Mechanism of Qishen Decoction inhibition of macrophage M1 type polarization by targeting TGR5-mediated NLRP3 inflammasome
2024-01-19GAOShanGAOJiaweiYANGLiuxinZHURuizengZHANGYaliYUANXingxing
GAO Shan, GAO Jia-wei, YANG Liu-xin✉, ZHU Rui-zeng, ZHANG Ya-li, YUAN Xingxing,3✉
1. Daqing Hospital of Traditional Chinese Medicine, Daqing 163311, China
2. Heilongjiang University of Chinese Medicine, Harbin 150040, China
3. Heilongjiang Academy of Traditional Chinese Medicine, Harbin 150006, China
4. Zhang Yali National Expert studio of Renowned Traditional Chinese Medicine Practioners, Harbin 150006, China
Keywords:
ABSTRACT Objective: To observe the effect of Qishen decoction on TGR5-mediated activation of NLRP3 inflammasome, so as to clarify the molecular mechanism of its inhibition of macrophage M1-type polarisation to ameliorate non-alcoholic steatohepatitis; Methods: Mouse macrophage cell line RAW264.7 was randomly divided into a control group, model group, Qishen decoction group, TGR5 agonist group and Qishen decoction + TGR5 agonist group.Except for the control group, the remaining groups were constructed the macrophage NLRP3 activation model by palmitic acid induction, and the corresponding drugs were given to intervene.ELISA was used to detect the levels of TNF-α, IL-6, IL-1β and CXCL2 in macrophage supernatants,flow cytometry was used to detect the expression levels of macrophage polarisation marker molecules CD86 and iNOS, and Western blot was used to detect the expression of the TGR5/STAT1/STAT6 signaling pathway and the expression of NLRP3 inflammasome -associated proteins, respectively.Results: Compared with the control group, the contents of macrophages TNF-α, IL-6, IL-1β, CXCL2 and the proportion of macrophages with positive expression of CD86 and iNOS were significantly increased in the model group, and the differences were all statistically significant (P<0.01).Compared with the model group, the contents of TNF-α,IL-6, IL-1β, CXCL2 and the proportion of macrophages with positive expression of CD86 and iNOS were significantly decreased in the Qishen decoction group, and the differences were all statistically significant (P<0.01).In addition, the expression of NLRP3 and Pro-IL-1β proteins in the macrophage lysate and the expression of Caspase-1 p10, Caspase-1 p20 and IL-1β p17 proteins in the cell supernatant of the model group were significantly increased when compared with the control group, and the differences were all statistically significant (P<0.01).Compared with the model group, the expression of NLRP3 and Pro-IL-1β proteins in macrophage lysate and the expression of Caspase-1 p10, Caspase-1 p20 and IL-1β p17 proteins in cell supernatant of the Qishen decoction were significantly reduced,and the differences were all statistically significant (P<0.01); Conclusion: Qishen decoction can inhibit the activation of NLRP3 inflammasome in macrophages by inhibiting the TGR5/STAT1/STAT6 signaling pathway, thereby inhibiting macrophage M1 polarization and improving inflammatory response.
1.Introduction
Nonalcoholic steatohepatitis (NASH) is a metabolism-related liver disease characterised by hepatic fat accumulation, inflammatory response and varying degrees of fibrosis[1].Epidemiological studies have shown that the incidence of NASH has shown an increasing trend in recent years and has become one of the most common chronic liver diseases worldwide, and it is expected that NASH will become one of the leading causes of liver transplantation by 2030[2-3].The lack of an accurate, non-invasive diagnostic approach to the clinical management of NASH, and the fact that there is no specific medication available for its treatment, has resulted in an urgent need for further research to develop effective therapeutic strategies and Further research is urgently needed to develop effective therapeutic strategies and clinical tools to manage and treat NASH[4].
NLRP3 (NOD-like receptor family, pyrin domain-containing protein 3) inflammasome is a multi-subunit organelle structure that has been shown to play an important role in NASH liver inflammation[5-6].NLRP3 inflammasome is activated when cells are damaged or infected, which in turn promotes inflammatory signaling[7].In macrophage polarization, activation of NLRP3 inflammatory vesicles is considered to be one of the key factors promoting M1-type macrophage polarization[8].The group’s previous study showed that the effect of Qishen decoction on NASH can be achieved by inhibiting the inflammatory response[9].Meanwhile, astragalus ginseng soup can also inhibit M1-type polarization of macrophages by up-regulating the expression of fat mass and obesity-associated(FTO) genes through miR-495, which can play a role in suppressing inflammation and improving insulin resistance[10].Therefore, in this study, we further clarified the molecular mechanism of the inhibition of inflammation in the treatment of NASH by observing the effect of astragalus and ginseng soup on the activation of NLRP3 inflammatory vesicles mediated by Takeda G protein-coupled receptor 5 (TGR5).
2.Materials and methods
2.1Animal
Clean-grade BALB/C mice were purchased (6~8 w, male,18g~23 g) from Saiye (Gu’an) Biotechnology Co.Ltd (Production Licence: SCXK(Ji)2021-003).Animals were housed in the Animal Experiment Centre of Heilongjiang Academy of Traditional Chinese Medicine with humidity of 45%~58%, temperature of 21~24 ℃and cyclic light.
