Going straight for the gut: gut-brain axis pathology and treatment of Parkinson’s disease
2024-01-24DominiqueEbedesCesarBorlongan
Dominique Ebedes,Cesar V.Borlongan
This perspective focuses on the recent literature regarding the role of the gut-brain axis (GBA) in fecal microbiota transplantation (FMT) and stem cell therapy (SCT) in Parkinson’s disease (PD).PD is the second most common neurodegenerative disease in the United States,yet therapies remain limited.Current research suggests that the GBA may play a role in the pathogenesis of PD.GBAbased FMT as well as SCT offer promising new avenues for PD treatment.Probing the interactions between FMT and SCT with the GBA may reveal novel therapeutics for PD.
PD is characterized by damage to the dopaminergic neurons of the substantia nigra and the formation of α-synuclein-containing inclusion bodies called Lewy bodies,which accompany the characteristic motor symptoms of PD: bradykinesia,tremor,and rigidity (Armstrong and Okun,2020).Despite PD affecting more than 6 million individuals worldwide,effective treatment options are sparse.Current treatments to improve motor symptoms include dopamine-based therapies for tremor,rigidity,and bradykinesia.Non-motor symptom treatments include selective serotonin reuptake inhibitors for mood and cholinesterase inhibitors for cognition.Rehabilitative therapy,exercise,and palliative care can be used to complement these pharmacological treatments.In severe cases of PD,patients may suffer from worsening symptoms when the drug wears off or they experience medication-resistant tremor.In these instances,more advanced treatments,like deep brain stimulation can be used.While these treatments alleviate PD symptoms,they are not diseasemodifying.Thus,there is a need for increased understanding of PD to retard or even halt the disease.GBA represents a novel target for this disease-modifying treatment.
GBA and neurological disease:The GBA refers to the bidirectional communication between the central and enteric nervous systems.In recent years,GBA has emerged as a promising therapeutic target for various neurological disorders,including stroke,Alzheimer’s disease,and PD (Liu et al.,2021).The connection between the brain and the gut is particularly well established in stroke: stroke alters gut microbiota composition,and gut dysbiosis impacts stroke outcomes.Hemorrhagic transformation (HT)is a life-threatening complication of stroke in which peripheral blood extravasates across the brain-blood barrier into the brain (Spronk et al.,2021).Since HT increases morbidity and mortality in stroke,it is an important pathological event that needs to be sequestered to improve stroke outcomes.An association exists between microbiota alterations and HT in stroke models(Spronk et al.,2021).When stroke rats were treated with antibiotics,hyperglycemia-induced HT exacerbation was not observed.Additionally,when rats were colonized with HT rat microbiota,they were more susceptible to HT.Altogether,these results suggest that altered microbiota may play an important role in the pathogenesis of hyperglycemic HT in stroke and stroke-related Alzheimer’s disease-like cognitive function (Liu et al.,2021).
Other forms of brain injury appear to be influenced by gut dysbiosis.Patients with amnestic mild cognitive impairment,the prodromal stage of Alzheimer’s disease,exhibit altered restingstate brain activity that is associated with specific gut composition (Liu et al.,2021).Compared to controls,Bacteroidetes levels increase in amnestic mild cognitive impairment patients,which negatively correlates with the fractional amplitudes of low-frequency fluctuations value of the cerebellar vermis (Liu et al.,2021).This suggests that resting-state brain activity alterations coincide with Bacteroidetes infiltration of the gut in amnestic mild cognitive impairment patients.
Increasing evidence reveals significant upper and lower gastrointestinal dysfunction in patients with PD as evidenced in a meta-analysis of 22 studies using 16S RNA,which is a sequence of DNA encoding the RNA of the small subunit of the ribosome of bacteria (Romano et al.,2021).In 2003,Braak hypothesis advances the notion that PD starts in the gut.This bidirectional propagation of α-synuclein across the GBA occurs via the vagus nerve (i.e.,duodenum to the brainstem to the stomach;Van Den Berge et al.,2019).Alterations to normal microbiota may be a key step in the development of PD.Indeed,α-synucleinoverexpressing mice whose microbiomes were depleted with antibiotics display decreased motor symptoms compared to those with intact microbiomes (Sampson et al.,2016).Interestingly,treatment with short-chain fatty acids (SCFAs)restores the major motor features of PD in these microbiome-depleted mice (Sampson et al.,2016),suggesting that the presence of gut microbes and their metabolites may interact with genetics to promote motor and intestinal dysfunction in PD.
