微藻曝气强化短程硝化/厌氧氨氧化自养脱氮工艺性能
2023-12-30刘春爽张璐遥尹海李世文王明艳刘力嘉王胜渊孙志超
刘春爽 张璐遥 尹海 李世文 王明艳 刘力嘉 王胜渊 孙志超

摘要:采用序批式生物膜反應器(SBR),在温度为30 ℃条件下,在短程硝化/厌氧氨氧化生物膜的基础上耦合小球藻构建藻菌耦合生物膜体系,通过改变光照时长和曝气量组合条件的运行方式共运行85 d,分析组合条件下体系的脱氮性能、藻菌耦合体系特性和氮转化路径,以得到最佳脱氮条件及藻菌耦合脱氮机制。结果表明:当进水氨氮质量浓度为(400±50) mg/L时,光暗比(单位为h/h)设置为6/4,曝气强度为200 mL/min时,脱氮效果最好,NH4+-N及总氮平均去除率最高可达92.31%和87.56%;藻菌耦合体系运行过程中污泥质量浓度与小球藻干重calgae比始终约为5.5,表明生物膜中藻类和细菌的比例达到相对稳定状态,并形成良好的互利共生关系,集中在生物膜外部的小球藻通过光合作用产生的氧气被硝化细菌消耗,因此产生的厌氧环境和亚硝酸盐底物来维持厌氧氨氧化菌的活性;在氮的去除机制中,生物吸收量约占45.71%,PN/A(短程硝化-厌氧氨氧化)过程N2的生成量及氮损失约占54.29%。
关键词:小球藻; 藻菌耦合体系; 短程硝化/厌氧氨氧化; 生物膜; 自养脱氮
中图分类号:X 522 文献标志码:A
引用格式:刘春爽,张璐遥,尹海,等.微藻曝气强化短程硝化/厌氧氨氧化自养脱氮工艺性能[J].中国石油大学学报(自然科学版),2023,47(6):185-191.
LIU Chunshuang, ZHANG Luyao, YIN Hai, et al. Performance of autotrophic denitrification enhanced by microalgae aeration and anaerobic ammox[J].Journal of China University of Petroleum(Edition of Natural Science),2023,47(6):185-191.
Performance of autotrophic denitrification enhanced
by microalgae aeration and anaerobic ammox
LIU Chunshuang1, ZHANG Luyao1,2, YIN Hai3, LI Shiwen4, WANG Mingyan1, LIU Lijia1, WANG Shengyuan4, SUN Zhichao1
(1.College of Chemistry and Chemical Engineering in China University of Petroleum(East China), Qingdao 266580,China;
2.Judicial Bureau of Pingyi County, Linyi 273399,China;
3. Fengcheng Oilfield Operation Area of Xinjiang Oilfield Company, PetroChina, Karamay 834000, China;
4.Qingdao West Coast Utility Group Water Company, Qingdao 266000, China)
Abstract: A sequencing batch biofilm (SBR) reactor was used to cultivate an algae-coupled biofilm system based on short-range nitrification/anaerobic ammonia oxidation biofilm with Chlorella vulgaris at a temperature of 30 ℃. The system was operated for 85 d by changing the combination of light duration and aeration. The nitrogen removal performance, algae-coupled system characteristics and nitrogen conversion pathways were analyzed to obtain the optimal nitrogen removal conditions and algal-bacterial coupling nitrogen removal mechanism. The results show that when the influent ammonia nitrogen concentration is 400±50 mg/L, the light/dark ratio is 6 h/4 h, and the aeration intensity is 200 mL/min, the best denitrification effect was achieved, in which the average removal rates of NH4+-N and total nitrogen were up to 92.31% and 87.56%, respectively. The ratio of cMLVSS(wastewater mass concentration) to calgae(dry weight of chlorella vulgaris) is always around 5.5 during the operation of the algae coupled system, which indicates that the ratio of algae and bacteria in the biofilm reaches a relatively stable state and forms a good mutually beneficial symbiotic relationship. The oxygen produced by the photosynthesis of Chlorella vulgaris concentrated outside the biofilm is consumed by nitrifying bacteria, and nitrifying bacteria provided anaerobic environment and nitrite substrate to maintain the activity of anaerobic ammonia oxidizing bacteria. In the nitrogen removal mechanism, the biological uptake accounts for about 45.71%, and the amount of N2 production and nitrogen loss in the PN/A(partial nitrification-anaerobic ammonium oxidation) process accounts for about 54.29%.