2.2 Cell line
The mouse macrophage cell line RAW264.7 was purchased from the Cell Bank of the Chinese Academy of Sciences.
2.3 Reagents and instruments
DMEM medium was purchased from Gibco, USA (Item No.12430054); 10% fetal bovine serum (FBS) and penicillin &streptomycin double antibody were purchased from Thermo Fisher, USA (Item Nos.S8318 and 15140122, respectively).Palmitic acid was purchased from Sigma, USA (Item No.7619,purity 99.0%, CAS No.57-10-3); SB756050 was purchased from MedChemExpress, USA (Item No.HY-102016, purity 98.49%,CAS No.447410-57-3); mouse interleukin-6 ( Interleukin-6 (IL-6),Tumor Necrosis Factor-alpha (TNF-alpha), Interleukin-1β (IL-1β) ELISA kit and Ultra-sensitive ECL chemiluminescence kit were purchased from Shanghai Biyuntian Bio-technology Co.Ltd(item numbers PT513, PI326, PI301 and P0018S, respectively);Chemokine ligand 2 (CXCL2) ELISA kit was purchased from Jiangsu Enzyme Immunity Industry Co.The isothiocyanine fluorescein (FITC)-labelled CD86, iNOS antibody and HRPlabelled secondary antibody were purchased from CST (item#99879, 13120 and 7074, respectively).nLRP3, ASC, Caspase-1,IL-1β, TGR5, STAT1, p-STAT1, STAT6, p-STAT6 and β-actin were purchased from the UK.β-actin were purchased from Abcam,UK (item numbers ab263899, ab309497, ab138483, ab216995,ab72608, ab109320, ab109461, ab263947, ab32520 and ab8226,respectively).The cell culture incubator was purchased from Binder,Germany (model: CB260); the flow cytometer was purchased from Beckman Coulter, USA (model: CytoFlex); the enzyme labeller was purchased from Agilent, USA (model: Synergy H1); the gel imager was purchased from Shanghai Tanon (model: Tanon 1600);the electrophoresis instrument was purchased from Bio-Rad, USA(model.The electrophoresis instrument was purchased from Bio-Rad(Model: Mini-Protean Tetra).
2.4 Methodologies
2.4.1 Cell culture
The mouse macrophage cell line RAW264.7 was cultured in a 37℃and 5% CO2incubator in DMEM medium containing 1% double antibody and 10% fetal bovine serum.
2.4.2 Drug and drug-containing serum preparation
Qishen decoction (composed of raw Astragalus 15 g, Radix et Rhizoma Ginseng 10 g, Salviae Miltiorrhizae 10 g, Hawthorn 10 g,Zeaxanthus salinarius 10 g, Lotus leaf 10 g, Panax quinquefolius 5 g,Radix et Rhizoma Murrayiensis 8 g, Radix et Rhizoma Chasteberry 8 g, Gynostemma pentaphyllum 5 g, and Sheng Glycyrrhiza glabra 3 g) was purchased from the Bureau of Traditional Chinese Herbal Medicine of Nangang Campus of the Academy of Traditional Chinese Medicine in Heilongjiang Province, and the medicines were immersed, decocted twice, filtered to merge the medicinal liquids,and then concentrated and prepared into an infusion.
After one week of acclimatization, 30 BALB/C mice were randomly divided into a blank group and a Qishen decoction group,and the Qishen decoction -containing serum was prepared by gavage at a 4-fold concentration (75.2 g/kg) with reference to the optimal intervention dose (18.8 g/kg) derived from a previous study[11].The mice in the blank group were given equal volumes of saline by gavage at a dose of 1 mL each time, once a day, for 7 consecutive days.Four hours after the last gavage, 1% pentobarbital sodium (40 mg/kg) was injected intraperitoneally to anaesthetize the mice, and then blood was collected from the abdominal aorta, centrifuged at 3,000 rpm for 10 min, then collected, inactivated in a water bath,and then filtered through a 0.22 μm membrane, and then frozen in separate packages.
2.4.3 Cell grouping and pharmacological interventions
When RAW264.7 was in logarithmic growth phase, the cells were inoculated in 6-well plates at a density of 1×106cells/mL, 900 μL per well.The cells were and divided into the blank group, the model group, the Qishen decoction group, the TGR5 agonist group and the Qishen decoction + TGR5 agonist group, and 4 replicate wells were set in each group.The macrophage NLRP3 activation model was constructed by palmitic acid induction with reference to the method in the literature[12].The specific implementation plan and drug intervention methods were as follows: (1) Control group: 900 μL normal medium + 100 μL blank serum; (2) Model group: 900 μL normal medium + 100 μL blank serum + 200 mM palmitic acid; (3)Qishen decoction group: 900 μL normal medium + 200 mM palmitic acid + 100 μL astragalus ginseng tang-containing serum; (4) TGR5 agonist group: 900 μ L normal medium + 100 μL blank serum +200 mM palmitic acid + 200 mM SB756050; (5) Qishen decoction+ TGR5 agonist group: 900 μL normal medium + 200 mM palmitic acid + 100 μL Qishen decoction drug-containing serum + 200 mM SB756050.48 h later, the cells of each group were collected and used for the subsequent experiments.