The clinical importance of SCFA metabolites is implicated in PD patients who exhibit reduced SCFA in their feces compared to healthy controls(Unger et al.,2013).This altered SCFA is likely due to changes in the prevalence of different bacterial families,specifically,reductions inBacteroidetesandPrevotellaceaebut an increase inEnterobacteriaceae(Unger et al.,2013).This signature gut composition in PD patients shows a lower abundance of certain microbiota families and a significantly higher abundance of others across different geographic regions.These changes in the gut microbiome and SCFA production appear to alter the enteric nervous system that is unique to PD.Understanding how changes to the microbiome may mediate PD onset and progression may be used as a therapeutic target.Just as certain modifications to gut microbiota may exacerbate PD,others may ameliorate it.The microbiome can be altered via diet,probiotics,prebiotics,antibiotics,SCT,and FMT.
Gut-directed therapies for PD:FMT refers to the transplantation of feces from a healthy individual to a sickly individual to modify the sickly individual’s microbiome.Support for the use of FMT for PD is mostly from animal studies and a few case studies.FMT via transendoscopic enteral tubing during colonoscopy once per day for three days decreased one patient’s constipation and caused no negative reactions at three months follow-up (Huang et al.,2019).Perhaps even more interestingly,a transitory decrease in motor symptoms was also observed for three months following this FMT treatment.Similarly,at 4 weeks post-FMT,motor,non-motor,and constipation symptoms were improved in 5 out of 6 PD patients(Segal et al.,2021).While these case studies indicate the potential of treatments targeted to the gut for treating PD,randomized controlled trials are needed to rule out other explanations for symptomatic relief such as the placebo effect or the resolution of constipation and discomfort improving motor symptoms.Notably,the patient’s microbiome that closely resembled that of the donor at 1 week gradually drifts back to its prior composition in the subsequent weeks (Huang et al.,2019).Microbiome-based treatments with long-lasting effects will likely yield improved clinical efficacy.
Many questions must be answered before FMT becomes a mainstream treatment for PD.For instance,the best delivery route for FMT warrants investigation.While FMT has been delivered via the colon,FMT can also be delivered via nasaljejunal tube,endoscopy,oral capsules,or retention enema.Patients receiving FMT via colonoscopy experience therapeutic effects,while patients receiving the same treatment via nasal-jejunal tube do not.These site-specific outcomes suggest the complexity of advancing FMT from the bench to the clinic in PD.Again,randomized clinical trials are needed to increase the clinical safety and efficacy of FMT for PD,with emphasis on assessing transplant-associated factors,such as route of delivery,dose,and timing,as well as the need for immunosuppression.
An alternative approach to treating PD via the microbiome is by using antibiotics.In the laboratory,mice overexpressing α-synuclein when treated with an antibiotic display reduced PD symptoms,while microbial recolonization promotes the disease pathology (Sampson et al.,2016).This suggests that a specific signature of altered gut microbiota may be associated with motor deficits,microglia activation,and α-synuclein pathology (Sampson et al.,2016).Yet,as certain gut microbiota appear to contribute to PD pathology,other microbiota seem to ameliorate the disease,as discussed previously.Additional studies are needed to elucidate this signature gut microbiome in PD models and patients by probing FMT and treating with antibiotics.
SCT,PD,and GBA:Preclinical studies and limited clinical trials suggest that SCT may alleviate PD.Cell replacement and by-stander effects of the transplanted cells may mediate the improved clinical outcomes.While SCT is traditionally considered as directly rescuing the brain,new compelling evidence suggests that the transplanted stem cells may act on the gut (Lee et al.,2019a,b,2022).
SCT may modulate the gut microbiome in PD;specifically,the transplanted stem cells may alleviate PD pathology by altering the gut microbiome composition.In two rodent models of PD,infusion of human umbilical cord blood(hUCB)-derived stem cells and plasma enhances motor and gastrointestinal functions and reduces dopaminergic neuronal loss (Lee et al.,2019a,b,2022).PD rodents intravenously treated with hUCB stem cells and plasma display diminished proinflammatory microbiomes both in the nigra and the gut.These results suggest that correcting gut dysbiosis via SCT may sequester inflammation and neurotoxicity,thus ameliorating PD (Lee et al.,2019a,b,2022).
Current stem cell sources,such as hUCB,only partially recapitulate the phenotype and function of mature dopaminergic neurons.Thus,it is likely that hUCB grafts ameliorate the histological and behavioral deficits associated with PD via the secretion of therapeutic substances.However,the exact mechanism of action is unknown.With intravenous hUCB,the treatment affords an effect at a neurological and gastrointestinal level (Lee et al.,2019a,b,2022),suggesting that SCT may act both on the gut and the brain.The hUCB appears to migrate preferentially from the bloodstream to the gut and brain.Direct delivery to the gut,similar to an FMT,may yield better functional outcomes,but a recent study shows that such an invasive approach may not be therapeutic,and actually be detrimental,in PD (Lee et al.,2022).These conflicting results require additional studies in optimizing stem cell route of delivery,dose,andtiming of intervention during the disease.
