Advances in non-invasive imaging of proteinopathies in animal models of neurodegenerative diseases
2024-01-24LeiCaoBinJiRuiqingNi
Lei Cao,Bin Ji,Ruiqing Ni
Neurodegenerative diseases,including Alzheimer’s disease (AD),frontotemporal dementia,Parkinson’s disease,and dementia with Lewy bodies,represent tremendous unmet clinical needs.A common feature of these diseases is the aberrant cerebral accumulation of pathological protein aggregates,affecting selectively vulnerable circuits in a disease-specific pattern.Earlier studies have established a relationship between abnormal aggregation and neuronal dysfunction or loss,suggesting multifactorial pathogenesis mechanisms in these neurodegenerative disorders.Developing disease-modifying drugs requires a thorough molecular understanding of how the proteinopathies progressively spread and the link to neurodegeneration and cognitive impairment.Preventing and removing pathological protein aggregates has shown potential as an effective therapeutic strategy for these proteinopathies.Immunotherapies have demonstrated slowing the rate of cognitive decline by effectively removing pathological amyloidbeta (Aβ) deposits in patients with AD.Several immunotherapeutic approaches targeting tau,alpha-synuclein,and TAR DNA-binding protein 43 for the treatment of neurodegenerative disorders,including AD,primary tauopathies,alpha-synucleinopathies,and amyotrophic lateral sclerosis,are currently in clinical trials.Advances in neuroimaging technology have enabled the noninvasive detection of physiopathological events in the brains of living patients and disease animal models.Positron emission tomography (PET) and single-photon emission computed tomography(SPECT) with radioactive ligands for protein aggregates such as Aβ,tau,and alpha-synuclein with β-sheet structures have facilitated the early and differential detection of AD and primary tauopathies.Aβ and tau PET have demonstrated their clinical utility and validity and are considered surrogate efficacy endpoints in clinical trials of pharmacological or emerging nonpharmacological treatments.In the era of disease-modifying therapy,imaging biomarkers are expected to play an increasingly important role in early diagnosis,patient stratification,and monitoring,in addition to fluid biomarkers.
Animal models such as transgenic/knock-in/inoculated rodents recapitulating these features have been instrumental in deciphering the underlying disease mechanisms,although it is known that differences exist in the conformation of fibrillar aggregates between patients with disease and models.Forin vivoimaging in animal models,a single-scale approach has often been used and provides limited information in the understanding of multifactorial diseases.This perspective highlights recent developments in the non-invasive imaging of proteinopathies in animal models.
Ligands:Antibody-based ligands: The ligands for proteinopathy imaging are mainly characterized as small molecules that detect the β-sheet structure,which are used in the clinical setting;and antibodybased ligands bind specifically to specific epitopes.Depending on the imaging modalities and imaging mechanism,the commonly used ligands have fluorescence emitting properties for fluorescence imaging,are suitable for photoacoustic imaging or magnetic resonance imaging or are labeled with radionuclei such as [11C]/[18F] for PET imaging.Antibody-based Aβ targeted ligands are sensitive and specific for detecting Aβ pathology in mouse models of AD but face challenges in blood-brain barrier entrance.To improve the brain uptake of Aβ and alpha-synuclein antibody fragments,transferrin receptor,which enhances receptormediated transcytosis,has been used as an effective approach for immunoPET/SPECT and application in AD and Parkinson’s disease animal models (Sehlin et al.,2016).In addition,a recent study demonstratedin vivofluorescence imaging using a single-domain antibody-based ligands of smaller size that do not require additional facilitators for crossing the blood-brain barrier(Jiang et al.,2023;Figure 1).

Figure 1 |Non-invasive in vivo imaging of proteinopathies in animal models.