2.4.4 ELISA
The supernatants of cells in each group were collected after 48 h.The contents of TNF-α, IL-6, IL-1β and CXCL2 in the supernatants of cells in each group were detected with reference to the instructions of the kit.The experimental steps were carried out strictly according to the instructions of the kit.
2.4.5 Flow cytometry
The cells were collected after 48 h.After centrifugation at 1 000 rpm for 5 min, the supernatant was discarded, and the cells were resuspended in PBS, centrifuged at 1 000 rpm for 5 min, the supernatant was discarded, and the washing was repeated for two times.Macrophages were resuspended by adding PBS and incubated with FITC-labelled CD86 and iNOS antibodies for 30 min.Cells were collected from each group, washed with PBS and resuspended by adding PBS, and the percentage of positively expressing cells for CD86 and iNOS in each macrophage group was detected by flow cytometry.
2.4.6 Western blot assay
The cells of each group were collected after 48 h.After centrifugation at 1 000 rpm for 5 min, the cell supernatant and cell precipitate were collected.The cells were lysed by adding RIPI and then centrifuged at 1 000 rpm for 5 min, and the supernatant was collected as the cell lysate.Total protein was extracted from the cell supernatant and lysate respectively, and the protein concentration was determined by BCA method.Sampling, electrophoresis,membrane transfer, skimmed milk closure, add diluted NLRP3, ASC,Caspase-1, IL-1β, TGR5, STAT1, p-STAT1, STAT6, p-STAT6, and β-actin primary antibody, incubate at 4 ℃ overnight, and add the appropriate proportion of the diluted secondary antibody to continue to incubate for 2 h.After TBTS washing, dropwise add ECL After TBTS was washed, ECL was added dropwise, and the bands were developed and photographed on a gel imaging system.The bands were analysed in grey value using Image J software, and β-actin was used as the internal reference antibody, and the comparison between the target proteins and the internal reference was used to indicate the relative expression level of the target proteins.
2.5 Statistical methods
The data of this study were statistically analysed by SPSS 26.0 software, and the comparison of data between multiple groups was performed by one-way ANOVA, and the comparison of two-bytwo between groups was tested by the method of least significant difference.P<0.05 was used to indicate that the difference was statistically significant.
3.Results
3.1 Effect of Qishen decoction on macrophage inflammation and chemokine content
Compared with the control group, the contents of TNF-α, IL-6,IL-1β and CXCL2 in macrophages in the model group were significantly increased, and the differences were all statistically significant (P<0.01).Compared with the model group, the contents of TNF-α, IL-6, IL-1β and CXCL2 were significantly decreased in the Qishen decoction group, the TGR5 agonist group and the Qishen decoction + TGR5 agonist group, and the differences were all statistically significant (P<0.01).The levels of TNF-α, IL-6, IL-1βand CXCL2 were all significantly reduced in the Qishen decoction group and the TGR5 agonist group compared with the Qishen decoction group and the TGR5 agonist group, and the differences were all statistically significant (P<0.01).See Table 1.
Tab 1 Comparison of macrophage inflammatory and chemokine content between groups(±s, pg/mL)
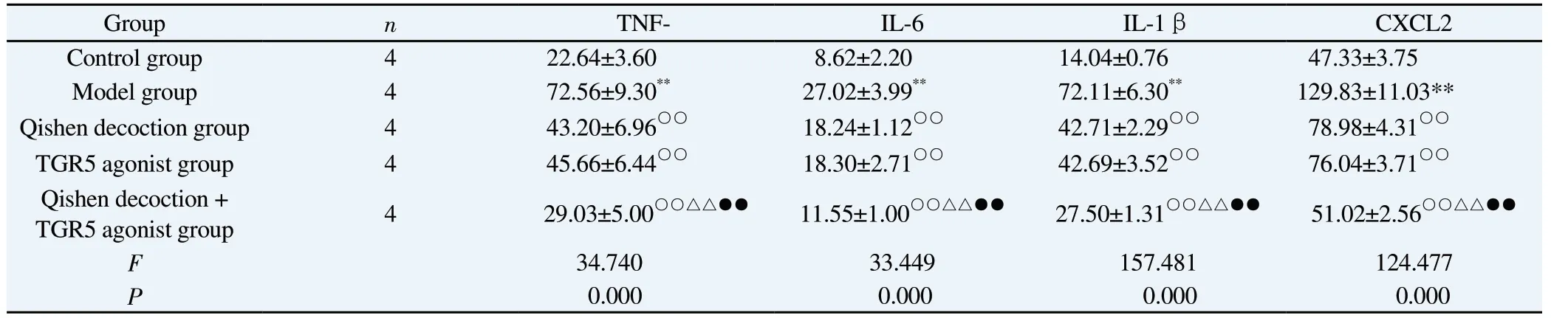
Tab 1 Comparison of macrophage inflammatory and chemokine content between groups(±s, pg/mL)
Note: Compared with the control group, *P<0.05, **P<0.01; compared with the model group, P<0.05, P<0.01; compared with the Qishen decoction group,△P<0.05, △△P<0.01; compared with the Qishen decoction + TGR5 agonist group, ●P<0.05, ●●P<0.01.Same as below.
