A high level of extra-pair paternity in the Chestnut Thrush(Turdus rubrocanus)
2024-01-22HunLiuYunFngYingqingLouYuehuSun
Hun Liu, Yun Fng, Yingqing Lou,*, Yuehu Sun,**
aKey Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
Keywords:Chestnut Thrush Extra-pair paternity Microsatellite Morphological traits
ABSTRACTExtra-pair copulation (EPC) can potentially maximize individual reproductive fitness, and this process may involve sexual selection of male and female traits that reflect individual quality.Previous studies have implied that adult characteristics are associated with the probability of extra-pair paternity (EPP), but it differs between species.Moreover, there are relatively few examples of the adaptive rationale for females’ engagement in EPCs based on an examination of these more traditionally recorded traits, in combination with female flight-mediated traits such as wing length.We investigated whether EPP existed in the wild Chestnut Thrush(Turdus rubrocanus)population during three breeding seasons(2019–2021),and whether paternity was related to morphological traits of males and females.Eight highly variable microsatellite loci were used to identify paternity, and generalized linear mixed models were used to analyze the relationship between paternity and morphological traits.We found that EPP existed in the Chestnut Thrush.53.3% (N ¼ 41/77) of the broods contained at least one extra-pair offspring (EPO), and 34.6% (N ¼ 72/208) of the nestlings were EPO.We also found that male wing length was negatively associated with the probability of EPP and the proportion of EPO.Female body length was positively related to the probability of EPP.Both female body condition and mass were negatively associated with the proportion of EPO.While other traits of male and female did not relate to the probability of EPP or the proportion of EPO.Extra-pair males had better body condition compared to the males they cuckolded.EPO did not differ from their half-siblings in terms of body size or body condition.The results suggest that body size and body condition were associated with EPP in the Chestnut Thrush.This study provides fundamental information for further studies on the evolution and maintenance of EPP in the Chestnut Thrush, and it is also useful for the comparison of EPP among Turdus species.
1.Introduction
Extra-pair paternity(EPP)is a common phenomenon in avian species,having been detected in 76%of all known social monogamous bird species that exhibit biparental care (Brouwer and Griffith, 2019).EPP rates vary among species(Griffith et al.,2002;Wang et al.,2021),being particularly high(up to 80%)in cooperative breeding species(Hughes et al.,2003),yet close to zero for other species such as Tawny Owls (Strix aluco) (Saladin et al.,2007).The gain of EPP or loss of paternity is often related to individual characteristics, such as body condition, body size and age (Hsu et al., 2015; Adams and Wilkinson, 2020; Desrosiers et al., 2021).It has been found that body condition positively correlates with individual immunocompetence,survival rate and reproductive fitness(Bustnes et al.,2002;Blums et al.,2005;Gleeson et al.,2005).For example,males in good body condition may be better at defending territory(Nystr€om,1997),or at providing paternal care (Dearborn, 2001), which are beneficial for females.Body size also has a positive effect on reproductive success, since large individuals may do well in acquiring territory (Desrosiers et al.,2021),guarding their mates(Møller,1987),and caring for their offspring(Williams,2018).Why females engage in extra-pair copulations(EPCs)has been the subject of much debate since EPC is a process of participation of both sexes,and may be involved in sexual selection of both male and female traits, which may signal individual quality (Costanzo et al., 2017).Furthermore, previous research has revealed that females with better flight-related traits such as wing loading (body mass/wing area) may be more capable of EPC resistance(Plaza et al.,2019).Since wing length can affect flight capacity by influencing wing load (Videler, 2006), which would be expected to be positively related to EPP in females where there is sexual conflict with males(Plaza et al.,2019),the inclusion of such flight related traits alongside other characteristics such as body size and condition, may help us better understand the adaptive rationale for female involvement in EPCs.
Numerous adaptive and nonadaptive hypotheses have been proposed to explain EPP variation between individuals,populations and species,such as good gene(Møller,1988),breeding density(Westneat,1990),and breeding synchrony(StutchburyandMorton,1995).Thegood gene hypothesisisone of most frequently explored hypotheses,which predicts that females benefit indirectly from EPCs,preferring high-quality males as extra-pair mates to gain‘good gene’for their offspring,and males may advertise their genetic‘quality’through various behavioral or morphological traits(Møller,1988).Thus,studies testing goodgene hypothesisusually look forbehavioral(such as song)and/or morphological (such as ornaments and body size)differences between extra-pair and within-pair males (reviewed in Akcay and Roughgarden,2007).Male traits including age,body size,ornaments and song are also positively associated with reproductive success,which is often interpreted as evidence that females seek benefit for their offspring(Griffith et al., 2002).A review of studies on testing this hypothesis suggests that extra-pair males would be,on average,of larger body size than within pair males, but do not differ in body condition and secondary characteristics(Akcay and Roughgarden,2007).