Keywords: chlorella; algal and bacterial coupling system; short-cut nitrification and anammox; biological membrane; autotrophic nitrogen removal
污水中氮的过量排放大大加剧了水体的富营养化,对水质构成威胁[1-2]。氮污染控制已经成为全球关注的问题[3-4]。短程硝化与厌氧氨氧化(PN/A)工艺相结合,首先好氧氨氧化菌(AOB)在特定溶解氧等条件下将部分NH4+-N转化为NO2--N,再通过Anammox菌代谢作用将NH4+-N和NO2--N转化为N2,该工艺可以在不添加有机物的情况下实现废水高效自养脱氮[5-6],同时还具有工艺流程简单、占地面积小的优点[7-8]。然而在PN/A工艺中,溶解氧(DO)是影响系统稳定性的重要因素,因为AOB和Anammox菌对溶解氧的需求不同。控制DO浓度是实现稳定运行PN/A工艺的必要条件。藻菌共生系统被应用于处理营养物质丰富的废水[9]。在光照下,微藻利用溶解在水中或通过细菌呼吸释放的二氧化碳,通过光合作用为细菌产生氧气,而细菌反过来为微藻的生长提供代谢物和无机碳源[10-11],藻菌共生系统可以节省大部分或全部曝气能耗[12-13]。用于废水处理的微藻和细菌组合可以比单一微藻或细菌系统更好地去除营养物质[14-15]。将微藻与好氧细菌结合以实现有效去除NH4+-N和有机物,需要额外有机物来实现总氮的去除[16-17]。将微藻与PN/A工艺相结合,微藻光合作用所产生的氧气被AOB利用将部分NH4+-N转化为NO2--N,在硝化细菌消耗氧气产生的厌氧环境中,厌氧氨氧化菌利用剩余NH4+-N和亚硝酸盐底物进行反应,此过程可大大减少外源有机物的添加,并实现高效的自养脱氮。