Group n TNF- IL-6 IL-1β CXCL2 Control group 4 22.64±3.60 8.62±2.20 14.04±0.76 47.33±3.75 Model group 4 72.56±9.30** 27.02±3.99** 72.11±6.30** 129.83±11.03**Qishen decoction group 4 43.20±6.96○○ 18.24±1.12○○ 42.71±2.29○○ 78.98±4.31○○TGR5 agonist group 4 45.66±6.44○○ 18.30±2.71○○ 42.69±3.52○○ 76.04±3.71○○Qishen decoction +TGR5 agonist group 4 29.03±5.00○○△△●● 11.55±1.00○○△△●● 27.50±1.31○○△△●● 51.02±2.56○○△△●●F 34.740 33.449 157.481 124.477 P 0.000 0.000 0.000 0.000
3.2 The effect of Qishen decoction on the expression of CD86 and iNOS in macrophages
Compared with the control group, the proportion of macrophages with positive expression of CD86 and iNOS were significantly increased in the model group, and the differences were all statistically significant (P<0.01).Compared with the model group,the proportions of macrophages with positive expression of CD86 and iNOS were all significantly decreased in the Qishen decoction group, the TGR5 agonist group and the Qishen decoction + TGR5 agonist group, and the differences were all statistically significant(P<0.01).Compared with the Qishen decoction group and TGR5 agonist group, the proportions of macrophages with positive expression of CD86 and iNOS in the Qishen decoction + TGR5 agonist group were all significantly reduced, and the differences were all statistically significant (P<0.01).See Table 2 and Figure 1.
Tab 2 Comparison of macrophage CD86 and iNOS expression between groups(±s, %)
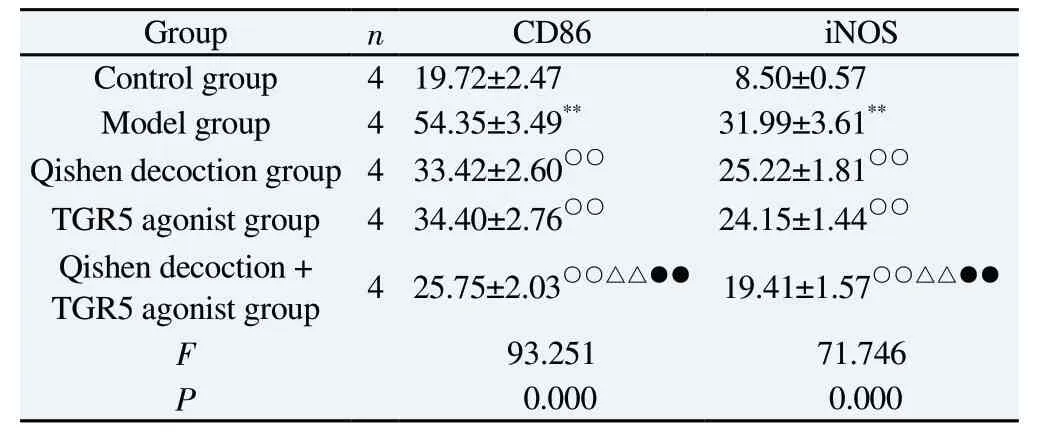
Tab 2 Comparison of macrophage CD86 and iNOS expression between groups(±s, %)
Group n CD86 iNOS Control group 4 19.72±2.47 8.50±0.57 Model group 4 54.35±3.49** 31.99±3.61**Qishen decoction group 4 33.42±2.60○○ 25.22±1.81○○TGR5 agonist group 4 34.40±2.76○○ 24.15±1.44○○Qishen decoction +TGR5 agonist group 4 25.75±2.03○○△△●● 19.41±1.57○○△△●●F 93.251 71.746 P 0.000 0.000
3.3 Effect of Qishen decoction on macrophage NLRP3 inflammatory vesicle activation
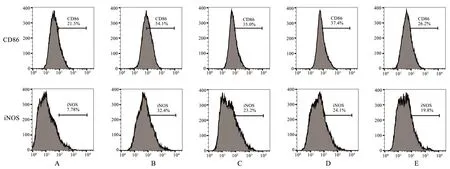
Fig 1 Comparison of macrophage CD86 and iNOS expression between groups
Compared with the control group, the expression of NLRP3 and Pro-IL-1β proteins in macrophage lysate and the expression of Caspase-1 p10, Caspase-1 p20 and IL-1β p17 proteins in cell supernatant were significantly increased in the model group, and the differences were all statistically significant (P< 0.01), whereas the expression of ASC and Caspase-1 p45 protein expression in macrophage lysate were not significantly changed, and the differences were not statistically significant (P> 0.05).Compared with the model group, the expression of NLRP3 and Pro-IL-1β proteins in macrophage lysate and the expression of Caspase-1 p10,Caspase-1 p20 and IL-1β p17 proteins in cell supernatant of the Qishen decoction group, the TGR5 agonist group and the Qishen decoction + TGR5 agonist group were significantly reduced, and the differences were all statistically significant (P < 0.01) ), while the expression of ASC and Caspase-1 p45 proteins in macrophage lysate did not change significantly, and none of the differences were statistically significant (P> 0.05).The expression of NLRP3 and Pro-IL-1β proteins in macrophage lysate and the expression of Caspase-1 p10, Caspase-1 p20 and IL-1β p17 proteins in cell supernatant were significantly reduced in the Qishen decoction +TGR5 agonist group compared with the Qishen decoction group and the TGR5 agonist group, and the difference was statistically significant in both groups (P< 0.01), while the expression of The expression of ASC and Caspase-1 p45 proteins in macrophage lysate did not change significantly, and the differences were not statistically significant (P > 0.05).See Table 3-4 and Figure 2.
