Interaction of water droplets with pyrolyzing coal particles and tablets
2023-12-27AnastasiaIslamovaPavelTkachenkoPavelStrizhak
Anastasia Islamova,Pavel Tkachenko,Pavel Strizhak
National Research Tomsk Polytechnic University, Heat and Mass Transfer Laboratory, 30 Lenin Avenue, Tomsk, 634050, Russia
Keywords:Droplet Particle Solid substrate Collision Agglomeration Separation
ABSTRACT The paper presents the experimental research findings for the patterns of collisions of water droplets with pressed tableted samples used as substrates and with small particles of a pyrolyzing solid fuel.Brown coal samples were used.Droplet-substrate interactions were studied when varying the droplet diameter in the range from 1 to 4 mm and velocity from 0.5 to 4 m/s.That corresponded to the Weber number range of 7-830.The coal tablet surface temperature was varied from 20 to 700 °C.In the interactions of water droplets (0.7-1.5 mm in diameter,pre-collision velocity from 1 to 3 m/s) with coal particles (with a size of 0.2-1 mm,pre-collision velocity 0.7-2 m/s),the temperature of the latter was varied in the range of 330-480 °C.The following regimes of the interaction of droplets with solid particles during chemical reactions and phase transformations were distinguished: spreading/agglomeration and break-up/separation.Differences in the characteristics of the interaction of water droplets with coal particles at varying temperatures were identified.Droplet-particle interaction regime maps for B(We), We(Oh) and We(Ca) were constructed.The collision regime boundaries were described using fitted curves that can be utilized to develop the existing mathematical models of droplet-particle collisions in gas.It was established that the gaseous volatile production in coal pyrolysis has a modest effect on the regimes and characteristics of the droplet destruction in the temperature range under consideration (20-700 °C).
1.Introduction
The characteristics of the interaction between a droplet and a solid surface depend on a number of factors: liquid and solid surface properties [1-3];droplet sizes [4];gas,liquid and solid wall temperatures [5,6];resultant velocity before the collision [7,8];geometry;medium (liquid,gas,dispersed) [9,10],etc.One of the key factors in the collisions of liquid droplets with a solid is its surface type[2,3].The collisions of droplets with metal[11-13]and inorganic [13,14] surfaces with varying roughness degrees have received much attention over the past few decades.The interaction of liquid droplets with solid surfaces is important not only in the context of fundamental research [15] but also in its practical application in such technologies as coating[16],spray cooling[17],fuel spraying[18,19],development of heat shield materials for highspeed aircrafts [20,21],reduction of erosion damage to jet engine components[22],and others.Moreover,the destruction of thermal protection during the flight of hypersonic aircrafts in dense atmospheric layers (including dust-laden ones) is driven by a range of extreme thermal factors and fluid dynamics affecting the thermal protection [23].As a result of this impact,a number of internal processes in the thermal protection volume arise as a reaction to the above factors.Extensive erosion damage to the surface layer of a heat shield is a barrier to the future development of technologies.There are studies on the choice of optimal heat shield materials.These include carbon materials with high thermal conductivity,conventional carbon fiber coatings and promising high-impedance ceramic materials maintaining the original geometry of the body[20,21].
Composite slurry fuels are a promising replacement for coal and fuel oil according to environmental,economic,energy and social criteria [24,25].In Refs.[26,27],a separate supply of slurry fuel components (water and oil droplets,coal and biomass particles)into a combustion chamber was proposed.When fuel components are injected separately,e.g.,in a precombustor space,the collisions of solid coal particles with liquid droplets can produce a multicomponent gas-vapor-droplet aerosol.To date,the research findings for the interaction of liquid droplets with organic surfaces,including coal,are limited.Reliable experimental data are required to develop the physical and mathematical models,as well as for subsequent predictive modeling of processes occurring in the collisions of fuel components in the precombustors of boilers and boiler units.The conditions of the collisions of droplets with pyrolyzing solid particles and massive surfaces,e.g.,unground solid fuel,remain understudied.
There are studies into the wettability of coals of different ranks[28,29].This is a crucial factor in the conditions and characteristics of the collisions of droplets with particles and with massive solid surfaces.For instance,Zhu et al.[28] investigated the way roughness of coking coal with an ash content of 7.71% affects its wettability and floatability.The contact angle was shown[28]to decrease with greater surface roughness,whereas the adhesion force between the water droplet and the coal surface increases due to a greater area of contact and emergence of pinning.These conclusions [28] are in line with the findings by Chen et al.[29].Several studies[30,31]establish that an increase in the mineral roughness weakens wettability.A number of authors [32,33] considered the effect of chemical properties of coal on wettability.Xia et al.[32]examined the way the heating conditions affect the wettability of coals of different ranks.It was established that heating reduces the contact angles(θ)for bituminous coal and anthracite,and increases those for lignite.This effect is conditioned by the changing content of C/CH,CO,C=O and COOH groups typical of coals of different ranks exposed to temperature.These compounds can be adsorbed on coal surface due to hydrogen bonds and the van der Waals force[34,35].Xia and Xie [33] found that heating anthracite to 600°C increases the content of hydrophilic functional groups on the coal surface.High-temperature heating was shown [33] to invert coal wettability (from hydrophobic to hydrophobic behavior).
Although coal wettability has been extensively studied[28-33],there is still no research into the dynamic interaction of droplets with a pyrolyzing coal particle surface.The collision of liquid droplets with a heated surface is a complex phenomenon whose dynamics depends on the hydrodynamic characteristics and regimes of heat exchange with large spatial and temporal gradients of variables [36].Experimental [4,5,36-40] and modeling [41-43]results were published for the interaction of liquid droplets with heated metal surfaces.Depending on the spraying characteristics,wettability properties,surface morphology and substrate temperature,different outcomes of the droplet-wall interaction were recorded.A comprehensive investigation was carried out into the effect of the surface temperature [36-38],the Weber number[36,38] and surface roughness [39] on collision characteristics and regimes.Liang and Mudawar [5] and Breitenbach et al.[4] identified four types of phase transformations: evaporation at a surface temperature below the boiling temperature (film evaporation),nucleate boiling,transition boiling and film boiling.The ambient gas component composition significantly affects the heat transfer,phase transition and hydrodynamics of a droplet[15].Xu et al.[44]established that a pressure decrease can prevent the droplet splashing on impact with a solid surface.Herbert et al.[41] developed a numerical model describing the hydrodynamics,heat exchange and evaporation of a droplet impacting on a hot wall in a steam atmosphere.The effect of the Reynolds number(Re=500-1500)on heat exchange and evaporation was examined.Heat transfer characteristics differ greatly at different stages of the droplet-surface interaction [41].The substrate morphology may have a significant effect on the heat transfer when a droplet impacts on a surface.This is caused by convection enhanced by velocity fluctuations in the near-wall film flow due to surface roughness.Rapid evaporation near the contact line plays a significant role too,if some areas of the textured surface are partly dry [45,46].Heat removal is enhanced on porous substrates [47,48],microtextured[46] and macrotextured [46,49] surfaces.Unlike it is with metals,heating coal to a temperature above 450°C enhances its pyrolysis and the corresponding volatile release.No studies have been published on the effect of chemical reactions (volatile release) occurring during the thermal treatment of coal on the interaction with droplets.Understanding these processes is instrumental in predicting the characteristics of processes during the spraying of a slurry fuel in a boiler furnace and its subsequent combustion.The specified aspects became the motivation for this research.The purpose of this research is to identify the key patterns of collisions of water droplets with a massive surface and fine particles of pyrolyzing coal,as well as to study the effect of the volatile release on the interaction characteristics and regimes.