Near-infrared (NIR) II fluorescent amyloid imaging ligands: Most of the available amyloid fluorescence imaging ligands reside below the emission wavelength of 950 nm.The fluorescence signal is affected by intense light scattering and signal cross-talk with superficial tissues,rendering quantification of the distribution of ligands in deep brain areas of the animal model difficult.In addition,given that autofluorescence from endogenous molecules in the brain may hamper the accuracy,the practical efficacy of such a method needs to be taken into consideration.NIRII (1000–1350 nm) amyloid binding ligands have shown great potential in noninvasive imaging in living animals with greater penetration depth,signal-to-noise ratio,and sensitivity (Miao et al.,2022).Moreover,ligands with aggregationinduced emission properties are expected to not only support thein vivodetection of amyloid aggregate distribution in the brain with antiquenching properties but also provide theranostic possibilities and even dual-targeted therapy,such as targeting reactive oxygen species and amyloid aggregated simultaneously.
Structure-based artificial intelligence (AI)-assisted screening: AI has revolutionized the field of medical imaging,influencing all processes from ligand discovery,screening,optimization of binding properties,pharmacodynamics and pharmacokinetics modeling,as well as streamlining image processing,analysis,and reporting (Figure 2).Rapidly advancing AI-assisted screening empowers the rapid development of imaging ligands based on the known structures of amyloids.Through the utilization of deep learning algorithms,AI can assist in identifying potential candidate molecules or antibody fragments with high binding affinities and specificity for pathological proteinopathies of specific conformations.This not only expedites the screening process and reduces the cost and time but also enhances the accuracy of the discovery step.Recent cryo-electron microscopy studies have revealed structural polymorphisms and disease-specific folding of amyloids in different diseases,such as alpha-synuclein in multiple system atrophy and Parkinson’s disease (Yang et al.,2022).Cryo-electron microscopy enables us to shed light on the potential multiple binding sites for imaging ligands such as the tau ligand PM-PBB on tau fibrils from primary tauopathies and AD brains (Tagai et al.,2021) as well as the discovery of the alpha-synuclein ligand F0502B on alphasynuclein fibrils (Xiang et al.,2023).Combining AIassisted screening,optimization,and experimental assays,including binding property characterization and validation,will speed up the development cycle,particularly for challenging targets without clinically available ligands such as alpha-synuclein and TAR DNA-binding protein 43.