3.4 Effects of Qishen decoction on TGR5/STAT1/STAT6 signalling pathway in macrophages
Compared with the control group, the expression of macrophage TGR5 and p-STAT6 proteins in the model group was significantly reduced, and the expression of p-STAT1 protein was significantly increased, and the differences were all statistically significant(P<0.01).Compared with the model group, the expression of macrophage TGR5 and p-STAT6 proteins was significantly increased and the expression of p-STAT1 protein was significantly decreased in the Qishen decoction group, the TGR5 agonist group and the Qishen decoction + TGR5 agonist group, and the differences were all statistically significant (P<0.01).Compared with the Qishen decoction group and the TGR5 agonist group, the expression of macrophage TGR5 and p-STAT6 proteins in the Qishen decoction +TGR5 agonist group was significantly increased, and the expression of p-STAT1 protein was significantly decreased, and the differences were all statistically significant (P< 0.01).See Table 5 and Figure 3.
Tab 3 Comparison of macrophage lysate NLRP3, ASC, Caspase-1 p45 and Pro-IL-1β protein expression between groups(±s)
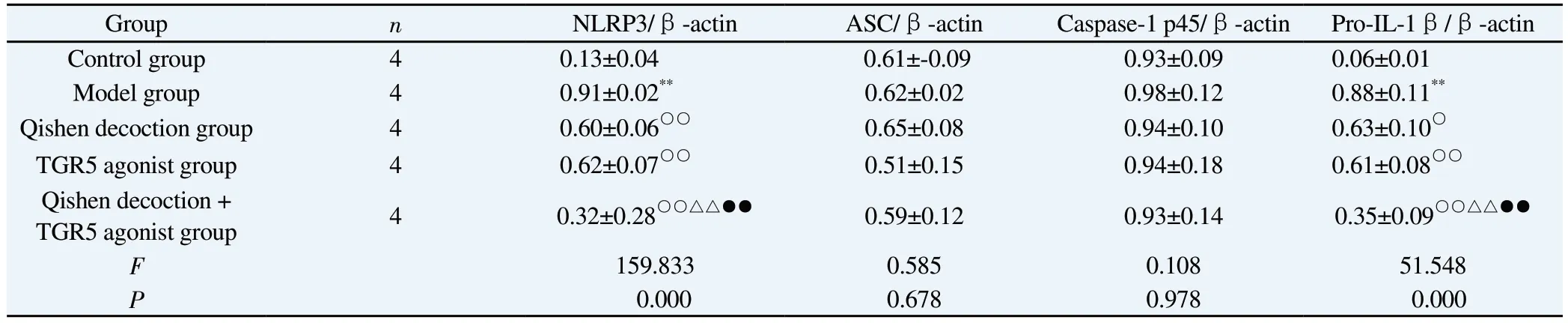
Tab 3 Comparison of macrophage lysate NLRP3, ASC, Caspase-1 p45 and Pro-IL-1β protein expression between groups(±s)
Group n NLRP3/β-actin ASC/β-actin Caspase-1 p45/β-actin Pro-IL-1β/β-actin Control group 4 0.13±0.04 0.61±-0.09 0.93±0.09 0.06±0.01 Model group 4 0.91±0.02** 0.62±0.02 0.98±0.12 0.88±0.11**Qishen decoction group 4 0.60±0.06○○ 0.65±0.08 0.94±0.10 0.63±0.10○TGR5 agonist group 4 0.62±0.07○○ 0.51±0.15 0.94±0.18 0.61±0.08○○Qishen decoction +TGR5 agonist group 4 0.32±0.28○○△△●● 0.59±0.12 0.93±0.14 0.35±0.09○○△△●●F 159.833 0.585 0.108 51.548 P 0.000 0.678 0.978 0.000
Tab 4 Comparison of protein expression of supernatant Caspase-1 p10, Caspase-1 p20 and IL-1β p17 in macrophages in each group (±s)