2.Experimental technique
2.1.Materials
The liquid under study was water,as it is a widely used component in composite fuel composition.Its temperature in the experiments was 20°C.The thermal and physical properties of water at this temperature were the following: density ρ=998 kg/m3;surface tension σ=0.07269 N/m;dynamic viscosity μ=0.001 Pa·s,thermal conductivity λ=0.599 W/m°C.
Tableted substrates of brown coal were used as samples to study the droplet-surface collisions.Brown coal was chosen because it produces a greater amount of volatiles(more than 41%)in pyrolysis than other coal ranks do.Tablets (substrates) 12 mm in diameter,with a height of 2 mm and a mass of 0.4 g represented coal surfaces.Dried coal ground to a particle size of less than 200 μm was put into a die to produce tablets.A hydraulic press applied a pressure of 2 MPa to the die to form coal tablets.In the experiments with solid particles,coal was preliminarily dried to be ground in a rotor mill and riddled through two sieves with a mesh size of 0.9 mm and 1.0 mm to obtain particles of equal sizes with slight deviations.A Hitachi 3000 M scanning electron microscope was employed to examine the microtexture of coal tablet and particle surfaces.Three-dimensional characteristics of roughness were determined,namely,root mean square roughness (Sq) and maximum height of surface (Sz).
2.2.Experimental methods
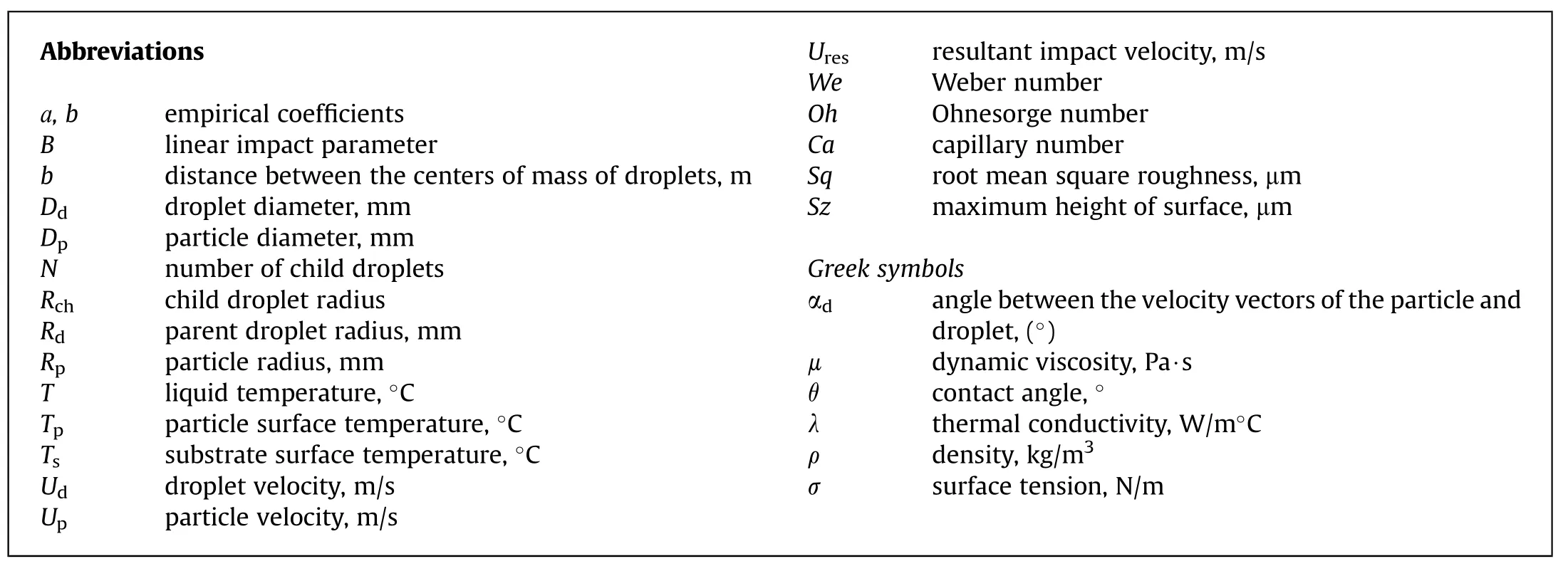
The characteristics of collisions of distilled water droplets with coal tablets and particles were studied employing a setup (Fig.1)and using shadowgraphy.The liquid was supplied with a Cole Parmer high-accuracy electronic syringe pump using a silicone tube,2 mm in diameter,connecting the pump to the nozzle.Detachable nozzles 1 made it possible to vary the size of generated droplets(Dd)from 1 to 4 mm.In the interaction of water droplets 2 with coal tablets 3,the nozzle was perpendicular to the surface under study(Fig.1(a)).The droplet velocity was varied in the range of 0.5-4 m/s.The droplet size range was chosen to provide minimum deformation of the droplet and its stable shape during its movement in gas.The velocity range was meant to provide the occurrence of different collision regimes.The effect of the surface temperature on the droplet dispersion was investigated.To that end,a tableted coal sample was heated to the temperature (Ts)ranging from 20 to 700°C.A coal substrate was placed on a copper holder that was heated using the alcohol burner 4.The temperature on the substrate surface was controlled by varying the height of the holder above the burner.The coal surface temperature was measured using a Testo 835-T2 pyrometer with an accuracy of 0.1°C and a type K thermocouple,whose junction was on the tablet surface,as well as a National Instruments measurement system(with an accuracy of ±1°C).