Tang等[18]通过单因素试验研究表明,0.2 L/min(空气)是藻菌体系所需的最佳曝气量;Zhang等[19]在无曝气辅助条件下探究藻类-细菌共生最佳光照时长表明当间歇光时间(L)/暗时间(D)(L/D,单位为h/h)设置为8/2,NH4+-N的平均去除速率为8.9 mg/(L·h),TN的平均去除率最高为63%。相同的DO质量浓度可以在生物膜系统的不同部位形成好氧区和缺氧区[20],为不同类型的细菌提供适宜的生长环境。此外生物膜具有抗冲击性及高生物多样性的特点[21]。目前关于生物膜形式的藻菌共生系统的研究较少,笔者利用序批式反应器(SBR)将小球藻与PN/A工艺结合,构建藻菌耦合体系,解析该系统在不同光照及曝气组合条件下的脱氮性能、藻菌耦合体系特性及氮转化路径。
1 试验材料与方法
1.1 进水和污泥
试验用水采用人工配水,其组成为 NH4Cl(提供NH4+-N),MgSO4·7H2O、CaCl2、NaHCO3和KH2PO4质量浓度分别为0.03、0.2、3和0.16 g/L,微量元素浓缩液 Ⅰ、Ⅱ,各加入2和1mL/L,具体成分与Xie等[20]报道相同。进水pH控制在8.0±0.2。
一体式短程硝化厌氧氨氧化污泥来自课题组前期培养的母反应器生物膜,生物膜上的生物量(挥发性悬浮物质量/载体质量)为2393 mg/g,接种藻种选用BG11培养基培养的小球藻,按藻菌质量比1∶3进行接种。
1.2 试验装置和操作程序
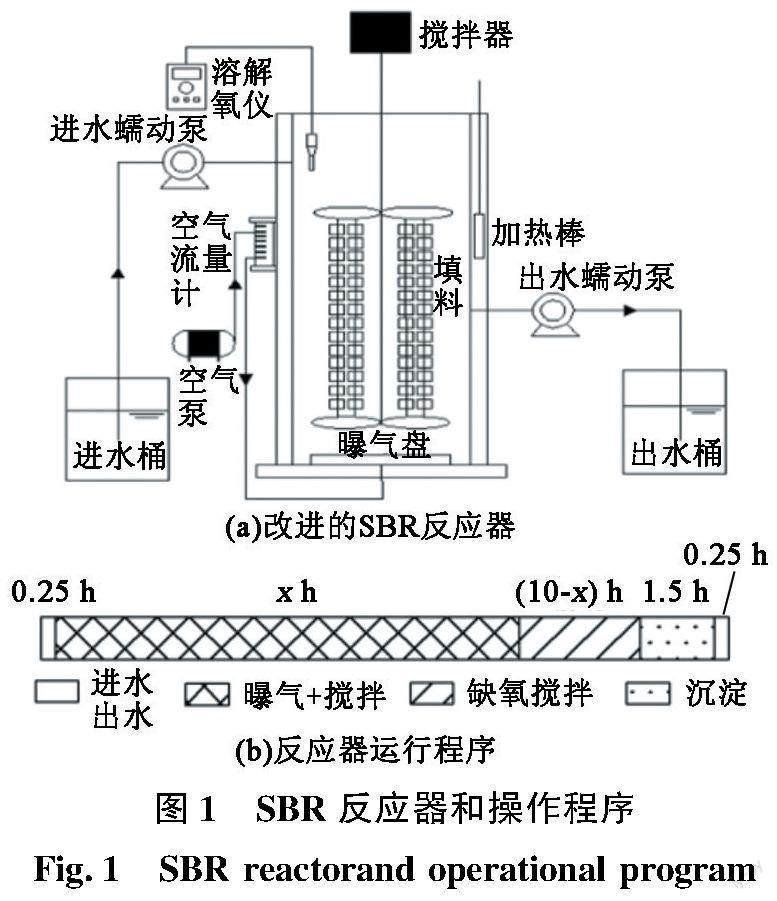