Although FMT and SCT have been viewed as standalone treatments,a combination treatment of these two approaches may render more beneficial effects in PD.Gut microbiota release metabolites,which may regulate gut homeostasis and dysbiosis,but also may control stem cell proliferation and differentiation.Stem cells perform a similar role to gut microbiota by maintaining a healthy gut microbiome composition.Indeed,stem cell targeting of the gut dampens deleterious immune response and inflammation in PD models (Lee et al.,2019a,b,2022).Interrogating the direct interaction of stem cells with the gut microbiome may further reveal the key role of GBA in PD.Future studies should probe whether this relationship is bidirectional,such that the gut microbiome impacts stem cell viability while the stem cells contribute to a healthy gut microbiome.Equally important is recognizing whether this crosstalk between the microbiome and stem cells closely accompanies PD onset and progression,which should advance our understanding of the disease pathology and treatment.To this end,the combined effects of FMT and SCT on the GBA represent a fertile ground for therapeutic development (Figure 1).
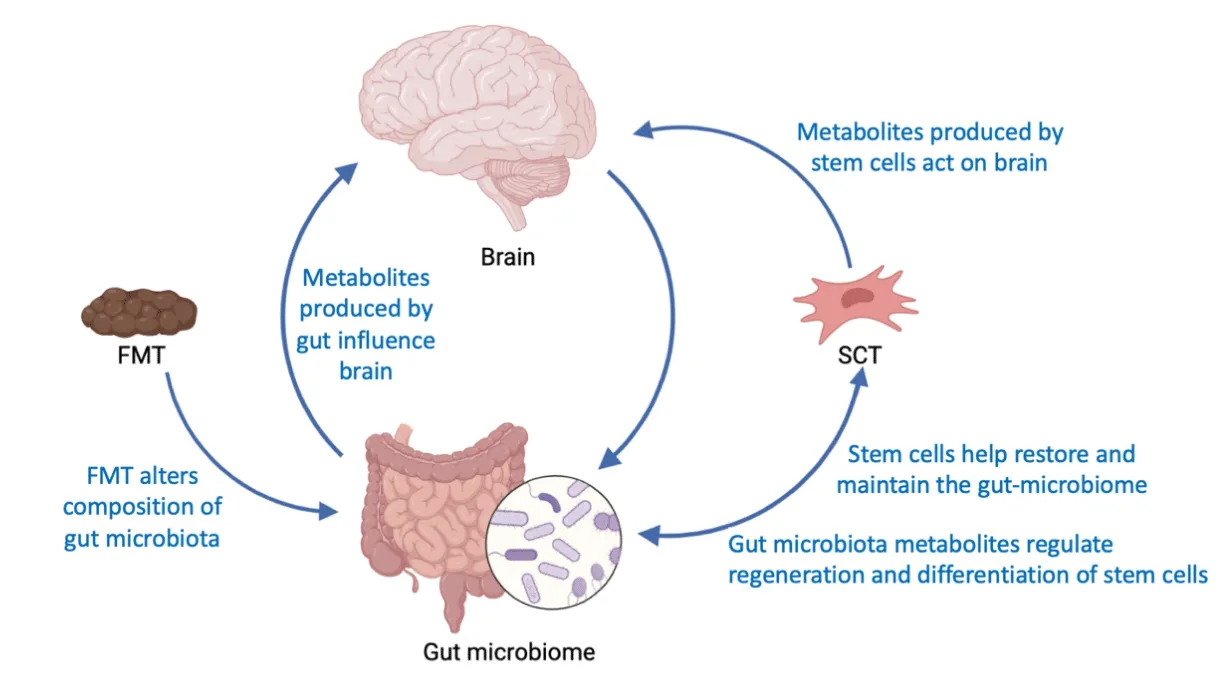
Figure 1 |The brain and gut microbiome communicate with one another in PD.
As the elderly population at risk for PD increases,developing a disease-modifying treatment for PD and other age-related neurodegenerative diseases is vital.Accumulating studies on the efficacy of FMT and SCT to ameliorate PD pathology both at the level of the brain and the gut make the GBA an appealing therapeutic target for PD.
Dominique Ebedes,Cesar V.Borlongan*
University of South Florida Morsani College of Medicine,Tampa,FL,USA (Ebedes D)
Center of Excellence for Aging and Brain Repair,Department of Neurosurgery and Brain Repair,University of South Florida Morsani College of Medicine,Tampa,FL,USA (Borlongan CV)
*Correspondence to:Cesar V.Borlongan,PhD,cborlong@health.usf.edu.
https://orcid.org/0000-0002-2966-9782(Cesar V.Borlongan)
Date of submission:July 29,2023
Date of decision:November 17,2023
Date of acceptance:November 30,2023
Date of web publication:January 8,2024
https://doi.org/10.4103/1673-5374.392885
How to cite this article:Ebedes D,Borlongan CV(2024)Going straight for the gut:gut-brain axis pathology and treatment of Parkinson’s disease.Neural Regen Res 19(10):2111-2112.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