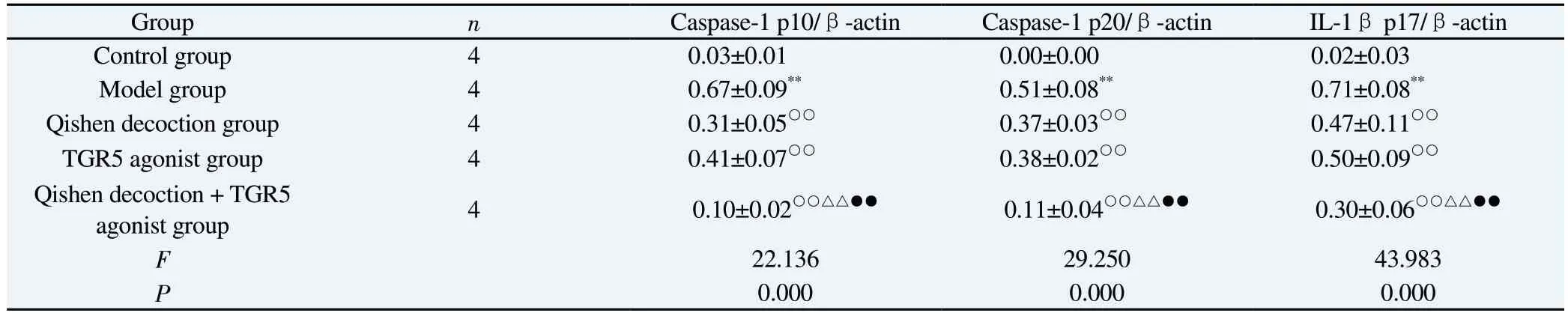
Tab 4 Comparison of protein expression of supernatant Caspase-1 p10, Caspase-1 p20 and IL-1β p17 in macrophages in each group (±s)
Group n Caspase-1 p10/β-actin Caspase-1 p20/β-actin IL-1β p17/β-actin Control group 4 0.03±0.01 0.00±0.00 0.02±0.03 Model group 4 0.67±0.09** 0.51±0.08** 0.71±0.08**Qishen decoction group 4 0.31±0.05○○ 0.37±0.03○○ 0.47±0.11○○TGR5 agonist group 4 0.41±0.07○○ 0.38±0.02○○ 0.50±0.09○○Qishen decoction + TGR5 agonist group 4 0.10±0.02○○△△●● 0.11±0.04○○△△●● 0.30±0.06○○△△●●F 22.136 29.250 43.983 P 0.000 0.000 0.000
Tab 5 Comparison of protein expression in the TGR5/STAT1/STAT6 signalling pathway in macrophages in each group (±s)
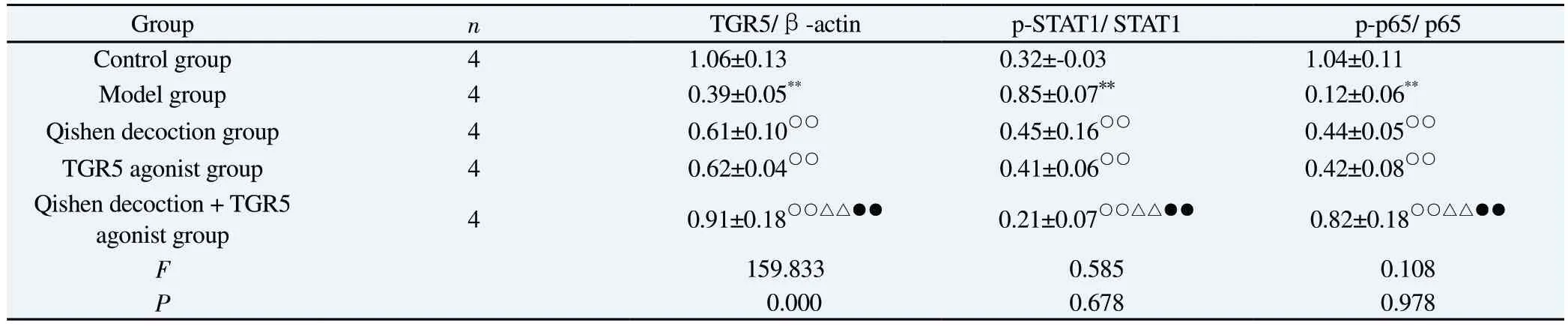
Tab 5 Comparison of protein expression in the TGR5/STAT1/STAT6 signalling pathway in macrophages in each group (±s)
Group n TGR5/β-actin p-STAT1/ STAT1 p-p65/ p65 Control group 4 1.06±0.13 0.32±-0.03 1.04±0.11 Model group 4 0.39±0.05** 0.85±0.07** 0.12±0.06**Qishen decoction group 4 0.61±0.10○○ 0.45±0.16○○ 0.44±0.05○○TGR5 agonist group 4 0.62±0.04○○ 0.41±0.06○○ 0.42±0.08○○Qishen decoction + TGR5 agonist group 4 0.91±0.18○○△△●● 0.21±0.07○○△△●● 0.82±0.18○○△△●●F 159.833 0.585 0.108 P 0.000 0.678 0.978
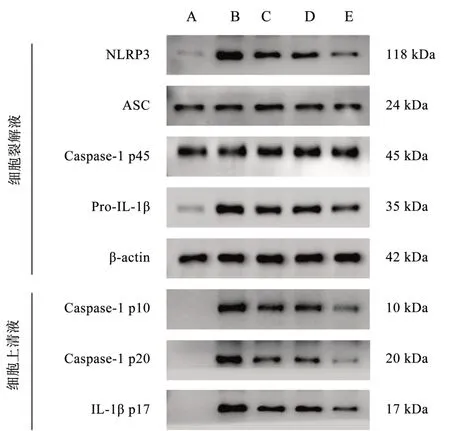
Fig 2 Comparison of macrophage NLRP3 inflammatory vesicle activation protein expression among groups of macrophages
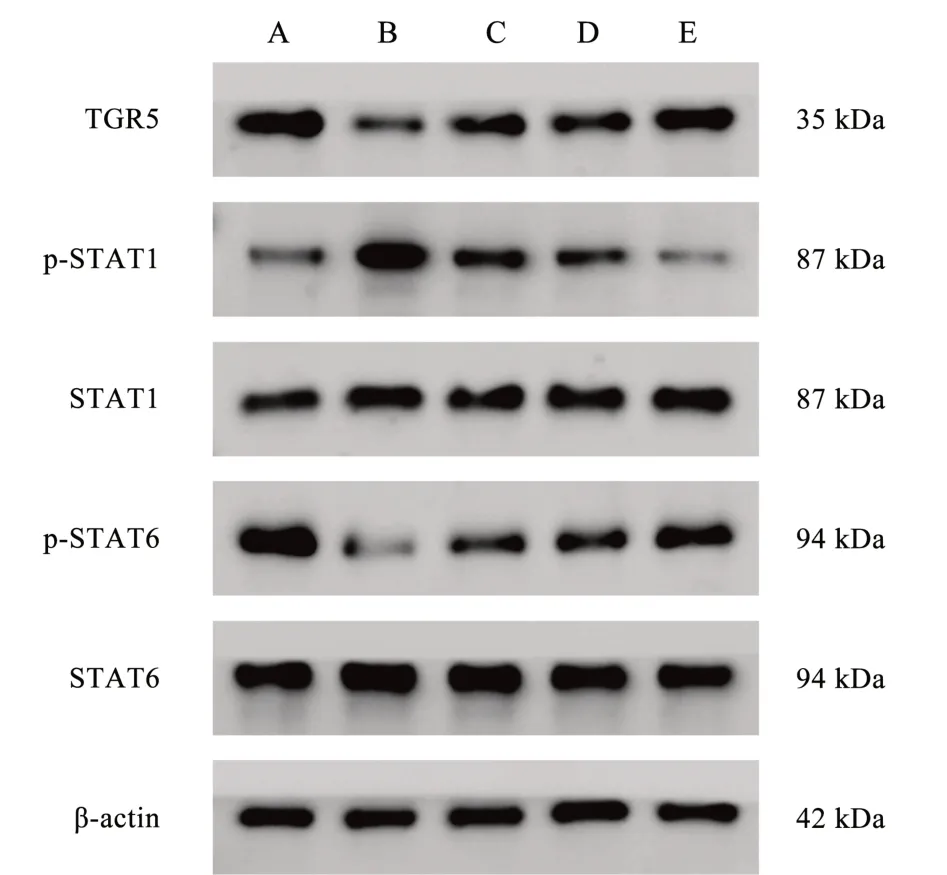
Fig 3 Comparison of protein expression in TGR5/STAT1/STAT6 signalling pathway in group macrophages
4.Discussion