试验采用改进的SBR反应器(图 1(a)),其有效容积为6.0 L,外柱为水浴加热层,将恒温加热棒固定于外层壁,加热水温保持在(30±1) ℃,并在水浴加热层中预留的4个和反应器高度相同的有机玻璃空管放置LED灯管。选择聚氨酯海绵作为反应器中藻菌共生生物膜的载体。SBR反应器配有搅拌器和曝气盘,搅拌器控制在20 r/min的速度,曝气强度根据试验所需调节。每周期水交换体积比为50%。
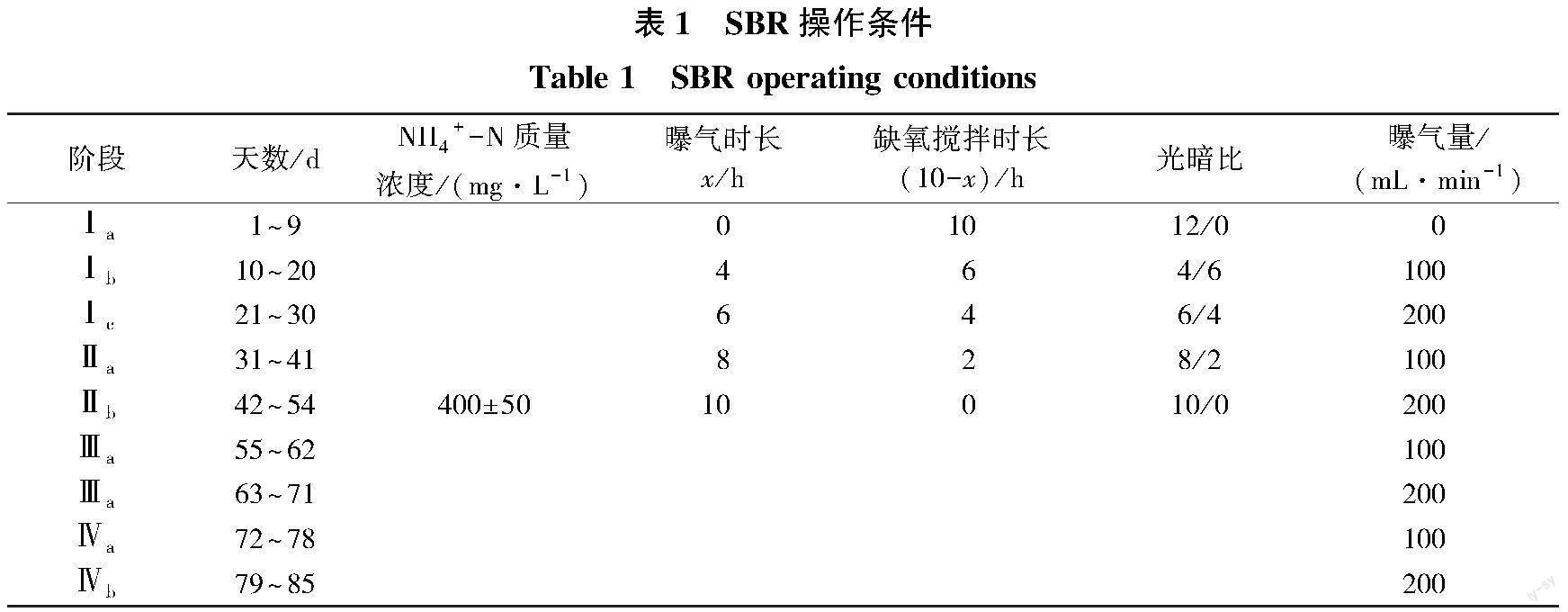
在接种小球藻后,小球藻随出水会有流失,持续间歇光照以促进小球藻在生物膜上的生长(持续9 d),待出水稳定后进行光照及曝气组合条件对系统脱氮变化的探究。SBR反应器每天运行2个周期,每周期运行12 h,其中反应时间固定为10 h。L/D设置为4/6、6/8、8/2、10/0,每个光暗比阶段曝气量分别采用100和200 mL/min。反应器运行程序如图1(b)所示,其中x为反应期间光照及曝气组合时间,x1、x2、x3、x4分别为4、6、8、10 h。不同反应条件下反应器的操作条件如表1所示。
1.3 化学分析方法
NH4+-N 分析采用纳氏试剂比色法[21],NO2- -N 采用乙二胺分光光度法[22],NO3--N采用酚二磺酸法[22]。pH值、污泥固体悬浮物(VSS)和VSS质量浓度采用常规测定方法[22]。EPS(胞外聚合物)根据根据Hao等[23]方法测定。叶绿素质量浓度的提取和测定参考文献 [24],小球藻叶绿素质量浓度与小球藻干重之间的关系为
calgae=0.62835cchl(a+b)+0.05557,R2=0.9923.
式中,calgae为小球藻干重,g/L;cchl(a+b)为叶绿素质量浓度,mg/L。
藻菌耦合SBR中氮平衡式为
Nin=Nout= Nuptake +Nv+Nd+Neff.