Furthermore, the good gene hypothesis predicts that the extra-pair offspring (EPO) have higher fitness than their maternal half-siblings,the within-pair offspring (WPO) (Møller, 1988).The use of a range of characteristics related to fitness is understandable given the inherent difficulties in measuring offspring fitness in most cases.For example,body condition is positively associated with survival to independence(Hochachka and Smith, 1991), tarsus length is observed to predict individual quality(Kempenaers et al.,1992;Hall et al.,2013),wing length is a strong predictor of timing of fledging of nestlings (Michaud and Leonard,2000) and it is also positively related to probability of recruitment (McCarty, 2001).Numerous studies have shown that EPO tend to have superior fitness-related traits such as growth rate (O'Brien and Dawson, 2007), recruitment (Schmoll et al., 2005), and body size(Bouwman et al., 2007) than their maternal half-siblings, yet other studies have failed to find any differences in morphology or immune-competence between EPO and WPO(Charmantier et al., 2004;Wilk et al.,2008).
In this study, we investigated whether EPP exists in a wild Chestnut Thrush (Turdus rubrocanus) population over three-year period, and explored the relationships between EPP and morphological traits.The Chestnut Thrush is a socially monogamous passerine bird that exhibits sexual dimorphism in morphological traits (Chen, 2016) and plumage color traits(Lou et al.,2022).The breeding period of the Chestnut Thrush in our study area ranges from early May to late July,and most individuals raise only one successful brood.Field observations reveal that the Chestnut Thrush has no obvious territorial behavior, and the distance between the nests is relatively short (the nearest distance was 10 m).Therefore,this study has four objectives:(1)to determine the presence of EPP in the Chestnut Thrush population; (2) to determine whether body size related traits and body condition of males and females are predictors of EPP within the population;(3)to test whether extra-pair males differ significantly in their body size related traits and body condition compared to within-pair males;and(4)to investigate whether EPO differ from their maternal half-siblings in several quality traits (tarsus length,wing length and body condition).
2.Materials and methods
2.1.Study area and species
Fieldwork was conducted in the Lianhuashan Nature Reserve,Gansu Province, China (34◦40′67′′N, 103◦30′84′′E) from April to July in 2019–2021.The study area is located at the north edge of the Lianhuashan Nature Reserve and it is covered by shrublands,croplands and villages (Sun et al., 2003).The Chestnut Thrush builds open-up nests usually in trees and shrubs,approximately 1–5 m above the ground,and are easily detected by experienced observers within a certain distance.The clutch size varies from 1 to 5(usually 3 or 4),and brood size ranges from 1 to 4.Only females participate in incubation, and both parents participate in nest defense and nestling provisioning.Nests of the Chestnut Thrush were found by systematical searches in the study area.
Before clutch initiation, we used mist nets to capture adult birds randomly as many as possible to find potential extra-pair males.Mist nets were also used to catch adults during nestling period when the nestlings were 5–8 days old.Nestlings were measured at 9–10 days old,and they fledged at 14–15 days old.For each captured bird,we measured the body mass (0.01 g), bill length (0.01 mm), tarsus length (0.01 mm), wing length (0.01 mm), body length (0.01 mm), and tail length (0.01 mm).Body condition was estimated by scaled mass index (SMI), which calculated in formula:SMI=Mi×(L0/Li)b.Miand Liare the body mass and tarsus length of each individual; L0is the arithmetic mean value of tarsus length; b is the regression coefficient by standardized major axis regression of lnM on lnL (Peig and Green, 2010).Furthermore, each individual was marked by a metal band and color bands.Before releasing,blood samples of all individuals were taken from the brachial vein and stored in absolute ethanol.Individuals were released near the nests where they were caught.Cameras(EZVIZ S2)were used to verify social parents during the nestling provisioning period.In our study area, it is difficult to distinguish the age of wild Chestnut Thrushes because the recruitment is rather low.
