Actin(g) toward a revised understanding of the role of cytoskeletal dynamics in neuronal bioenergetics
2024-01-24SabrinaHollandGianlucaGallo
Sabrina M.Holland,Gianluca Gallo
Neurons are energy-demanding cells.Disruptions in energy metabolism can quickly interrupt neuronal function,leading to cell death and neurodegeneration.For instance,ischemia rapidly depletes adenosine triphosphate (ATP)thereby disrupting energy-dependent cellular processes crucial for homeostasis,and axon degeneration is preceded by a collapse of axonal ATP levels.The initial extension and regeneration of axons are also considered to require significant bioenergetic utilization.Therefore,understanding neuronal bioenergetics and the regulation of intracellular ATP levels is crucial for both our basic understanding of neuronal function and ultimately developing new therapies to facilitate neuronal regeneration after injuries.Work performed over 20 years ago,using available approaches,lead to the hypothesis that actin filament dynamics are a major “sink” of ATP utilization in embryonic ciliary ganglion neurons (Bernstein and Bamburg,2003) and platelets (Daniel et al.,1986).However,neither of these studies directly measured ATP and relied on indirect measurements or quantitative estimation of ATP.Our recent work (Holland and Gallo,2023) revisited this hypothesis using modern live imaging ratiometric intra-cellular reporters of ATP and the ATP/ADP ratio and failed to obtain data consistent with the hypothesis that actin filament dynamics are a sink of ATP utilization in embryonic sensory neurons.Thus,these recent data indicate that the contribution of cytoskeletal dynamics and other bioenergetic sinks would benefit from additional scrutiny.
The actin filament cytoskeleton has important roles in axon extension/regeneration and morphogenesis,the establishment and function of synapses,and a multitude of additional cellular processes.Actin filaments are formed through nucleation of cytosolic actin subunits and elongate through subsequent polymerization until depolymerized (Figure 1A),a cycle referred to as actin filament turnover.Actin subunits bound to ATP are primed for nucleation and polymerization into actin filaments (for a review on actin dynamics see Pollard,2017;Figure 1A).Following polymerization into a filament,the hydrolytic activity of actin increases,leading to ATP hydrolysis along the filament (Figure 1A).Consequently,based on the conclusions of Bernstein and Bamburg (2003) and Daniel et al.(1986),actin filament turnover has been hypothesized to be a significant drain of cellular ATP (recent reviews discussing this hypothesis include DeWane et al.,2021;Bülow et al.,2022).
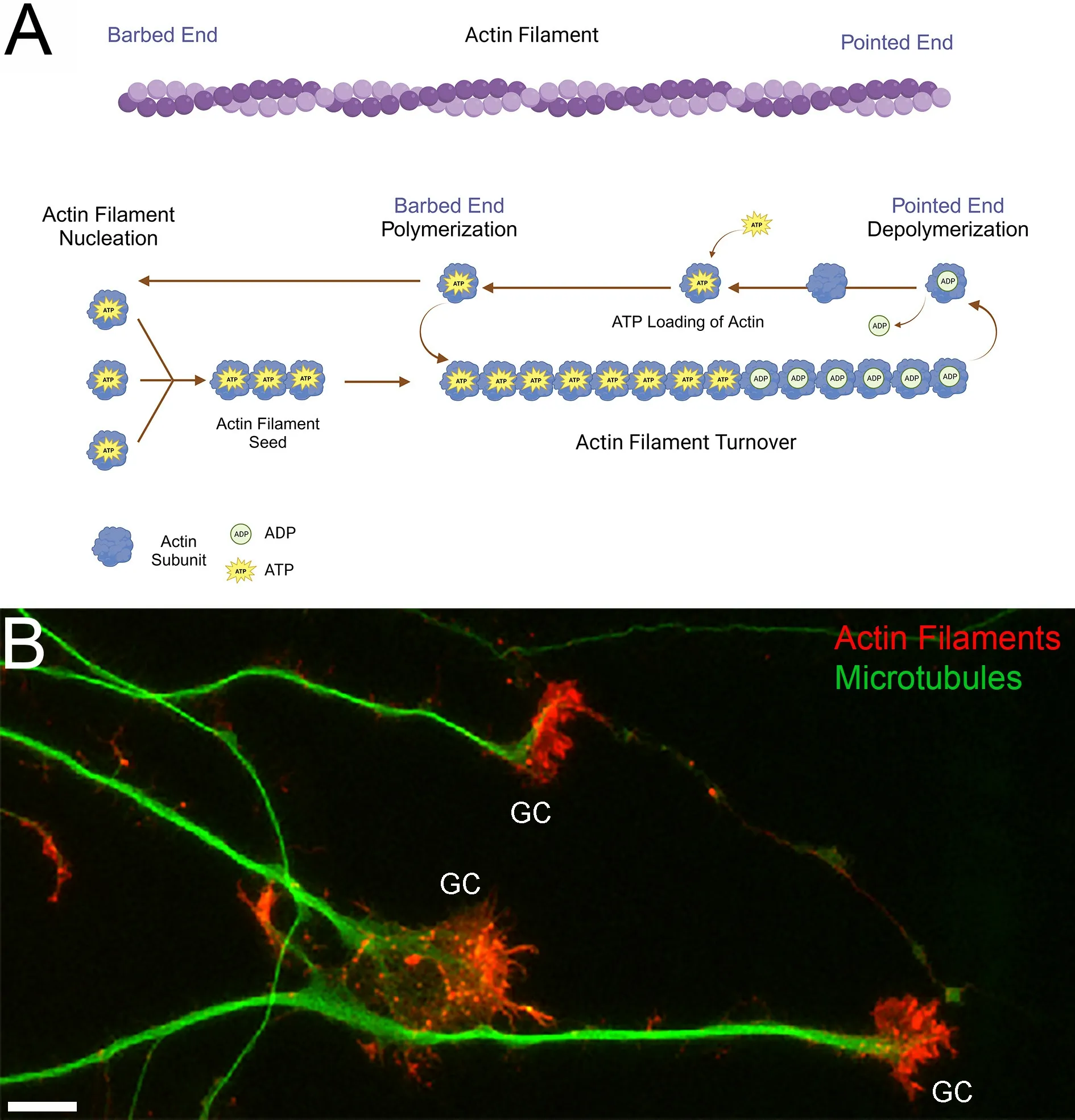
Figure 1 |Actin filament dynamics and growth cones.
Embryonic sensory neurons extend their axons over long distances before reaching their target tissues.In developing neurons,the region with the highest density of actin filaments and filament dynamics is the growth cone of extending axons(Figure 1B).Growth cones exhibit actin filamentdependent dynamic filopodia and lamellipodia that mediate axon growth and guidance.While mitochondria are distributed throughout axons,their highest density occurs in the growth cones of these neurons.The role of mitochondrial oxidative phosphorylation and glycolysis in regulating growth cone dynamics and extension has begun to be investigated (reviewed in Gallo,2020;Ketschek et al.,2021).However,the local regulation of growth cone bioenergetics by cytoskeletal dynamics remains minimally understood.Therefore,in our recent study (Holland and Gallo,2023),we sought to further investigate the impact of actin dynamics on neuronal energy homeostasis focusing on growth cones based on the rationale that growth cones represent a site of high actin filament dynamics.