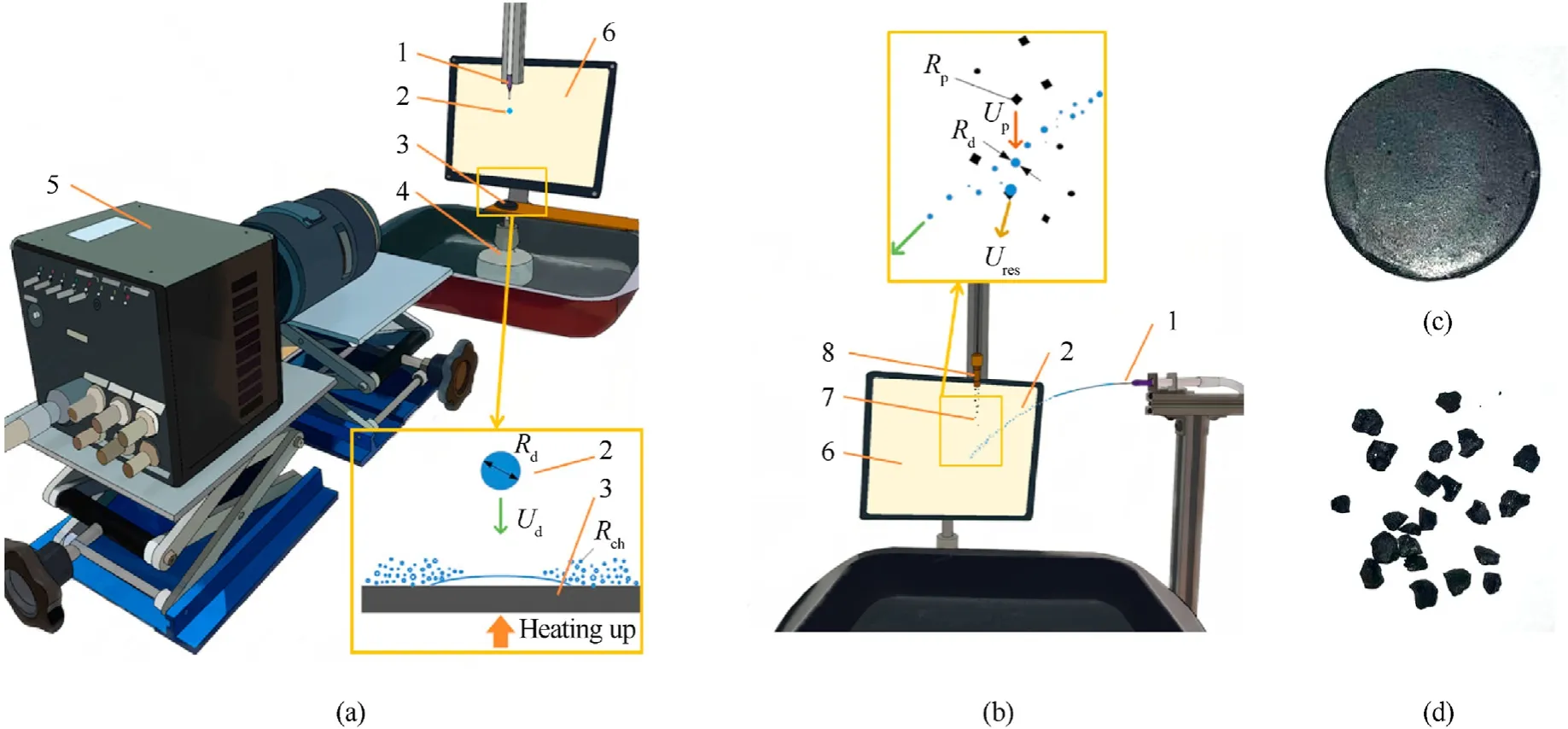
Fig.1.Layout of setup to study the collisions of liquid droplets with substrates (a) and particles (b) during rapid pyrolysis: 1 -Nozzle;2 -Water droplet;3 -Coal tablet;4 -Alcohol burner;5-High-speed video camera;6-Dffusion screen;7-Coal particles;8-Container for coal particles.Appearance of the(c)tableted sample and(d)particles of coal.
To study the collisions of water droplets 2 with coal particles 7,the setup was slightly modified(Fig.1(b)).The nozzle 1 was located in the horizontal plane.The syringe pump varied the droplet delivery rate from 1 to 3 m/s.The droplet diameter ranged from 0.7 to 1.5 mm.Coal particles were placed into a copper container 8 with a cover that was heated with a gas burner.Their size (Dp) was 0.2-1 mm,their velocity(Up)varied from 0.7 to 2 m/s.The particle temperature was in the range of 20-480°C.The temperature inside the copper container was measured with a type K thermocouple and a National Instruments measurement system.As soon as the temperature reached the required level,the container 8 cover was opened and the particles fell under gravity,thus impacting on distilled water droplets.
The patterns of the collisions of water droplets with a solid particle surface and a tableted sample were recorded using a highspeed Photron Fastcam Mini UX 100 video camera 5 and a Navitar Zoom 7000 lens (18-108/2.5).The recording was performed at a frame rate of 5,000 fps and an inter-frame delay of 1/20,000 s,resolution 1,280×1,000 pixels.To accurately measure the interaction characteristics,the camera was centered relative to the coal substrate.The recording area was arranged so as to monitor the parameters of the initial droplet and those of fragments formed after the collision (often referred to as satellite droplets or child droplets).Photron Fastcam Viewer determined the droplet velocity(Ud),size (Rd),spread diameter (Dmax),number of secondary fragments(N)and their sizes(Rch).The sizes(average radii)of droplets were determined using shadowgraphy[50].The diffusion screen6provided a contrast shadow image of droplets.Droplet sizes were determined allowing for the scale factor obtained at the stage of the measurement system calibration.
The resultant velocity before the droplet-particle interaction(Ures) was calculated using the cosine law:whereUdandUp-are the initial velocities of droplets and particles (m/s),respectively.The linear impact parameter was determined using formula:B=b/(Rd+Rp),wherebis the distance between the centers of mass of droplets,allowing for the resultant velocity vector projection (m),RdandRpare the diameters of the droplet and particles(mm),respectively).To plot the regime maps,the dimensionless similarity criteria were calculated:the Weber number (We),the Ohnesorge number (Oh) and the capillary number (Ca).The obtained parameters were used to plot the maps of regimes (bounce,separation,disruption and coalescence) in theB(We),We(Oh) andWe(Ca) coordinate systems.The surface areas of liquid fragments formed after the droplet-substrate interaction (S1) and of droplets before the interaction (S0) were calculated.The surface area of a water droplet before the collision with a coal surface was calculated as the area of a sphere:S0=The area of child droplets was given byS0=4∑π(Rch)2.
The error of determining the characteristics of the collisions of droplets with a substrate and particle came from the instrument error conditioned by the possibilities of the shadowgram processing methods and the systematic error.Sets of five experiments were conducted under identical initial conditions.The systematic errors of droplet size and velocity measurement were 2.1% and 3.4%,respectively.The random error was 2.1% for droplet size measurement and 3.4%for velocity measurement.As part of the droplet size analysis,several measurements were performed to determine the average radius and minimize the random error.The error of determining the droplet size was calculated by means of the three Sigma rule.The mean square deviation of the random component of direct (sizes and angles) and indirect (dimensionless numbers)measurement errors does not exceed 3% and 7%,respectively.
3.Results and discussion
3.1.Interaction of droplets with a wall
The technological complications(nozzle clogging,inner surface wear due to maintaining high pressure during injection and increased friction),arising in the combustion of coal-water slurries with petrochemicals and without them in boiler units,can be avoided if solid and liquid components are supplied separately into combustion chambers.It is a primary task to reliably determine the threshold conditions of coalescence,agglomeration and separation of liquids and solid particles in the composite fuel spray composition.The experiments involving the collisions of distilled water droplets with pressed coal substrates at varying initial sizes and velocities of droplets and substrate surface temperatures revealed the main patterns of the developing processes and showed the contribution of each decisive factor.The most interesting findings were obtained at a substrate surface temperatureTsof over 300°C,since that is when volatile components of coal are produced due to its rapid thermal decomposition.Other noteworthy results were obtained in the experiments with the collisions of water droplets with solid substrates heated toTs=500-700°C,because the release of volatile components of coal becomes more active and their gas-phase ignition is enhanced.