2.2.Paternity analysis
DNA extraction was performed using the TIANamp Blood DNA Kit(Tiangen Biotech, Co., Ltd).Since no microsatellite locus has been previously characterized in the Chestnut Thrush, we firstly tested 20 microsatellite loci isolated from its related species European Blackbird(Turdus merula) with cross-species amplification (Simeoni et al., 2009).Polymerase chain reaction(PCR)was run in 20 μL volume including 0.4 μL genomic DNA,0.5 U Taq DNA polymerase,2.5 mM dNTPs,0.4 μL of each forward and reverse primers, 2 μL 10 × Taq buffer (with 15 mM MgCl2).PCR was performed under following conditions: 5 min initial denaturation at 95◦C, 35 cycles of 30 s at 95◦C, 30 s at locus-specific annealing temperature (Table 1), 30 s at 72◦C, followed by a final extension of 72◦C for 5 min.PCR products were run on an ABI-3730 XL DNA analyzer with an internal lane size standard (GS500 LIZ, Applied Biosystems.Inc).Alleles fragments were binned and sized using Gene-Marker 2.2 software.All alleles were also confirmed manually.PCR amplifications and genomic analyses were repeated at least two times to confirm genotypes.
Basic statistical indices of microsatellites were obtained for 50 (25 males and 25 females) presumably unrelated adult individuals.The number of alleles(A), expected heterozygosity (He), observed heterozygosity (Ho), Hardy-Weinberg equilibrium (HWE) and linkage disequilibrium (LD) were estimated using Genepop on the Web version 4.7(Raymond and Rousset, 1995; Rousset, 2008), and a Bonferroni correction was applied (Rice, 1989).We calculated the null allele frequency(Fnull),polymorphic information content(PIC),probabilities of exclusion based on the genotype of no parent known (Excl 1), probabilities of exclusion based on the genotype of one parent known (Excl 2) using Cervus 3.0 (Kalinowski et al., 2007).Null alleles have no significant effect on paternity analysis when their frequency is less than 0.2 (Dakin and Avise,2004).
We first manually confirmed the genotypes between parents and nestlings to detect EPO by comparing the nestlings’ presumed paternal alleles with the genotype of the social father.Nestlings with 2 or more mismatches in their genotypes with the social father were identified as EPO.Then, we applied Cervus 3.0 (Kalinowski et al., 2007) usingmaximum likelihood method to perform paternity assignment.We searched for the most likely genetic father using inferred parental genotypes based on its genetic nestlings and their associated social mother requested for assignments at 95%confidence level.
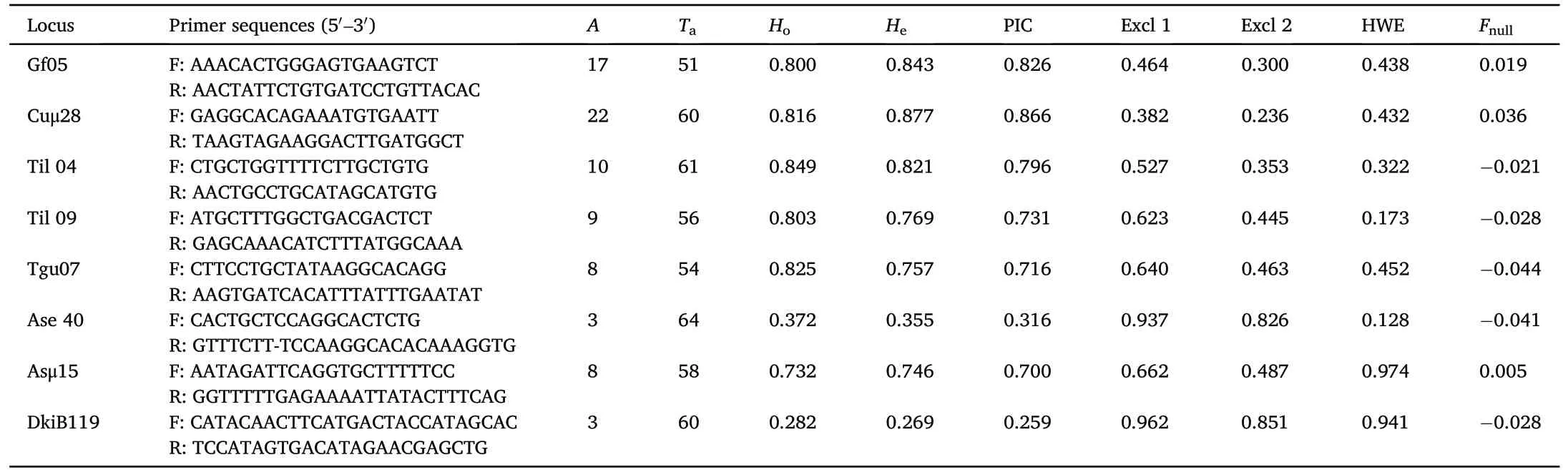
Table 1Microsatellite loci genotyped in the Chestnut Thrush population at the Lianhuashan Nature Reserve, Gansu Province, China.