式中,Nin为反应器氮输入量即进水总氮量,mg/L,等于进水氨氮质量浓度;Nout為反应器氮输出量,mg/L;Nd为PN/A的N2生成量及氮损失,mg/L;Nuptake为生物吸收量,mg/L;Neff为出水总氮量,mg/L;Nv为NH3挥发量,mg/L。
通过假设藻类的化学计量法[25]确定Nuptake。Neff为出水NH4+-N、NO2--N、NO3-N的质量浓度,氨的挥发现象为NH4+-N在高pH条件下(pH>10)生成氨气溢出系统相较于其他转化路径,Nv可以近似为0。故PN/A的N2生成量及氮损失为Nd=Nin-Nuptake+Nv-Neff。
2 结果分析
2.1 反应器运行效能
在接种小球藻后,阶段Ⅰa 在不曝气的条件下提供间歇光照,以促进小球藻的生长,待出水稳定后开始试验。之后调节不同光暗比为4/6、6/4、8/2、10/0以及曝气量设置为100和200 mL/min以探究藻菌耦合脱氮最佳操作条件,共运行85 d,结果如图2所示。在L/D=4/6時,提供 100 mL/min的曝气量时系统表现出对NH4+-N的去除能力,出水NH4+-N浓度大幅度降低,当曝气量增大到200 mL/min时,出水NH4+-N质量浓度进一步降低,NH4+-N及总氮平均去除率为72.00%和68.63%,说明此前系统受溶解氧限制导致氨氮去除能力较低,在提供曝气后系统脱氮能力逐渐恢复。同时由于厌氧氨氧化反应产生的部分NO3--N及少量未被利用的NO2--N在系统中,所以总氮去除率低于NH4+-N去除率。在L/D=6/4时得到同样的规律,但在100 mL/min的曝气强度下,较上一阶段曝气量减少了25%,NH4+-N平均去除率由72.0%提升到至78.09%,总氮平均去除率由68.63%提升至74.49%,这是因为随着光照时间的增加,藻类产生更多的DO,有利于提高硝化细菌的活性,从而提高NH4+-N的去除率。但在L/D=8/2以及L/D=10/0时,虽延长了光照及曝气时间,在与之前同样的曝气强度下,出水NH4+-N质量浓度却升高,这是因为当外界所提供的溶解氧质量浓度增大时,虽然可以增强硝化细菌的供氧来源促进其活性,但对厌氧氨氧化菌可能产生一定程度的抑制,使其不能充分利用NH4+-N。同时如果一味增大曝气强度,藻类的光合作用被限制,藻类活性受抑制,即打破了藻菌之间一致的生长速率,破坏了共生系统的平衡。因此当光暗比设置为6/4、曝气强度为200 mL/min时,出水NH4+-N平均质量浓度为34.38 mg/L,NH4+-N及总氮平均去除率最高可达92.31%和87.56%。
2.2 藻菌耦合体系特性
图3为藻菌共生生物膜表观形态,图4为第50 d生物膜扫描电镜结果。可以看出,反应器内的生物膜在接种小球藻后,不同时期生物膜外观差别较大。随着培养过程的进行,生物膜外小球藻分布逐渐均匀,由最初的黄褐色加深为深绿色。从第50 d不同放大倍数的扫描电镜观察结果可知,与内部相比,外部生物膜较厚且小球藻主要分布在外部,这为小球藻利用光照产氧提供了有利条件并形成生物膜从外到内溶解氧逐渐减少的氧气梯度,细菌形态主要为杆状、球状并有少量长杆状或丝状形态。
在培养过程中,生物膜上所负载的生物参数也有不同程度的变化:载体负载生物量由2056 mg/g载体升至3784 mg/g;污泥质量浓度(cMLVSS)及calgae从第1 d的3.02和0.56 g/L升至第85 d的0.56和2.45 g/L,两者比值始终约为5.5(表2),表明生物膜中藻类和细菌的比例达到相对稳定状态,并形成了良好的互利共生关系。此外S-EPS、TB-EPS和LB-EPS的含量逐渐上升(图5),蛋白质(PN)分别从最初的11.36、11.07和6.23 mg/g上升至30.86、35.10和35.40 mg/g。在生物膜生长繁殖阶段,EPS具有聚集、连接菌藻细胞以及作为暂存和传递营养物质媒介的作用,构成了菌藻耦合生物膜的骨架[26]。
2.3 氮转化路径分析
藻菌共生系统主要是通过藻菌协同作用实现高效脱氮,一部分是活性污泥菌群的短程硝化/厌氧氨氧化作用脱氮,另一部分是微藻生物同化吸收氮。整个系统中氮形态的变化是联动的,利用氮元素质量守恒原则分析藻菌共生SBR稳定运行阶段每周期氮的迁移转化路径,结果见图6。
藻菌耦合SBR系统在稳定运行阶段的氮平衡分析数据见表3。
SBR稳定阶段,进水NH4+-N质量浓度为449.73 mg/L时,NH4+-N去除率约为92.03%,TN去除率约为87.43%,其中生物吸收量约占45.71%,PN/A过程N2的生成量及氮损失约占54.29%。系统中氮损失可能有两方面原因:一是异养硝化反应过程会产生中间产物从而造成氮损失,硝化过程中产生N2、N2O和NO,其比例可达氮除去率的 10%以上[26-28];二是可能存在同时硝化反硝化现象 (SND),即硝化反应和反硝化反应在同一反应器中、相同操作条件下同时发生[26-28]。
3 结 论
(1)采用SBR反应器,以聚氨酯海绵负载的一体式短程硝化厌氧氨氧化污泥为接种污泥,在温度为(30±1) ℃,通过改变光照时长及曝气量方式,连续运行85 d。当进水氨氮质量浓度为(400±50) mg/L、光暗比设置为6/4、曝气强度为200 mL/min时,NH4+-N及总氮平均去除率最高可达92.31%和87.56%。
(2)藻菌耦合体系生物膜外观为深绿色,小球藻主要集中在生物膜外部。藻菌耦合体系运行过程中cMLVSS与calgae比值始终约为5.5,表明生物膜中藻类和细菌的比例达到相对稳定状态,并形成了良好的互利共生关系。系统运行过程中EPS含量逐渐上升,构成了菌藻共生生物膜的骨架。