Fig.1 presents the images of collisions of distilled water droplets with pressed coal substrates without preliminary heating.In this case,the effect of droplet velocities (thus,the Weber number) on the characteristics of the interaction with a coal substrate was investigated.The initial sizeRdof droplets was 1.5 mm.Fig.2(a)and Supplementary material A show that a water droplet moving at a velocityUdof 1 m/s (We≈40) collides with a substrate to spread on its surface without fragmentation.The stain of contact of liquid on the surface increases approx.2.5 times relative to the initial droplet size (Dmax/Dd).An increase in the droplet velocityUdto 2.5 m/s (We≈200) (Fig.2(b)) did not affect the droplet-coal substrate interaction regime,yet it increased theDmax/Ddratio to 4.A further increase in the velocityUdto 3.5 m/s(We≈400)(Fig.2(c))changed the interaction regime and led to the fragmentation of the liquid after the collision with the coal substrate.The number(N=10-12)and size(Rch=0.1-0.15 mm)of child droplets at this velocityUd(thus,at thisWe)slightly increase the free surface area of liquid.The heat exchange process efficiency is enhanced insignificantly.
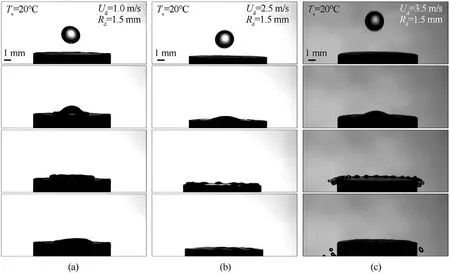
Fig.2.Images of collisions of distilled water droplets with pressed coal substrates, Rd=1.5 mm, Ts=20°C: (a) Ud=1 m/s;(b) Ud=2.5 m/s;(c) Ud=3.5 m/s.
Heating the substrate to a temperatureTsof 500-700°C(Fig.3)at constant droplet sizes(Rd=1.5 mm)and velocities(Ud=2.5 m/s)produced some interesting findings.Thus,compared to the collisions of a droplet with a substrate at room temperatureTs=20°C(Fig.3(a)),the collisions with a substrate heated toTs=500°C(Fig.3(b)) were accompanied by fragmentation,other conditions being equal.When the substrates were heated toTs=600°C(Fig.3(c)) andTs=700°C (Fig.3(d)),the fragmentation intensity increased.This was represented in reduced sizes of child droplets(Rch=0.015-0.025 mm) and their increased number(N=150-200).It was also established that at a substrate temperatureTsof 500°C(Fig.3(b)),there was a liquid film on its surface after fragmentation.An increase in the substrate surface temperatureTsto 600-700°C (Supplementary material B and Supplementary material C) caused the liquid film to detach from the surface to form several large child droplets(Rch=0.15-0.45 mm).This phenomenon is related to the fast boiling of the liquid layer in contact with the surface and a sharp increase in the pressure of vapors between the solid surface and the liquid stain.This leads to the destruction of the liquid film and formation of a great number of child droplets.From the moment a droplet detaches from the nozzle and until its contact with a carbonaceous particle/substrate (about 0.2 s),the droplet is affected by the thermal energy from the substrate.The impact of the corresponding heat changes the physical properties of water.In particular,the viscosity and density decrease.Different physical properties of water lead to the surface deformation and emergence of convective flows inside the droplet.However,given the initial water temperature (20°C),droplet diameter (1-4 mm) and the short time the droplet travels before the contact (up to 0.2 s),the thermal field impact can be neglected in the case under study.Kuznetsov et al.[51] showed that a water droplet with the initial temperature of 80°C,moving in gas with a temperature of 20°C for 1 s,cooled down by 1-2°C in the near-surface layer,which changes the physical properties of water only slightly.
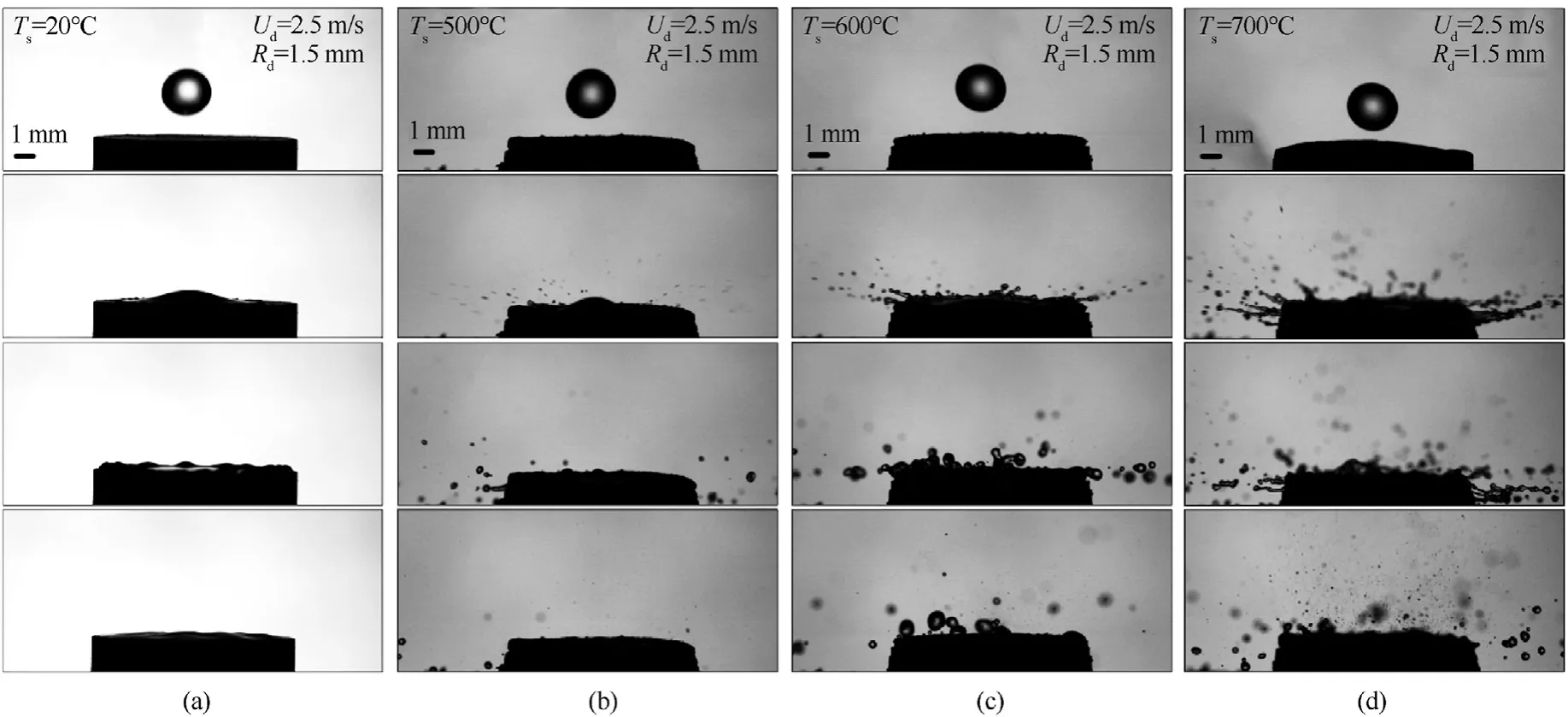
Fig.3.Images of collisions of droplets(Rd=1.5 mm,Ud=2.5 m/s)with substrates at varying surface temperatures:(a)Ts=20 °C;(b)Ts=500 °C;(c)Ts=600 °C;(d)Ts=700 °C.