2.3.Statistical analysis
We used univariate generalized linear mixed models (GLMMs, with binomial distribution and logit link function) to test the relationships between the probability of EPP within nests and morphological variables of males and females,respectively.We included bill length,wing length,tarsus length, tail length, body length, mass and body condition as predictors, respectively, clutch size and year as covariates, individual identity as a random effect in these models.
We conducted univariate linear mixed models (LMMs) to test the relationships between the proportion of EPO and morphological variables of males and females,respectively.In these models,the proportion of EPO was included as a response variable, bill length, wing length,tarsus length, tail length, body length, mass and body condition were predictors, respectively, clutch size and year were covariates, and individual identity was a random effect.
Three LMMs were used to compare the difference between EPO and WPO with wing length,tarsus length and body condition of the offspring as response variables,offspring status(EPO versus WPO)as a predictor,year as a covariate, and nest identity as a random effect in broods containing both EPO and WPO.Paired t-tests were used to investigate the differences of morphological traits between extra-pair males and males they cuckolded.
We estimated 95%confidence intervals(CI)for the probability that a brood contained at least one EPO and the frequencies of EPO per brood by fitting two generalized linear models(GLMs)with the intercept as the only fixed effect.This estimates the overall mean EPP rates across years.GLMMs,GLMs and LMMs were fitted with glmer and lmer functions from the R package lme4(Bates et al.,2014).All statistical analysis and graphs(with ggplot in the ggplot 2 package)were conducted using R version 4.1.3(R Core Team,2022).
3.Results
From the initial set of 20 primers, 14 primers amplified specific products and 6 failed to amplify any product or amplified a non-specific product.All 14 loci were then amplified in the Chestnut Thrush to test for polymorphism, 5 were monomorphic and 9 were polymorphic.Among the 9 polymorphic loci,1 was excluded because it deviates significantly from Hardy-Weinberg equilibrium (P < 0.05).Both observed and expected heterozygosity of the 8 microsatellites were high,with a majority of loci exceeding 0.7 (Table 1).No statistically significant linkage disequilibrium was found for 28 comparisons in Genepop after Bonferroni correction (P > 0.05).In our study, null allele frequencies of 8 microsatellites were all<0.2.The combined exclusion probability for the first parent (both parents unknown) of all loci was 97.78%, and the combined exclusion probability for the second parent (one parent known)of all loci was 99.82%,which indicated that the 8 microsatellites are sufficient to identify paternity.Table 1 shows detailed information on these 8 microsatellites.
In total, we analyzed the paternity of 208 nestlings from 77 broods over 3 years(24 in 2019,24 in 2020,and 29 in 2021).EPP was found in 41 of 77 broods (53.3%, 95% CI: 41.8–64.6%), and 72 of 208 nestlings(34.6%, 95%CI:26.9–44.7%) were EPO.The proportion of EPO within these broods ranged from 25% to 100%, and frequency distribution of the proportion of EPO for 77 broods is shown in Fig.1.We were able to assign paternity to specific extra-pair males for 18 EPO.
Male wing length showed significantly negative correlation with the probability of EPP(GLMM:estimate±SE=-0.556±0.277,χ2=4.022,P=0.045;Table 2;Appendix Fig.S1C),and the proportion of EPO(LMM:estimate±SE=-0.102±0.046,χ2=4.777,P=0.029;Table 3).Female body length was positively related to the probability of EPP (GLMM:estimate±SE=0.697±0.292,χ2=5.682,P=0.017;Table 2;Appendix Fig.S2F).The proportion of EPO was negatively associated with female body condition(LMM:estimate±SE=-0.105±0.046,χ2=5.156,P=0.023; Table 3), and female mass (LMM: estimate ± SE = -0.093 ±0.046,χ2=4.110,P=0.043;Table 3).Other body size traits and body condition of male and female did not relate to the probability of EPP(P>0.05;Table 2;Appendix Figs.S1 and S2),as well as the proportion of EPO(P>0.05;Table 3).
A paired comparison revealed that extra-pair males had significantly better body condition than their cuckolded males(t=-2.718,P=0.017;Table 4), but there was no difference in other morphological traits between extra-pair males and within-paired males (P > 0.05; Table 4).Furthermore,we did not find any significant difference between EPO and their maternal half-siblings in all characteristics (P > 0.05; Appendix Table S1).