Using modern genetically encoded ratiometric reporters we measured the ATP/ADP ratio(PercevalHR;Tantama et al.,2013) and ATP levels(mRubyiATPSnFR;Lobas et al.,2019) locally in the growth cones and cell bodies of live cultured embryonic chicken sensory neurons.Disrupting actin filament polymerization and turnover did not impact the ATP/ADP ratio or ATP levels under normal growing conditions.Furthermore,manipulations of actin dynamics did not impact the decrease in the ATP/ADP ratio or ATP level induced by the blockade of ATP production.This result stands in contrast to the results of Bernstein and Bamburg (2003) that used the same experimental design but measured ATP levels in live neurons through indirect methodologies (i.e.,changes in cytosolic magnesium level as a proxy for ATP,see Bernstein and Bamburg for details).Prior work established that actin filament level and dynamics in sensory growth cones are increased by acute nerve growth factor treatment,which leads to an increase in surface area and morphologic complexity of the growth cone together with hyperpolarization of axonal mitochondria.However,even though we detected increased growth cone expansion and hyperpolarization of mitochondria located in the growth cones,the ATP/ADP ratio remained stable even when nerve growth factor signaling and actin dynamics were at their maximum.In addition,luciferase-based quantification of cellular ATP in forebrain neurons also failed to reveal any effect of actin filament depolymerization at steady-state or in response to shutdown of ATP synthesis.Microtubule dynamic instability may constitute another energy-depleting process and can indirectly regulate actin filament turnover.While the suppression of microtubule plus tip dynamics during nerve growth factor signaling did not impact the ATP/ADP ratio in growth cones in our recent study,it did prevent growth cone elaboration dependent on increased actin filament level and dynamics.In conclusion,our data fail to provide evidence in support of the hypothesis that actin dynamics are a major drain of ATP in the growth cones and cell bodies of developing neurons and underscore the need to reevaluate established theories using modern techniques.Furthermore,the data emphasize the necessity for additional exploration into additional possible sinks of ATP utilization in developing neurons.
Beyond cytoskeletal dynamics,other sources of energy expenditure such as protein synthesis,lipid turnover,membrane pump activity,and mitochondrial proton leak warrant further investigation through the use of live imaging reporters with subcellular resolution.Apart from energy-consuming processes,the mechanism by which cells maintain a steady level of ATP and ATP/ADP ratio is not clear and will require further analysis.In our study treatment with the AMP-activated protein kinase activator AICAR increased the ATP/ADP ratio in growth cones.This observation implies the potential involvement of the AMP-activated protein kinase in establishing the setpoint for the cytoplasmic ATP/ADP ratio in growth cones.Work by Baeza-Lehnert et al.(2019) points to the sodium pump as a major mediator for the maintenance of the ATP level in cultured hippocampal neurons that have formed synaptic networks.The regulation of mitochondrial superoxide flashes could contribute to sustaining bioenergetic homeostasis as observed in other electrically active cells (Wang et al.,2017).
