (Doust et al.,2023).Future work should investigate cell-specific TNF-α containing EVs across acute and chronic timepoints.
IL-10
Interleukin-10 (IL-10) is generally thought of as an antiinflammatory cytokine post neural injury.IL-10 is commonly investigated post TBI as part of inflammatory cytokine panels along with IL-6 and TNF-α.IL-10 can be produced by all leukocytes (Saraiva et al.,2009),microglia,astrocytes,oligodendrocytes (Peferoen et al.,2014) and additionally under specific circumstances by keratinocytes and epithelial cells (Itakura et al.,2011).Generally,IL-10 is thought to play a neuroprotective role enhancing cellular survival and attenuating the response of pro-inflammatory cytokines (Porro et al.,2020).In preclinical studies,IL-10 has been shown to decrease neuroinflammation (Shanaki-Bavarsad et al.,2022).Whether or not this reduction in inflammation corresponds with improved functional outcome has raised conflicting results.Intriguingly,one study demonstrated IL-10 was lower in repetitive mTBI relative to single TBI on days 1,3,7,14,and 30 (Gao et al.,2017).While others have shown IL-10 levels correlate with severity and mortality in severe TBI (Schneider Soares et al.,2012).
In clinical studies plasma IL-10 is seemingly increased in TBI patients in the acute stages with different studies reporting a peak at 3 hours (Hensler et al.,2002) and 5–6 days (Helmy et al.,2012).An additional study also reported an initial elevation for the first 22 days post-injury followed by a second peak occurring later (Csuka et al.,1999).More recently IL-10 has been explored in EVs post TBI.A report by Gill and colleagues showed in a that in military personnel with mild TBI,EV IL-10 levels were correlated with PTSD symptoms (Gill et al.,2018).A separate study examining military breachers with chronic career exposure to low-level blasts found although there were not differences in serum levels of IL-10,neuronal derived EV IL-10 levels were decreased relative to controls (Edwards et al.,2022).More studies are needed to elucidate the cell type responsible for these changes in EV levels of IL-10.
IL-1RA
Acute TBI is known to exhibit dramatic neuroinflammatory changes.Inflammatory cytokines that mediate this response include IL-1α and IL-1β (Thome et al.,2020).These cytokines primarily target IL-1 receptor type 1 (Symons,1995).In the brain this receptor is known to be expressed in astrocytes,microglia,neurons and endothelial cells (Liu et al.,2019).Activation of IL-1 receptor type 1 triggers a cascade leading to activation of transcription factors including nuclear factorkappa B and activator protein 1,which then promote the generation of pro-inflammatory cytokines including IL-6 and IL-1 (Thome et al.,2020).IL-1 receptor antagonist (IL-1RA) can act as a blocking ligand for this cascade (Newell et al.,2018)and as such has shown promise as a potential therapeutic for TBI in animal models (Fomicheva et al.,2022).In human patients post TBI,Increased levels of IL-1RA in blood samples correlated with lower levels of BBB leakiness as measured by Dynamic Contrast-Enhanced Magnetic Resonance Imaging (To et al.,2023).In sports-related concussion EV-depleted samples,IL-1RA was significantly increased post injury compared to both individual player baseline levels and controls;however,no difference was observed in EV levels of IL-1RA (Meier et al.,2022).Given its therapeutic potential,more studies are needed to explore IL-1RA release post-TBI and its cellular origin.
MicroRNAs
MicroRNAs (miRNAs) are small,non-coding RNA molecules ranging from 21–23 nucleotides in length that bind mRNA molecules to regulate expression (Du and Zamore,2007).Importantly,one miRNA can have many mRNA targets,allowing them to exert broad effects on signaling pathways when present.Given their small size,miRNAs are frequently detected as EV cargo and are thought be instrumental in the cell-cell signaling capabilities of EVs (Mir and Goettsch,2020).With respect to neurological disease,changes to miRNA cargo have been reported in studies of stroke,TBI and neurodegenerative disease (Mir and Goettsch,2020;Fullerton et al.,2022;Natale et al.,2022).Below we discuss specific examples of miRNA cargo alterations post TBI and their potential cell-signaling implications.