(3)在稳定运行的第45~55 d,NH4+-N去除率约为92.03%,TN去除率约为87.43%;在氮的去除机制中,生物吸收量约占45.71%,PN/A过程N2的生成量及氮损失约占54.29%。
参考文献:
[1] MITCHESON Y S D, TO W L, WONG N W, et al. Emerging from the murk: threats, challenges and opportunities for the global swim bladder trade[J]. Reviews in Fish Biology and Fisheries, 2019,29(4):809-835.
[2] ZGA B, WJB C, YZA D, et al. Revisiting seasonal dynamics of total nitrogen in reservoirs with a systematic framework for mining data from existing publications[J]. Water Research, 2021,201:117380.
[3] KAISE J. The other global pollutant: nitrogen proves tough to curb[J]. Science, 2001,294(5545):1268-1269.
[4] LIU Xuejun, ZHANG Ying, HAN Wenxuan, et al. Enhanced nitrogen deposition over China[J]. Nature, 2013,494:459-462.
[5] MIAO L, YANG G, TAO T, et al. Recent advances in nitrogen removal from landfill leachate using biological treatments: a review[J]. Journal of Environmental Management, 2019,235:178-185.
[6] CAO S, YAN W, YU L, et al. Challenges of THP-AD centrate treatment using partial nitritation-anammox (PN/A):inhibition, biomass washout, low alkalinity, recalcitrant and more[J]. Water Research, 2021,203:117555.
[7] DOSTA J, VILA J, SANCHO I, et al. Two-step partial nitritation/Anammox process in granulation reactors: start-up operation and microbial characterization[J]. Journal of Environmental Management, 2015,164:196-205.
[8] SUN H W , BAI Y , PENG Y Z , et al. Achieving nitrogen removal via nitrite pathway from urban landfill leachate using the synergetic inhibition of free ammonia and free nitrous acid on nitrifying bacteria activity[J]. Water Science & Technology, 2013,68(9):2035-2041.
[9] RAMANAN R, KIM B H, CHO D H, et al. Algae–bacteria interactions: evolution, ecology and emerging applications[J]. Biotechnology Advances, 2016,34(1):14-29.
[10] FALLAHI A, REZVANI F, ASGHARNEJAD H, et al. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: a review[J]. Chemosphere, 2021,272:129878.
[11] ARUN S, MANIKANDAN N A, PAKSHIRAJAN K, et al. Novel shortcut biological nitrogen removal method using an algae-bacterial consortium in a photo-sequencing batch reactor: process optimization and kinetic modelling[J]. Journal of Environmental Management, 2019,250:109401.
[12] LI B, BAO M, LIU Y, et al. Novel shortcut biological nitrogen removal using activated sludge-biofilm coupled with symbiotic algae[J]. Journal of Water Process Engineering, 2021,43:102275.