Macrophages are remarkably heterogeneous and can be classified into different subpopulations based on their surface markers,cytokines produced and gene expression profiles.As important immune cells in the liver, macrophages perform different immune functions in tissue homeostasis and disease progression.They regulate local inflammatory responses by recognising, ingesting, and degrading foreign pathogens or cellular debris, thereby promoting,or inhibiting the progression of hepatic fibrosis.TNF-α is a multicellularly produced inflammatory factor, which promotes hepatic inflammatory responses by participating in oxidative stress and lipid peroxidation in different ways[13].Not only that, TNF-α also promotes hepatic steatosis through the insulin signaling pathway and plays an important role in the progression of NASH[14].IL-6,also known as stem cell stimulating factor, is produced by activated monocytes and plays an important role in hepatic inflammatory response, metabolism, and liver tissue regeneration through the modulation of adaptive immunity[15].IL-1β, which is produced by monocyte-macrophage cells, is not only able to promote the hepatic recruitment of inflammatory cells, but also to induce the expression of adhesion molecules in epithelial cells, thus aggravating hepatic inflammatory injury.Findings showed that serum IL-1β expression was significantly increased in NASH patients[16].CXCL2 is a member of the CXC class of chemokines, which accelerates the hepatic inflammatory response by recruiting and activating neutrophils and releasing a variety of inflammatory factors.In addition, macrophages promote the activation of intracellular NLRP3 inflammatory vesicles by secreting CXCL2 and acting on CXCR2 on hepatic stellate cells[17].The results of the present study showed that palmitic acid induction was able to significantly increase the levels of TNF-α, IL-6, IL-1β and CXCL2 in macrophages, whereas the serum containing Astragalus Ginseng Soup was able to significantly reduce the secretion of TNF-α, IL-6, IL-1β and CXCL2 in macrophages, exerting an inhibitory effect on inflammation.