Similarly,to the collisions of water droplets with coal substrates without heating (Ts=20°C),an increase in the droplet velocities from 2.5 to 3.5 m/s before the collision with a coal substrate heated toTs=700°C enhanced the secondary atomization of liquid.However,in this case,droplet fragmentation proceeded faster and under a different scenario due to the additional contribution of the high surface temperature.Fig.4 presents the images of the collisions of water droplets with pressed coal substrates heated toTs=700°C.A rise in the droplet velocity with a higher substrate temperature increased the number of child droplets.The statistical processing of the images revealed that the number of child droplets atUd=3.5 m/s increased by 18-20% relative toUd=2.5 m/s.It is obvious that the number of child dropletsNis almost proportional to the resultant velocity of dropletsUd.

Fig.4.Images of collisions of distilled water droplets with pressed substrates heated to Ts=700 °C,Rd=1.5 mm at varying droplet velocities:(a)Ud=2.5 m/s;(b)Ud=3.5 m/s.
The patterns developing in the collisions of water droplets of different sizes with heated coal substrates were identified by varying the diameter of nozzles fitted at the syringe pump outlet.This allowed the droplet sizeRdvariation in the range of 1.2-1.8 mm.Fig.5 shows the images of the collisions of distilled water droplets with pressed coal substrates at varying initial sizes of droplets but at a constant temperatureTs=500°C and velocityUd=2.5 m/s.Droplets with a sizeRdof 1.2 mm (Fig.5(a))collided with a coal substrate to form 30-50 child droplets.The predominant size of the fragmentsRchwas 0.05-0.1 mm.An increase in the droplet sizeRdto 1.5 mm (Fig.5(b)) resulted in 50-70 child droplets with a sizeRchof 0.05-0.1 mm.The number of child droplets with a sizeRchof 0.15-0.2 mm increased.A further increase in the droplet sizeRdto 1.8 mm(Fig.5(c))accounted for the formation of a greater (by 12-15%) number of large child droplets(Rch=0.15-0.2 mm) than there were atRd=1.5 mm.The total number of child droplets after the collision of the water droplet with the coal substrate increased too.The main reason for a greater number and size of child droplets is an increase in the liquid volume in the droplets.Recording the variations in the number and size of liquid child droplets makes it possible to predict with greater accuracy the processes developing in the technological equipment during the collisions of liquid droplets with solid fragments and surfaces undergoing phase transformations and participating in chemical reactions in this gas environment.
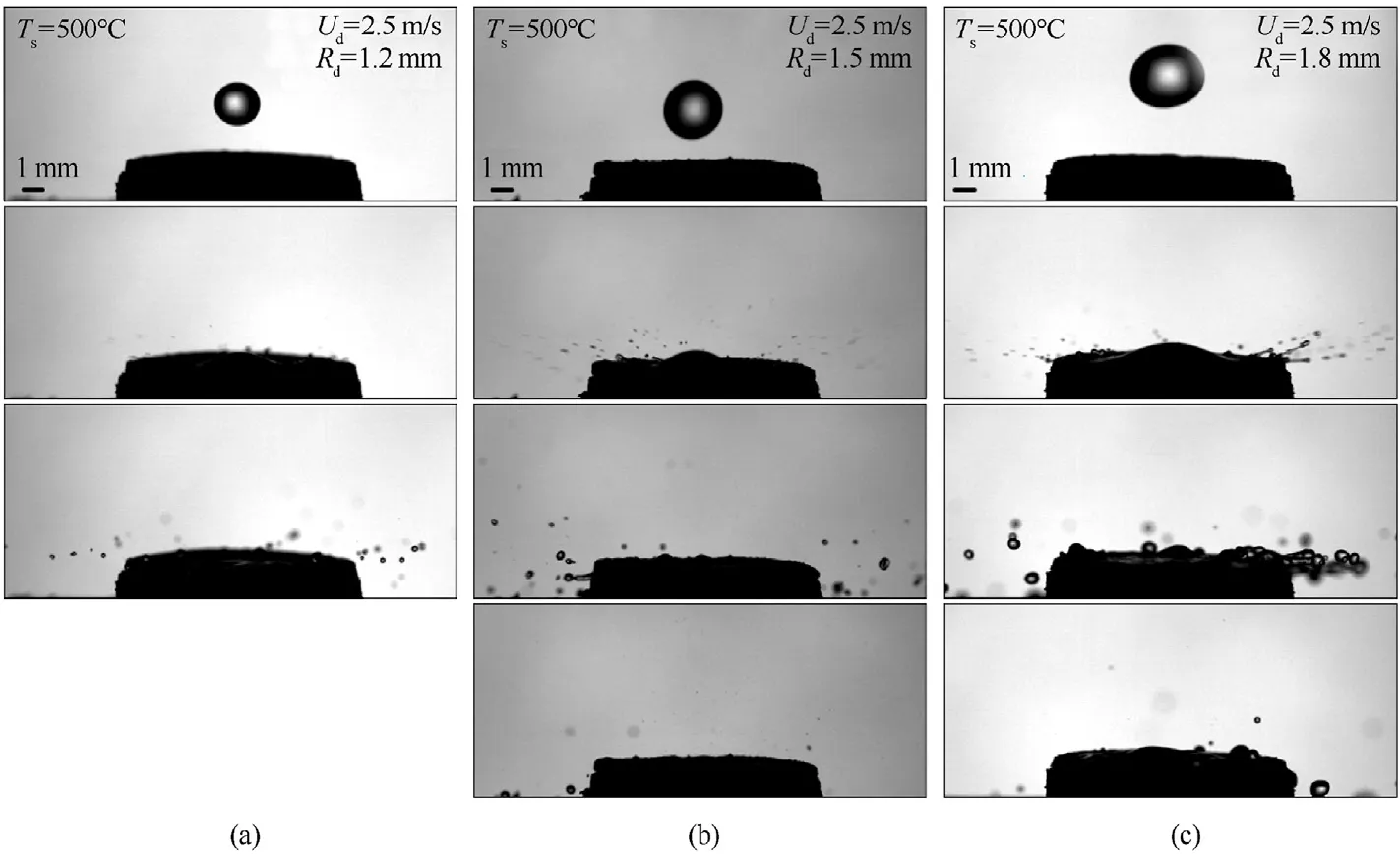
Fig.5.Images of collisions of distilled water droplets with pressed substrates at varying initial sizes of droplets with preliminary heating to Ts=500 °C; Ud=2.5 m/s: (a)Rd=1.2 mm;(b) Rd=1.5 mm;(c) Rd=1.8 mm.