4.Discussion
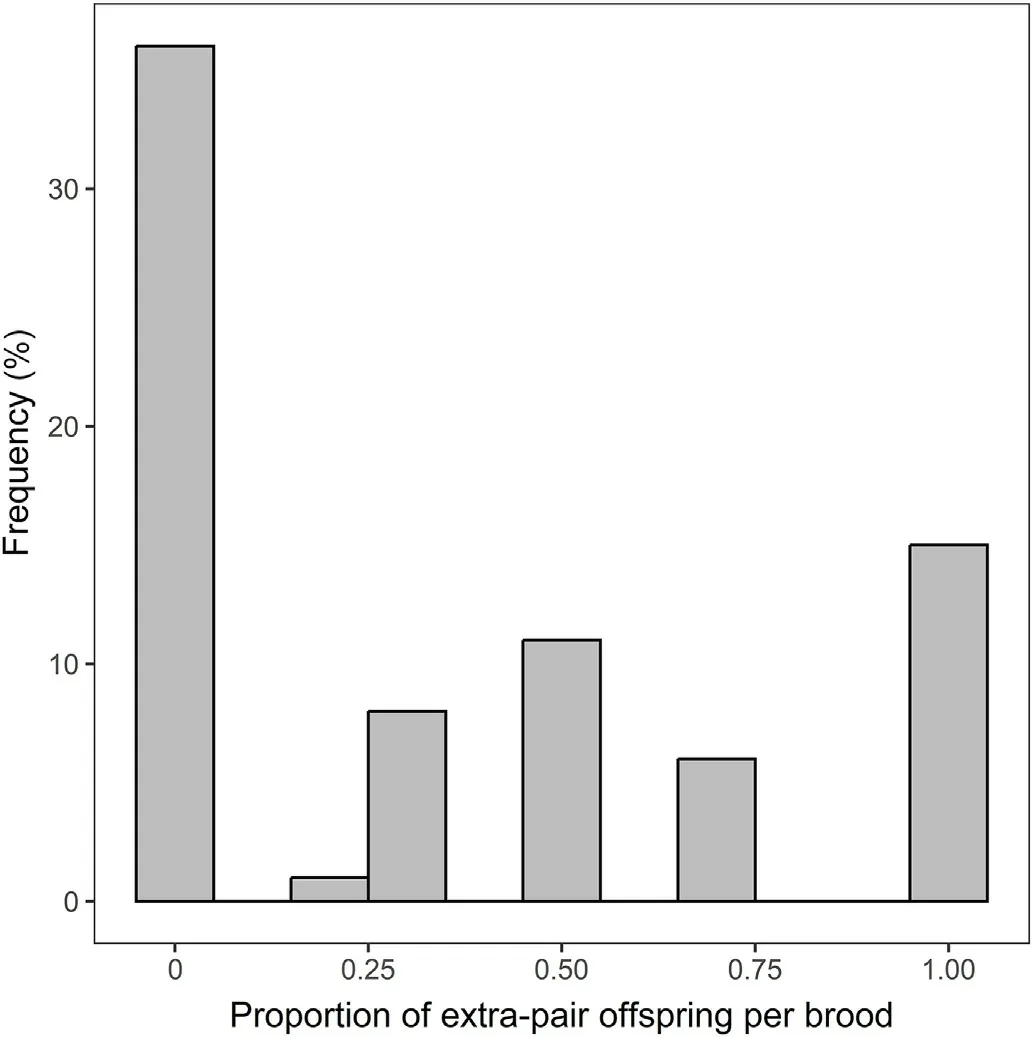
Fig.1.Frequency distribution of the proportion of extra-pair offspring per brood in Chestnut Thrushes.