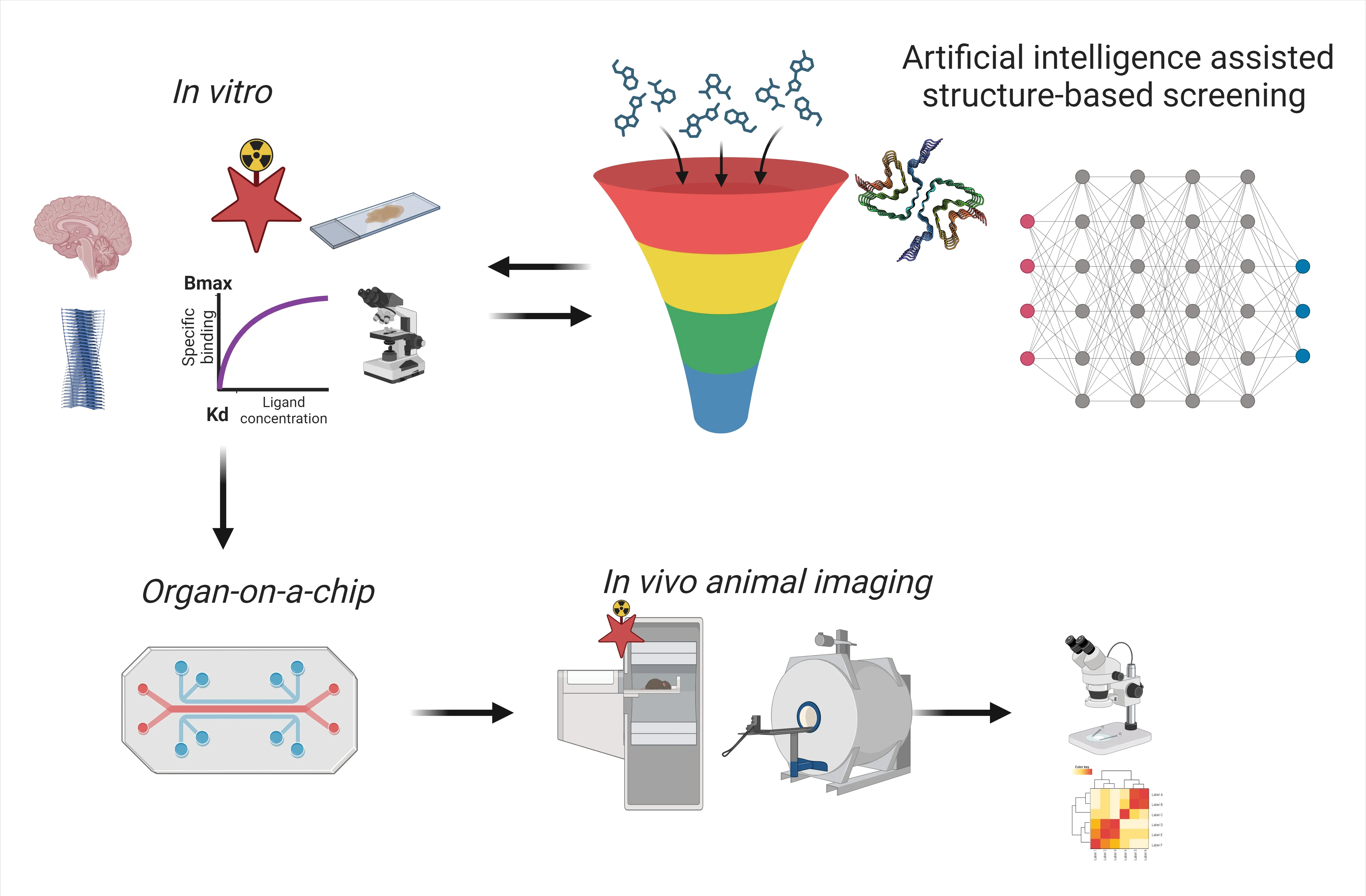
Figure 2 |Scheme of the development of imaging biomarkers for proteinopathies in neurodegenerative diseases.
Organ-on-a-chip:Organ-on-a-chip technology has emerged as a transformative approach for studying complex physiological processes beforein vivoimaging in animal models (Figure 1).It promotes the 3R principles (replace,reduce,refine) for the development and use of alternatives to animal experiments and has started to gain regulatory acceptance for toxicity testing.For imaging ligands targeting proteinopathies,the organ-on-a-chip platform allows forin vitroandin vivoextrapolation,i.e.,to investigate the ligand uptake,distribution,and binding kinetics even in multiple organs in a physiologically relevant environment.This immensely reduces the need forin vivoimaging in animal models for toxicity as well as for physiologically based pharmacokinetic/pharmacodynamic studies.
Pushing the imaging resolution limits:In small animal models,PET/SPECT is able to contribute to new ligand development and provide a strong prediction for clinical results by using clinically available techniques at the preclinical phases(Tagai et al.,2021).While PET provides excellent accuracy in quantification,the small animal PET system has a limited spatial resolution relative to the mouse/rat brain,hindering accurate mapping of amyloid/tauopathy in different brain regions.The quantification of PET images is further affected by the partial volume effect for quantification.PET/SPECT,advanced optical imaging in animal models of neurodegenerative diseases,provides compensatory information for deepening the understanding of disease mechanisms.Recent long-termin vivoimaging using photoacoustic/optoacoustic tomography (Chen et al.,2022;Ni et al.,2022;Vagenknecht et al.,2022;Deán-Ben et al.,2023) has enabled high-resolution,real-timein vivoimaging beyond the 1-mm penetration depth typical of microscopy methods.We recently developed an Aβ imaging pipeline using a fluorescence volumetric optoacoustic tomography platform assisted with the Aβ fibril binding oxazine derivative AOI987 (Ni et al.,2022).AOI987 absorbs far-red light,unlike hemoglobin,but eliminates interference from blood vessels,a common problem with this type of microscopy in transgenic arcAb and APP/PS1 mouse models of AD amyloidosis.