One of the main parameters describing the dynamics of the interaction of a droplet with a solid surface is the maximum spreading factor which is the ratio of the maximum spread diameter(Dmax)to the initial droplet diameter before the collision(Dd).Fig.6 presents typical curves for theDmax/Ddversus the Weber number at a surface temperatureTs=20°C.In the collision of the droplet with a heated coal substrate,the droplet destruction was recorded within the whole Weber number range.In these experiments,it was impossible to capture the maximum spread diameter due to the rapid fragmentation of the liquid.The experimental curveDmax/Dd=f(We) looks similar to those in the earlier studies on the interaction of liquid droplets with substrates,such as[52,53].To compare the experimental data on the relationship of the water droplet spread diameters in the collision with a coal substrate,the curveDmax/Dd=f(We)was approximated by a power function:Dmax/Dd=0.54·(We)0.40,the rate of convergenceR2is 0.97.It was established that for the Weber numbers below 100,the values obtained in this research are in good agreement with the data by Liang et al.[52] and a theoretical relationship (Dmax/Dd)3-2·(0.052·We+1)·(Dmax/Dd)+4/3=0,obtained from the law of the conservation of energy [53].At higherWe,there is discrepancy between the data.This discrepancy might result from the fact that Liang et al.[52] used water,butanol,ethanol and 5.21% NaCl solution to study the collisions of droplets with a solid surface.The equationDmax/Dd=f(We)in Ref.[53]was derived for diesel droplet spreading.Moreover,the studies [52,53] considered the collisions of droplets with heated polished surfaces of steel with surface temperatures above the Leidenfrost point.In our case,a distilled water droplet interacted with rough porous coal surfaces(the root mean square roughnessSqwas 2.15 μm,the maximum heightSzwas 16.24 μm).The contact angle of the coal tablet was 66.7°±2.4°(θ <90°).If the surface is wetted,roughness leads to greater hydrophilicity.In turn,this leads to a rapid droplet spreading.
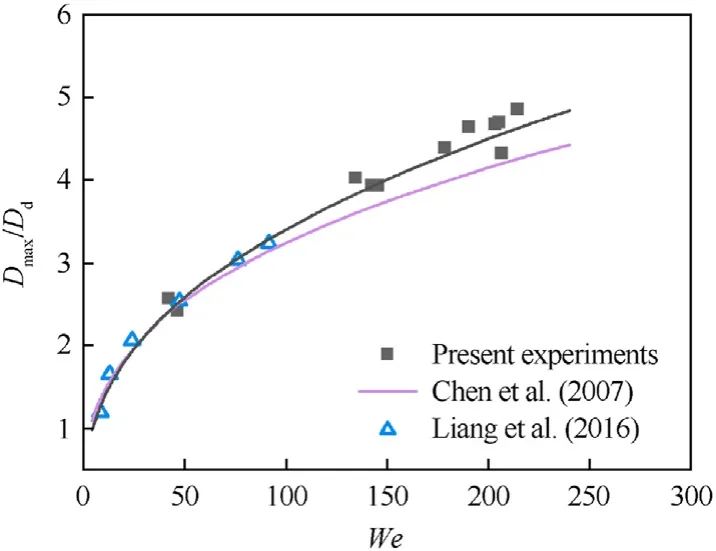
Fig.6.Ratio of maximum spread diameters of water droplets to initial droplet diameters in the interaction with a solid surface versus the Weber number (Ts=20 °C).
The collision regime map for water droplets and pyrolyzing coal substrate surfaces in theWe(Ts) coordinates is presented in Fig.7.Fig.7 also shows the results obtained by Liu et al.[2] for the collisions of a Milli-Q water droplet with a heated polished silicon surface.Liu et al.[2]identified 12 regimes.They can be grouped into spreading,rebound and break-up.In our case,two regimes were recorded: spreading (or agglomeration),and atomization (or separation).Bounce was not present in the collisions of water with a tableted coal sample in the investigated temperature and Weber number ranges.This can be due to the material and due to surface roughness.In Ref.[2],the surface roughness did not exceed 2 Å,whereas the coal surface featured considerable roughness(Sq=2.15 μm,Sz=16.24 μm).It is clear in Fig.7 that the transition boundary between spreading (agglomeration) and atomization(separation) for the findings [2] does not depend on the Weber number.The collision outcome(child droplets formation)depends on the silicon surface temperature.The transition occurs atTs≈140°C.In our case,the occurrence of a regime depends not only on the coal surface temperature,but also onWe.The surface texture has a significant effect on the collision outcome.The boundary between spreading and atomization in the interaction of water with a coal surface is described by an exponential functionTs=233.8·e-0.0068·We,the rate of convergenceR2is 0.95.
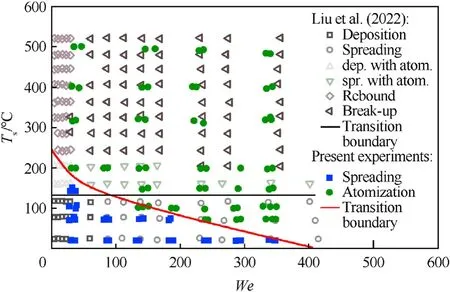
Fig.7.Collision regime maps for water droplets interacting with coal and silicon [2]substrates.
Fig.8 presents the child droplets distribution by size in the collisions of a distilled water droplet with pressed coal substrates,when varying the surface temperature under identical interaction conditions (We=830).

Fig.8.Child droplet distribution by size in the collisions of a distilled water droplet with pressed coal substrates (We=830) at varying surface temperatures.
The number of child droplets was found to increase exponentially with a coal surface temperature rise from 320 to 550°C.In the experiments with a surface temperature over 360°C,the porous texture of the colliding droplet caused the liquid to spread over the surface,penetrate into the pores and cool it.The coal surface image obtained using a scanning electron microscope at 2000× magnification in presented Fig.9(a).Child droplets detachment occurs as a result of the transformation of kinetic,potential and surface energies of the droplet into the energy of deformation and viscous dissipation.The number of newly formed fragments,0.1-0.5 mm in diameter,did not exceed 25.The bulk of the liquid remained on the substrate surface and evaporated.Boiling was recorded.With the temperature rising to over 400°C,the collision of the droplet with the substrate resulted in rapid boiling.Due to thermal conductivity,the droplet instantly heated(the spread time on the coal surface atTs=400°C was approx.1.5 ms),and the liquid surface tension decreased.A large number of child droplets withRcd>0.1 mm broke off.Droplet impact images show that all the child droplets with a size of less than 0.1 mm result from the boiling of the droplet colliding with a heated substrate.In the temperature range of 550-700°C,there was explosive boiling with the formation of a great number of large (>0.3 mm) child droplets (Fig.8,Supplementary material C).As the temperature rises from 550 to 650°C,the number of secondary fragments increases insignificantly (less than 5%).When a drop of a substrate heated to high temperatures touches,the evaporation of water is intensified.The vapor fills the space between the drop and the substrate.A buffer vapor layer is formed.The spreading water film is destroyed due to the ascending steam flow.Vapor bubbles pass through the film.A certain volume of liquid is retained in the pores of the substrate and exits in the form of vapor after drying its surface.AtTs=650°C,the maximum number of secondary droplets is formed.At a temperature of 700°C,the value of this parameter decreases by almost 15%.The result is due to several reasons.First,with increasing temperature,the volume of evaporated liquid from the substrate surface increases until decomposition.Secondly,the partial vapor pressure increases with increasingTs.There is a rebound of a certain volume of a drop from a heated surface.This lowers the temperature of the liquid and slows down its destruction.

Fig.9.Images of (a) coal and (c) substrate and (b),(d) particle surface: (c) Sq=2.15 μm; Sz=16.24 μm;(d) Sq=1.87 μm; Sz=13.82 μm.