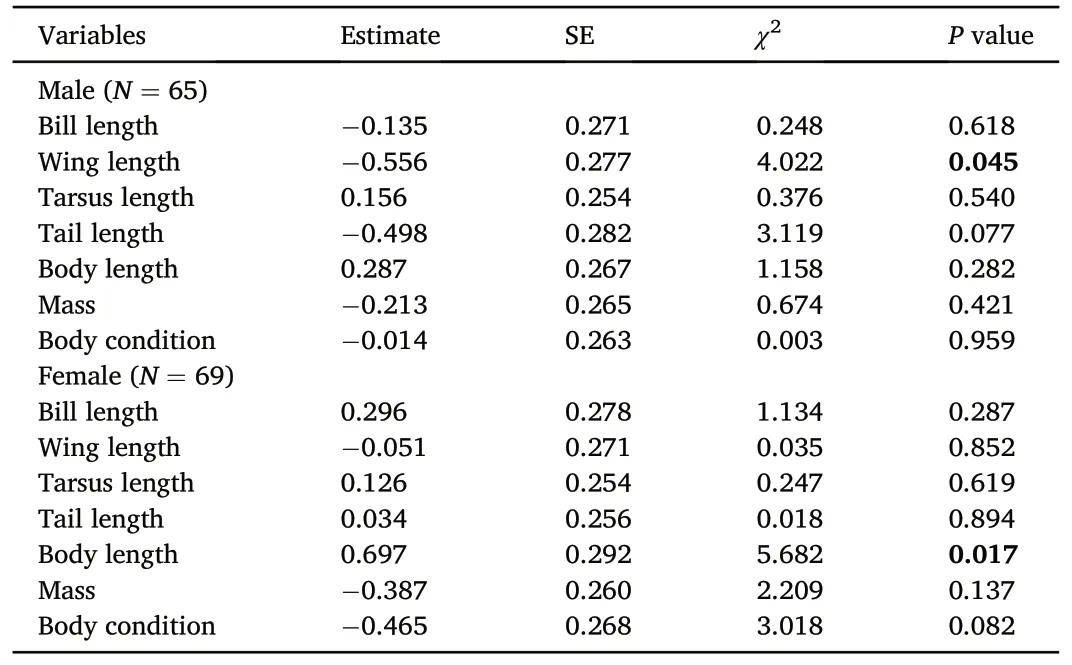
Table 2Summary of univariate tests of the relationships between the probability of extrapair paternity and morphological traits for males and females.
In this study,we explored the existence of EPP in the Chestnut Thrush and investigated the relationships between morphological traits and EPP.Our results showed that this species is not strictly genetically monogamous, with more than half of the broods containing EPO.Variation in the rate of EPP was related to morphological traits of females and males, which demonstrated that EPP may be body size- and body condition-dependent.A comparison between extra-pair males and within-pair males revealed that extra-pair males were in better body condition than the within-pair males.We found that EPO was not superior than WPO within a clutch in terms of phenotypic quality.
One of the main finding is that 53.3%broods of the Chestnut Thrush contained EPP, which is an intermediate level among Turdus species,similar to the Fieldfare (Turdus pilaris) (Kleven et al., 2019), but much lower than the American Robin (Turdus migratorius) (71.9% broods containing at least one EPO) (Rowe and Weatherhead, 2007).Furthermore, a recent review showed that only 14% of socially monogamous birds with EPP rate greater than 30%(Brouwer and Griffith,2019),while the EPP rate for the Chestnut Thrush was approximately 35%.There are three explanations for the relatively high EPP level in the Chestnut Thrush population at Lianhuashan.First, EPC can increase the male'stotal reproductive success in monogamous species (Møller and Ninni,1998; Balenger et al., 2009).In our study, most birds raise only one successful brood during three-month breeding season, which may prompt males to engage in EPCs,as they could have nestlings in multiple nests.Second, EPP rates are positively correlated with local breeding density (Mayer and Pasinelli, 2013).In our population of the Chestnut Thrush,the average distance to the nearest active nest was 90 m,and in about 28% of nests the distance to the nearest active nest was less than 50 m (unpublished data).The high breeding density of the Chestnut Thrush in our population may increase the encounter rates of potential extra-pair mates.Third, high EPP rate at the individual level may also depend on the number of fertile females in the vicinity (Westneat and Stewart, 2003; Canal et al., 2012).A male's extra-pair reproductive success may not only be determined by the availability opportunity,but also the fertile stage of his social mate.It is possible that males sire more EPO during the fertile period of their social mates, because males reproductive-related behaviors and sperm production may peak during this period, as the “pair synchrony spillover” hypothesis suggested(Araya-Ajoy et al.,2016).