[13] WANG M, YANG H, ERGAS S J, et al. A novel shortcut nitrogen removal processusingan algal-bacterialconsortium in a photo-sequencing batch reactor (PSBR)[J]. Water Research, 2015,87:38-48.
[14] LI X, GUO S, PENG Y, et al. Anaerobic digestion using ultrasound as pretreatment approach: changes in waste activated sludge, anaerobic digestion performances and digestive microbial populations[J]. Biochemical Engineering Journal, 2018,139:139-145.
[15] JI X, JIANG M, ZHANG J, et al. The interactions of algae-bacteria symbiotic system and its effects on nutrients removal from synthetic wastewater [J]. Bioresource Technology, 2018,247:44-50.
[16] SARAVANAN A, KUMAR P S, VARJANI S, et al. A review on algal-bacterial symbiotic system for effective treatment of wastewater[J]. Chemosphere, 2021,271:129540.
[17] HE Q, SONG Q, ZHANG S, et al. Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sequencing batch reactor with mixed carbon sources: reactor performance, extracellular polymeric substances and microbial successions [J]. Chemical Engineering Journal, 2018,331:841-849.
[18] BING T, SONG H, BIN L, et al. Determination of the profile of DO and its mass transferring coefficient in a biofilm reactor packed with semi-suspended bio-carriers[J]. Bioresource Technology, 2017,241:54-62.
[19] ZHANG Y, WANG J, PENG S, et al. Autotrophic biological nitrogen removal in a bacterial-algal symbiosis system: formation of integrated algae/partial-nitrification/anammox biofilm and metagenomic analysis[J]. Chemical Engineering Journal, 2022,439:135689.
[20] MCQUARRIE J P, BOLTZ J P. Moving bed biofilm reactor technology: process applications, design, and performance [J]. Water Environment Research, 2011,83(6):560-575.
[21] XIE G, LIU T, CAI C, et al. Achieving high-level nitrogen removal in mainstream by coupling anammox with denitrifying anaerobic methane oxidation in a membrane biofilm reactor[J]. Water Research, 2018,131:196-204.
[22] RAGHOEBARSING A A, POL A, PAS-SCHOONEN K, et al. A microbial consortium couples anaerobic methane oxidation to denitrification[J]. Nature, 2006,440(7086):918-921.
[23] American Public Health Association. Standard methods for the examination of water and wastewater[M]. 21st ed. Washington, DC: American Water Works Associa-tion and Water Environment Federation, 2005:520-580.
[24] YE J, LIANG J, WANG L, et al. Operation optimization of a photo-sequencing batch reactor for wastewater treatment: study on influencing factors and impact on symbiotic microbial ecology[J]. Bioresource Technology, 2018,252:7-13.
[25] SCHUNURR P J, ALLEN D G. Factors affecting algae biofilm growth and lipid production: a review[J]. Renewable and Sustainable Energy Reviews, 2015,52:418-429.
[26] HANAKI K, MATSUO T, HONG Z. Production of nitrous oxide gas during denitrification of wastewater[J]. Water Science & Technology, 1992,43(6):1027-1036.
[27] 趙东风,李文斐,马文娟,等.NaCl对反硝化脱硫工艺运行效果的影响[J].中国石油大学学报(自然科学版),2017,41(5):176-180.
ZHAO Dongfeng, LI Wenfei, MA Wenjuan, et al. Effect of NaCl on denitrifying sulfide removal process [J]. Journal of China University of Petroleum(Edition of Natural Science),2017,41(5):176-180.
[28] 刘春爽,李伟,于海彤,等.反硝化厌氧甲烷氧化与厌氧氨氧化耦合颗粒污泥脱氮效能[J].中国石油大学学报(自然科学版),2022,46(1):177-182.
LIU Chunshuang, LI Wei, YU Haitong, et al. Nitrogen removal performance of coculture granular sludge based on nitrate/nitrite-dependent anaerobic methane oxidation and anaerobic ammonium oxidation[J]. Journal of China University of Petroleum(Edition of Natural Science),2022,46(1):177-182.