Fig.10 shows the child droplets distribution by size,obtained in the collisions of a distilled water droplet with pressed coal substrates when varying the Weber number.The conducted experiments revealed that at a constant surface temperature,the number of child droplets increases with an increase in theWenumber,which is consistent with the findings[11].The analysis of Figs.8 and 9 suggests that the droplet size depends on the Weber number to a lesser degree in the experiments with a heated surface.This indicates the presence of synergistic effects(latent vaporization heat and surface tension) during the droplet break-up,contrary to the droplet destruction as a result of the collision with an unheated surface,where inertia forces dominate.At a surface temperature above 500°C and the Weber numbers over 210,a crown is formed within a short time after the collision.It almost immediately breaks up due to the unstable film (Fig.5).
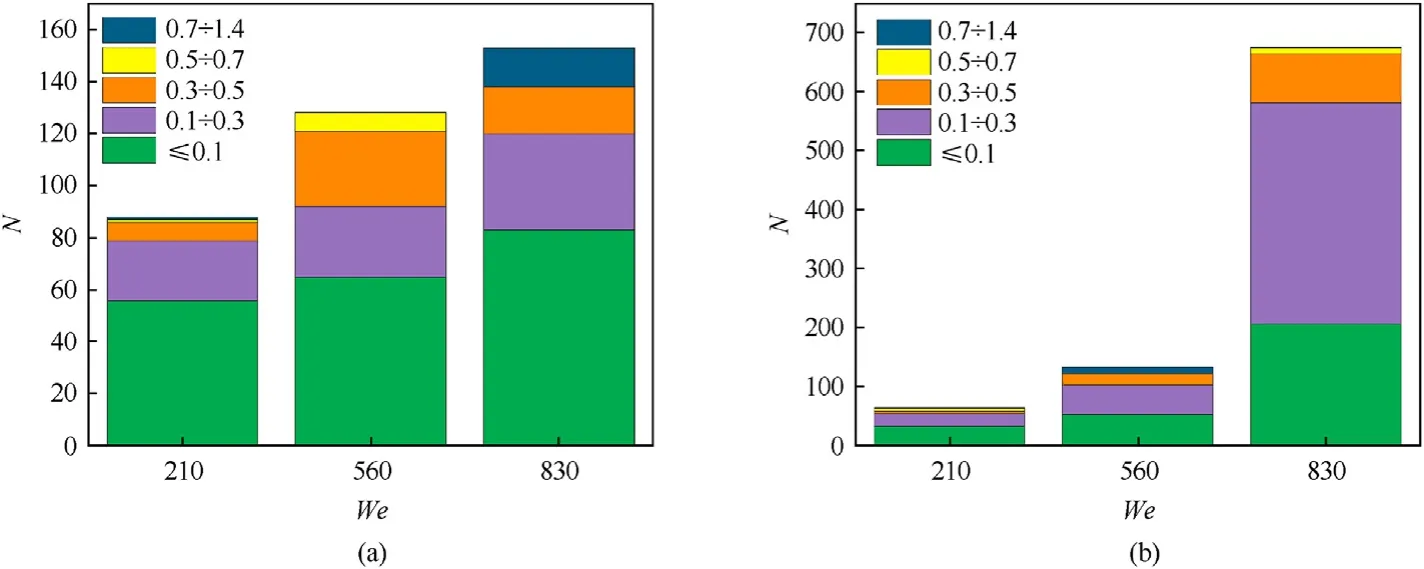
Fig.10.Child droplets distribution by size in the collision of a droplet with substrates at different We and temperature: (a) Ts=500 °C;(b) Ts=700 °C.
Fig.11 shows the ratio of the free surface areas of droplets after and before the atomization,when varying the substrate temperature and at the Weber number approx.830.TheS1/S0ratio was found to increase significantly with a higher temperature.An increase in the liquid temperature is known to increase the average distance between molecules and weaken the interaction between them.With the temperature growth,the surface tension coefficient of liquid decreases.Moreover,heating increases the saturation vapor density,and the physical properties of liquid and vapor become similar.When the liquid temperature approaches the critical value(at a pyrolyzing coal surface temperature of more than 550°C),the conditions of the interaction between the surface layer molecules are almost the same as inside the liquid,and the surface tension coefficient equals zero.Therefore,atTs>550°C,more than a twofold increase in the ratio of surface areas of droplets after and before the collision was recorded.

Fig.11.Ratios of the surface areas of droplets after(S1) and before(S0) atomization at different surface temperatures (We=830).
3.2.Interaction of droplets with particles
Fig.12 shows the collision regime maps for distilled water droplets interacting with heated coal particles,plotted following the experiments.In the collision of water droplets with coal particles with different temperatures,two interaction regimes,agglomeration and separation,were identified.The boundary between the distinguished regimes can be described by an exponential function likeB=a·e-b·We.The equations for the boundary between agglomeration and separation for each case of collision take the form:
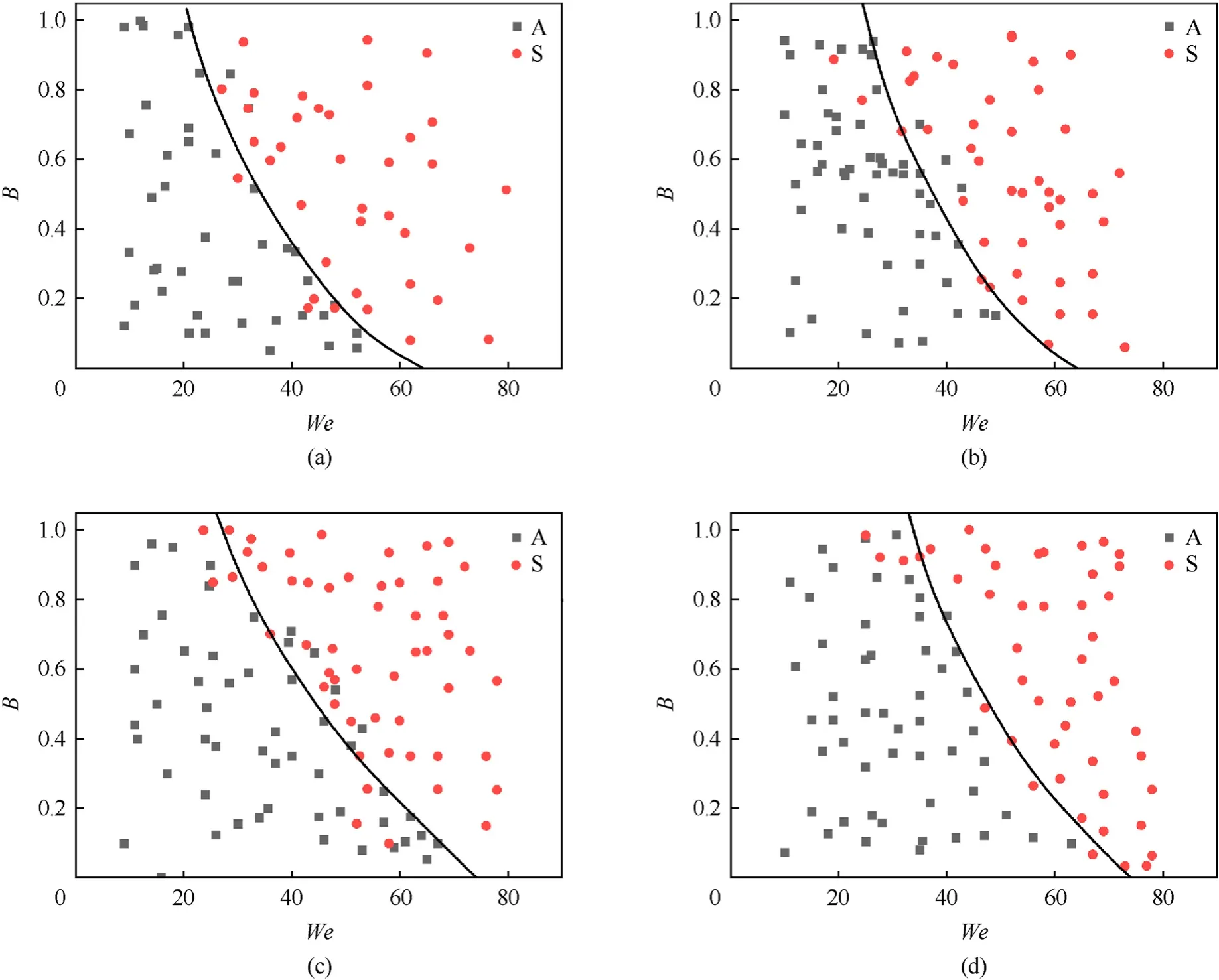
Fig.12.Collision regime maps for water droplets interacting with coal particles at different temperatures of the latter: (a) Tp=20 °C;(b) Tp=350 °C;(c) Tp=400 °C;(d)Tp=480 °C.A -Agglomeration;S -Separation.