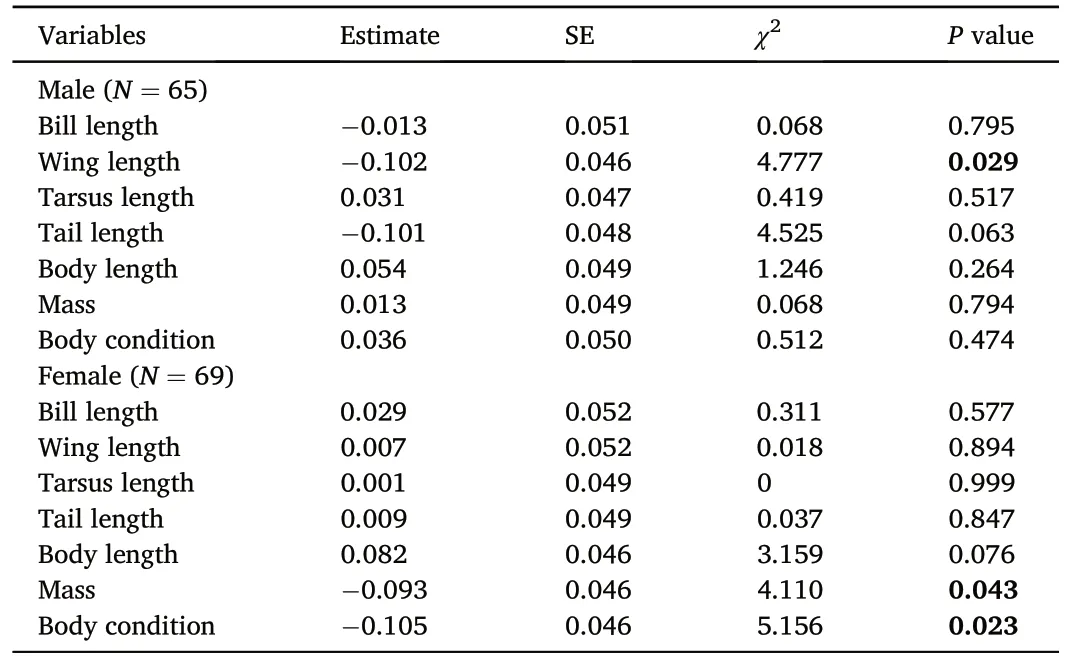
Table 3Summary of univariate tests of the relationships between the proportion of extrapair paternity and morphological traits for males and females.
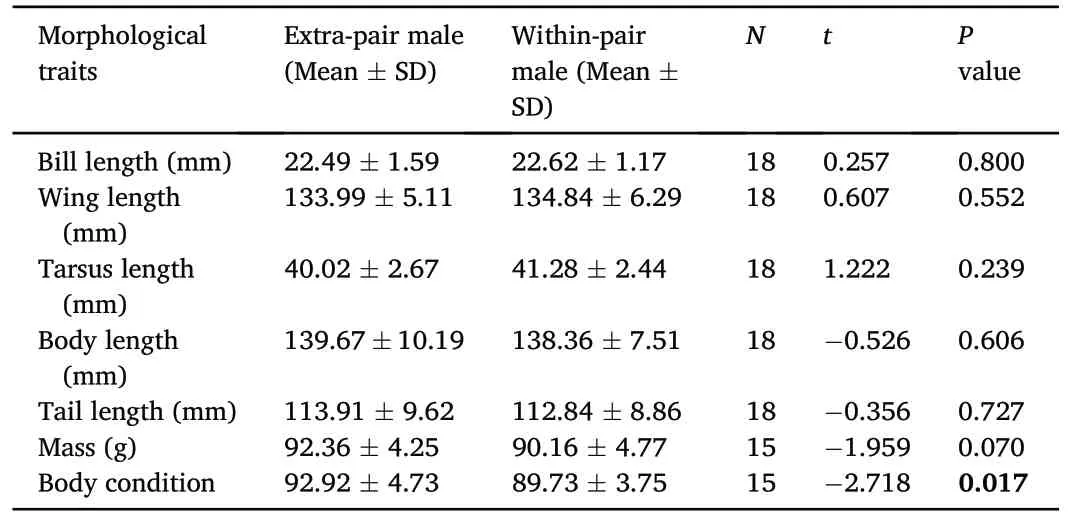
Table 4Pairwise comparisons of extra-pair males and within-pair males they cuckolded in the Chestnut Thrush.
We found that males with longer wings had lower paternity loss and less EPO.The effect of wing length on EPP could be that individuals with longer wings tend to display aggressive behaviors more frequently(Beltrão et al.,2021),and thus prevent intrusions from other males.Wing length may reflect overall body size (Ashton, 2002), which has been shown to be positively associated with EPP in other passerines (Morton et al., 1990; Yezerinac and Weatherhead, 1997).Males may be more willing to invade territories of individuals with smaller rather than larger body size, because in many species, larger body size signals greater aggressiveness and fighting ability (Funghi et al., 2015; Beltrão et al.,2021).The assessment of the body size may avoid increased risk of injury(Pereira et al., 2018).In Reed Buntings (Emberiza schoeniclus), older females tended to seek more EPCs when mating with males with shorter wing lengths (younger males) (Bouwman and Komdeur, 2005), suggesting that female decisions on whether to seek EPC may be based on male flight traits,a topic that merits further examination in future studies of Chestnut Thrush populations.Moreover, in some bird species, wing length of juveniles is shorter than that of adults and wing length increases with age(Merom et al.,1999).Age is also an important life-history trait that could explain EPP variation in birds.Individuals who survive to an older age may have a higher genetic quality(Brooks and Kemp,2001),or are more experienced(Cicho~n,2003),thus are more likely to be chosen as mates.Older males may be better able to force females to copulate or to be more competitive in male-male contest (Westneat and Stewart,2003), consequently, sire more EPO.The effect of age on EPP is not known in the Chestnut Thrush due to the difficulties in age determination.Furthermore, we did not find any relationship between paternity loss and other male morphological traits in the Chestnut Thrush.There may be a variation in extra-pair sexual selection on male traits or the information content of male traits may differ in different species.