In the setting of acute TBI,combinations of miRNAs (miR-203b-5p,miR-203a-3p,miR-206,and miR-18505p) in EVs can be used to distinguish TBI from healthy control blood samples(Ko et al.,2020).When assessing severity of TBI,differential expression of 11 miRNAs separates TBI with altered consciousness in comparison to controls or TBI patients without loss of consciousness (Puffer et al.,2020).miR-9-3p demonstrated the largest fold-change in expression in the acute phase and has been reported in a preclinical study of acute TBI as well (Ko et al.,2020).In preclinical models,EVs collected from brain isolates post-TBI had elevated levels of miR-21,miR-146,miR-7a,and miR-7b,while plasma post-TBI had elevated levels of miR-150-5p,miR-669c-5p,miR-488-3p,miR-22-5p,miR-9-5p,miR-6236,miR-219a-3o,and miR-351-3p (Harrison et al.,2016;Ko et al.,2020).The use of machine learning has enabled the development of miRNA combinations that better distinguish post TBI samples in comparison to conventional protein-based biomarkers assays(Ko et al.,2018).Collectively,these studies provide support for the use of EV-contained miRNAs as acute TBI biomarkers and potential differentiators of TBI severity and highlight the need for combinatorial approaches with multiple miRNA targets.
There may also be utility for miRNA biomarkers in predicting long-term outcomes post TBI as well.Post-TBI blood samples from chronic mTBI reported elevated levels of 12 different miRNAs with miR-139-5p (Guedes et al.,2021)the most strongly associated with post-traumatic stress disorder symptoms.Another study reported alterations to 45 EV-contained miRNAs in samples from chronic mTBI subjects,although associations with outcomes remain unclear (Ghai et al.,2020).Across both studies,upregulated miRNAs are associated with signaling pathways involved in neurodegeneration,metabolic dysfunction,blood-brain barrier function and inflammation.
Future studies are needed to determine the best timing and biofluid sampling for EV-miRNA measurement.Some targets like miR-404-5p have been reported in EVs from both blood and CSF samples post TBI (Guedes et al.,2021;Seršić et al.,2023),while many have only been measured in only one or the other.Validation and comparison of miRNA levels in CSF versus blood will help define appropriate study methods and collection windows for individual markers.The majority of studies to date have measured miRNA cargo in the total EV population without differentiating between neuronal,astrocytic,oligodendroglial,microglial or endothelial EVs.Another important step moving forward will be the differentiation of EV cell origin when measuring miRNA cargo,allowing for more accurate interpretation of cellular activity based on miRNA release.
Conclusions and Future Directions
EVs are emerging as a liquid biopsy into the cellular changes occurring in the brain following a variety of neurologic pathologies including TBI.To best harness the insights emerging from EV literature,it is paramount that the cellular origin of EVs be elucidated to the best of our abilities.A great example of this is the study by Kawata et al.(2018)assessing TBI in ice hockey players from a single season of play.Although this study was limited by a small sample size,their methodology of assessing EV labels enriched within neurons and the distinct types of glia,as well as registering copositivity of said labels,should be replicated in future studies with larger sample sizes (Kawata et al.,2018).In order to promote this future effort,we have included a list of EV labels enriched within distinct cell populations outlined inTable 2.Moving forward,future research should aim to classify as many biomarkers as feasible in their samples as it is probable that a panel of biomarkers will be more telling than any single biomarker alone.Understanding the changes in EV cargo from different cell types at different time points post TBI may play a critical role in understanding the cellular changes that may one day be therapeutic targets in TBI.
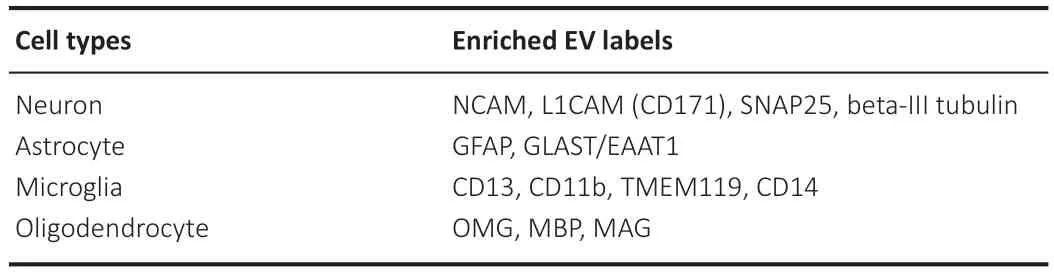
Table 2 |Cell enriched EV labels
Author contributions:NOB,ADR,WW,and JDW completed the relevant literature survey and contributed to writing the manuscript.Figures were created with BioRender.com by NOB.Supervision and expert guidance was carried out by SNW who also made significant contributions to writing.All authors read and approved the final manuscript for publication.
Conflicts of interest:None declared.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Ye Xiong,Henry Ford Hospital,USA.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