Bioenergetic homeostasis encompasses a delicate balance between energy-consuming and energy-producing processes at the level of subcellular domains.For instance,the positioning of mitochondria along axons is of functional significance,underscored by processes like axon regeneration or axon branching,both of which have been linked to precise mitochondrial positioning.Some glycolytic enzymes (e.g.,phosphofructokinase,aldolase,pyruvate kinase,lactate dehydrogenase,glyceraldehyde 3-phosphate dehydrogenase) contain actin filament binding domains that target the enzymes to specific subsets of actin filaments within cells.While oxidative phosphorylation and glycolysis constitute the primary ATP sources,cells also employ creatine kinase-based ATP regeneration to promptly replenish ATP within subcellular domains with high energy demands(as reviewed by Gallo,2020).The consideration of the specific local context is thereby suggested to be crucial for understanding the regulation of energy homeostasis.The availability and ongoing development of genetically encoded sensors for ATP,GTP,and various signaling pathways dependent on these energy sources,combined with modern imaging techniques,will enable the continuous revision and advancement of our understanding regarding the bioenergetics of subdomains in neurons.
Our recent report that actin filament dynamics are not the hypothesized major sink of ATP utilization in developing sensory neurons,as otherwise indicated by earlier work (Daniel et al.,1986;Bernstein and Bamburg,2003),suggests the need for continued analysis of bioenergetic availability and utilization at the subcellular level.As the literature on the topic of neuronal bioenergetics expands,it will be impactful to address the issue in a variety of cellular contexts and neuronal cell types.For example,little is known about whether in neurons extracellular matrix molecules,cell adhesion molecules,and other extracellular signals (e.g.,guidance cues) might impact bioenergetics,mitochondria biology,or glycolytic activity,or alter the ATP level or ATP/ADP ratio set point.As neurons develop,they transition from a strong dependence on glycolysis to mitochondrial oxidative phosphorylation as the major source of ATP (reviewed in Gallo,2020).It will thus be of interest to address neuronal bioenergetics at the subcellular level as a function of neuronal age and development.Finally,the multitude of issues related to neuronal bioenergetics will also need to be assessedin vivoand modern imaging tools and modalities will assist in this endeavor (e.g.,van Hameren et al.,2019).The availability of new tools holds great potential for the continued dissection of neuronal bioenergetics and the regulation thereofin vitroandin vivo.
In conclusion,prior work addressing the potential significance of actin filament and cytoskeletal dynamics in consuming ATP in developing neurons has been inspirational to the field and was the driving force behind our study.Our results indicate that this issue requires additional scrutiny.While cytoskeletal dynamics may not be a physiologically significant sink of ATP utilization in healthy developing neurons,they may well contribute significantly in specific contexts,neuronal types other than peripheral nervous system neurons,or as a function of neuronal age.It will be of interest to further consider the possible roles in cytoskeletal dynamics as a sink of ATP utilization in models of disease and injury states wherein the physiology of the neuron is aberrant.We hope our recent publication will lead others to consider this issue in their specific areas of expertise and model systems.
This work was supported by National Institute of Health awards NS118000 and NS128049(to GG).
Sabrina M.Holland,Gianluca Gallo*
Department of Neural Sciences,Shriners Pediatric Research Center,Lewis Katz School of Medicine at Temple University,Philadelphia,PA,USA
*Correspondence to:Gianluca Gallo,PhD,tue86088@temple.edu.
https://orcid.org/0000-0002-0777-379X(Gianluca Gallo)
Date of submission:September 7,2023
Date of decision:November 10,2023
Date of acceptance:December 3,2023
Date of web publication:January 8,2024
https://doi.org/10.4103/1673-5374.392863
How to cite this article:Holland SM,Gallo G(2024)Actin(g)toward a revised understanding of the role of cytoskeletal dynamics in neuronal bioenergetics.Neural Regen Res 19(10):2109-2110.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Modulation of p75 neurotrophin receptor mitigates brain damage following ischemic stroke in mice
- Conformational dynamics as an intrinsic determinant of prion protein misfolding and neurotoxicity
- Exploring the synergy of the eyebrain connection: neuromodulation approaches for neurodegenerative disorders through transcorneal electrical stimulation
- Pathogenic contribution of cholesteryl ester accumulation in the brain to neurodegenerative disorders
- Cognition and movement in neurodegenerative disorders:a dynamic duo
- Probing the endoplasmic reticulummitochondria interaction in Alzheimer’s disease: searching far and wide