Our results revealed that females in better body condition had lower proportion of EPO.Body condition may affect females’ability to escape from unwanted copulations by extra-pair males (Lundberg et al., 1987)and females with better body condition may be more capable of resisting male intruders, and thereby escape more EPC (but see Benítez Saldívar et al.,2022).We also found that females with lighter body mass tended to have higher proportion of EPO.The flight efficiency hypothesis proposed that lighter birds can be more energy-saving during flight (Norberg,1981;Nagy et al.,2007),which allow them to forage further from their nests, getting access to more potential extra-pair mates (Ferretti et al.,2018).The probability of a female mated out of pair was positively related to female body length, a trait found to be positively related to fecundity (clutch size) in some bird species (Li et al., 2015; Villa et al.,2018), which could explain the benefit for males seek EPCs with larger females.Just as a previous study suggested that female phenotypical traits which are visible to males are in relation to EPP, likely affecting male choice of social and extra-pair mates (Costanzo et al., 2017).Besides, larger females may be more inclined to EPCs because their better maternal performance can bear reduction in paternal investment(Whittingham and Dunn, 2010).Our results suggested that whether females involved in EPCs may relate to their morphological traits, which complements the current research on the relationship between EPP and female traits.
When directly comparing extra-pair and within-pair males, we only found that extra-pair males had a better body condition than within-pair males.The results indicated that females might seek extra-pair males based on their body condition, which may signal their quality (Wang et al.,2019)or reproductive success(Milenkaya et al.,2015).Otherwise,it could be that males in better condition can fly farther and encounter more fertile females to copulate, and thus gain EPP.Furthermore, there was no evidence that Chestnut Thrush EPO were larger or in better body condition than their within-pair half-siblings,which was not in line with the good gene hypothesis.Our results may suggest that females may engage in EPCs for other reasons than suggested by the good gene hypothesis.Failing to find support for the good gene hypothesis could also be that the genetic benefit of EPC is context-dependent and unfavorable environment may constrain the expression of good gene effect on phenotypic traits(O'Brien and Dawson,2007).Besides,lack of long-term fitness data on the Chestnut Thrush may also lead to null results, and further empirical testing of the good gene hypothesis may require more direct fitness testing.
In sum, we report the presence of EPP in a wild Chestnut Thrush population for the first time.Our findings suggest that body size and body condition may play a role in EPP.Furthermore, our work in China provides a model case study from Asia that not only promotes a broader geographical representation of EPP species, but also provides fundamental information for further research into the mechanisms of EPC behavior.
Funding
This work was supported by the National Natural Science Foundation of China(Project 32070452).
Authors’contributions
YS,YL and HL designed this study;HL,YL and YF collected data in the field; HL did molecular experiments, performed the data analysis and wrote the manuscript; YS and YL revised and improved the manuscript.All authors have read and approved the final manuscript.
Ethics statement
The experiments were conducted under the approval of the Animal Care and Ethics Committee and carried out in accordance with the guidelines for the use of Animals in Research issued by the Institute of Zoology,Chinese Academy of Sciences(Permission No.2013/108).
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful to the staffs of the Lianhuashan Nature Reserve,Gansu Province.We also thank local villages' support and volunteers'assistance for our work.We thank the editor and two anonymous reviewers for their constructive suggestions which greatly improved the paper.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://do i.org/10.1016/j.avrs.2023.100135.
杂志排行
Avian Research的其它文章
- Selecting the best: Interspecific and age-related diet differences among sympatric steppe passerines
- Morphology and morphometry of two hybridizing buntings at their hybrid zone in northern Iran reveal intermediate and transgressive morphotypes
- Quiet in the nest: The nest environment attenuates song in a grassland songbird
- Characteristics of cross transmission of gut fungal pathogens between wintering Hooded Cranes and sympatric Domestic Geese
- Fecal DNA metabarcoding reveals the dietary composition of wintering Red-crowned Cranes (Grus japonensis)
- Short-term night lighting disrupts lipid and glucose metabolism in Zebra Finches: Implication for urban stopover birds