Depending on the activation state, macrophages can be classified into classically activated (M1-type) and alternatively activated(M2-type) macrophages, of which M1-type macrophages are predominantly INF-γ and LPS signaling and play a proinflammatory role through the secretion of chemokines and proinflammatory factors.The NLRP3 inflammasome is an important component of the natural immune system, and is widely present in hepatic parenchymal and nonparenchymal cells[18].The inflammasome is composed of NLRP3, the adapter apoptosisassociated speck-like protein ASC containing the cysteine asparaginase recruitment domain, and the effector protease pro-Caspase -1.The activation of the NLRP3 inflammasome is divided into two phases: initiation and activation.First, pathogen-associated molecular patterns bind to pattern recognition receptors to upregulate the expression of NLRP3 and pro-IL-1β.Subsequently, NLRP3 molecules undergo oligomerization through homotypic interactions of the NACHT domain, and their oligomers recruit ASCs through PYD-PYD interactions, promoting the formation of ASC spots[19].Finally, the C-terminal caspase recruitment domain of ASC interacts with the precursor pro-Caspase-1 and undergoes selfcleavage through neighborhood-induced interactions between the p20 and p10 subunits of Caspase-1, thereby activating Caspase-1[20].The activated Caspase-1 further cleaves the precursors IL-18 and IL-1β to generate mature IL- 18 and IL-1β [21].In addition,activated Caspase-1 was able to cleave and activate GSDMD,which translocated to the cytoplasmic membrane and formed a pore, promoting the release of mature IL-18 and IL-1β into the extracellular[22].In the present study, palmitic acid induction was able to significantly increase the proportion of macrophages with positive expression of CD86 and iNOS, and the serum containing astragali and ginseng soup was able to significantly inhibit the proportion of macrophages with positive expression of CD86 and iNOS to achieve the inhibition of M1-type polarization of macrophages.In addition, palmitic acid significantly up-regulated the expression of NLRP3 and Pro-IL-1β proteins in macrophages and the expression of Caspase-1 p10, Caspase-1 p20 and IL-1β p17 proteins in the cell supernatant, while it had no significant effect on the expression of Caspase-1 p45 protein.Astragalus ginseng soup intervention was able to significantly down-regulate the expression of NLRP3 and Pro-IL-1β proteins in macrophages and the expression of Caspase-1 p10, Caspase-1 p20 and IL-1β p17 proteins in cell supernatant, thus inhibiting the activation of NLRP3 inflammatory vesicles.
TGR5 is a bile acid receptor widely expressed in several organs of the human body, which transmits extracellular signals to intracellular downstream cascade responses through multiple effector pathways.It has been demonstrated that TGR5 inhibits LPS-induced cytokine release from macrophages, thereby ameliorating liver injury[23].And in an alcohol-induced mouse model of liver injury, macrophage infiltration and inflammation in liver tissues of TGR5 knockout mice were significantly higher than that of wild-type mice[24].As one of the members of the STAT family, STAT1 and STAT6, which are important bridges for signaling between the cell membrane receptor proteins and the effector organelles, play an important role in macrophage polarization.Studies have shown that STAT1 expression is significantly upregulated in NASH mice, and inhibition of STAT1 expression significantly ameliorates hepatocyte steatosis,balloon-like degeneration, inflammation, and endoplasmic reticulum stress injury[25].In contrast to the effects of STAT1, STAT6 was able to protect hepatocytes and endothelial cells from damage by inhibiting inflammation[26].The results of this study showed that palmitic acid also significantly up-regulated the expression of TGR5 and p-STAT1 proteins in macrophages and down-regulated the expression of p-STAT6 protein in macrophages, while it had no significant effect on the expression of and STAT1 and STAT6 proteins.Astragalus ginseng soup and TGR5 agonist were able to significantly down-regulate the expression of TGR5 and p-STAT1 proteins in macrophages and up-regulate the expression of p-STAT6 protein in macrophages, and both had comparable effects.And the effect of astragalus ginseng soup + TGR5 agonist was significantly better than that of astragalus ginseng soup and TGR5 agonist group.In summary, Qishen Tang can inhibit the activation of macrophage NLRP3 inflammatory vesicles, thereby inhibiting macrophage M1-type polarization and improving the inflammatory response.The main mechanism by which Qishen Tang inhibited the activation of macrophage NLRP3 inflammatory vesicles was through the inhibition of the TGR5/STAT1/STAT6 signaling pathway.
杂志排行
Journal of Hainan Medical College的其它文章
- The prevention of high-fat diet-induced non-alcoholic fatty liver disease in mice by Fucoidan
- Epidemiological characteristics of hyperuricemia in metabolic syndrome and its different components in the physical examination population
- Analysis of E2F3 gene variants, expression and clinical significance in melanoma based on multiple databases
- Meta analysis and data mining of the method of yishenhuoxue in the treatment of nonproliferative diabetic retinopathy
- Evaluation of the diagnostic efficacy of noninvasive diagnosis in patients with chronic viral hepatitis B complicated with nonalcoholic fatty liver disease and significant liver fibrosis
- Discover the key genes for glomerular inflammation in patients with type II diabetic nephropathy based on bioinformatics and network pharmacology