Optical two-photon microscopy offers excellent spatial resolution and longitudinal observation for identical pathology or cells,enabling the assessment of inclusion turnover and the response of individual cells at different distances from pathological inclusions.However,two-photon imaging is afflicted by a small (submm) field of view and limited depth penetration.One recently developed approach using large field-of-view light microscopy has provided an opportunity to enlarge the FOV while maintaining high resolution (6–10 microns).Assisted by the luminescent conjugated oligothiophene dye HS-169,which binds Aβ and tau,light microscopy resolved individual plaques in APP/PS1 and arcAβ amyloidosis mice as well as tau deposits in P301L tau micein vivoin the cortex.The imaging depth issues in proteinopathy imaging in animal models remain to be solved,since a large proportion of tauopathy and the majority of alpha-synucleinopathy are located in subcortical brain regions such as the hippocampus or striatum and substantia nigra.Qin et al.(2022)recently developed an adaptive optics threephoton microscope based on analog lock-in phase detection for focus sensing and shaping.This method measures and compensates for both aberrations and scattering induced by specimens with high accuracy.The authors demonstratedin vivoimaging of microglia and hippocampal neurons at synaptic resolution in the aged AD mouse brain through an open skull window.With the recently developed NIR-II two-photon amyloid detection ligands such as ZW800-1C (Hou et al.,2023),furtherin vivoapplications in imaging Aβ and tau aggregates in deep brain regions in animal models are expected.This approach will enable anin vivounderstanding of the accumulation of amyloid/tau and the phagocytosis of aggregates and neurons mediated by microglia in a living mouse model and provide insight into the link between amyloid/tau,neuroinflammation,and atrophy in the hippocampus.
Contrast agent-free imaging:The aforementioned various imaging approaches are made possible with the assistance of imaging ligands.Contrast agent-free imaging of amyloid deposits has been feasible in recent years.Pansieri et al.(2019)developed approaches forin vivoandin vitroultraviolet imaging of Aβ in a mouse model based on the ultraviolet-visible-near-infrared optical properties of amyloid fibrils.Using continuouswave NIR fluorescence-enhanced diffuse optical tomography,the author demonstrated direct detection of amyloid deposits in the brains of living APP/PS1 mice at 18 months of age with abundant Aβ plaque accumulation.An increasedin vivoNIR signal (excitation wavelength 690 nm)was measured using fluorescence-enhanced diffuse optical tomography and could also be seen using noninvasive 2D NIR imaging (Fluobeam 800) performed on the head of living mice.The ultraviolet-visible-near-infrared optical properties of amyloids open new research avenues for amyloidosis as well as for next-generation biophotonic devices.Whether a similar approach can be applied to image other amyloids,such as tau and alpha-synuclein,remains to be demonstrated.
In conclusion,there have been rapid advances in the noninvasive imaging of proteinopathies in terms of new ligands,AI-assisted discovery,and the development of new multiscale imaging setups with improved imaging resolution and depth.Therefore,it would help to understand the underlying disease mechanism and aid in the preclinical drug discovery.
No conflicts of interest exist between Changes Technology Corporation Ltd.and publication of this article.
RN received funding from Swiss Centre for Applied Human Toxicology,and Helmut Hortun Stiftung.
Lei Cao,Bin Ji,Ruiqing Ni*
Changes Technology Corporation Ltd.,Shanghai,China;Fudan University,Shanghai,China (Cao L)School of Pharmacy,Fudan University,Shanghai,China (Ji B)
Institute for Biomedical Engineering,University of Zurich Ð Zurich Institute for Regenerative Medicine,University of Zurich,Zurich,Switzerland(Ni R)
*Correspondence to:Ruiqing Ni,PhD,ruiqing.ni@uzh.ch.
https://orcid.org/0000-0002-0793-2113(Ruiqing Ni)
Date of submission:August 12,2023
Date of decision:November 7,2023
Date of acceptance:December 1,2023
Date of web publication:January 8,2024
https://doi.org/10.4103/1673-5374.392886
How to cite this article:Cao L,Ji B,Ni R(2024)Advances in non-invasive imaging of proteinopathies in animal models of neurodegenerative diseases.Neural Regen Res 19(10):2115-2116.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