With a coal particle temperature increase,the boundary between agglomeration and separation shifts towards higher Weber numbers.This is accounted for by increased adhesion forces with a temperature rise.This increase in the adhesion forces is caused by an increase in the area of contact of the particle with the liquid surface due to deformation,when the heated particle is placed into a colder environment(the particle falls before the collision with the water droplet at an ambient temperature of approx.20°C).In the area of contact,adsorption and capillary condensation are also possible.Capillary forces,dominating in the interaction of the droplet with the coal surface due to its porous texture,increase(Fig.9(b)).At the initial temperature of particles above 350°C,intense water boiling occurs during their agglomeration.With an increase in the resultant velocity of droplets and particles,inertia forces dominate surface tension,and fragments separate.
Since the Weber number reflects both the inertia and surface tension,the collision regime maps for coal particles interacting with water droplets were plotted in theWe=f(Oh)andWe=f(Ca)coordinates for better understanding of the effect of viscous forces(the Ohnesorge number) and capillary forces (the capillary number) on the interaction characteristics(Fig.13).
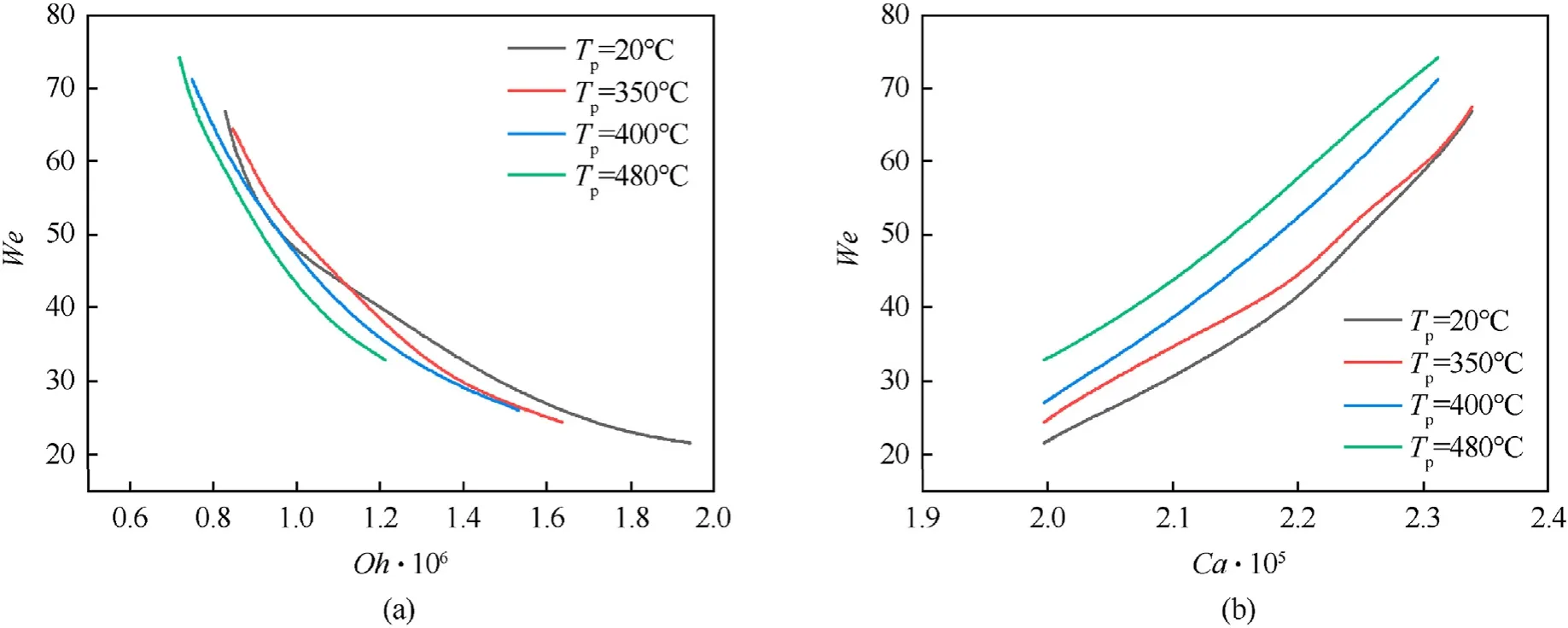
Fig.13.Collision regime maps of oil droplets interacting with coal particles at different temperatures of the latter in the coordinates: (a) We= f(Oh);(b) We= f(Ca).
The comparison of the curvesWe=f(Oh) shows that they are close to each other (Fig.13(a)).The difference between the Ohnesorge numbers at identical Weber numbers does not exceed 15%.The comparison of the curvesWe=f(Ca)(Fig.13(b))reveals a strong correlation: with a temperature increase,the boundary between agglomeration and separation shifts towards lower Weber numbers and capillary numbers.It can be assumed that inertia and surface tension forces dominate viscous forces.The curvesWe=f(Oh) can be described by a power function (We=a·Ohb):
The empirical power coefficient(b=-1.6)is constant for all the collision conditions.The physical properties of the basic liquid were the same in the experiments.This led to the assumption that the power indicator depends on water properties.The constant term in front of Oh varies by approx.15% with the temperature growth.It was assumed that a depends on the particle (Tp) and liquid (T)temperature.These coefficients are described by a second-degree polynomial forWe=20-75:
The regime mapsWe=f(Ca) (Fig.13(b)) andB=f(We) clearly show that the demarcation boundary between the regimes shifts towards higher We with the temperature growth.The maximum deviations between the curves for the temperatures 20°C and 480°C reach 38%.The capillary forces act at the liquid-air-particle interface,minimizing the surface energy at this interface.Thus,higher Weber numbers are required for separation to occur.TheWe=f(Ca)curves,like theWe=f(Oh)curves,can be described by a power function:
The constant term in front ofCa,like in the case withOh,is described by a second-degree polynomial as a function of the ratio of the particle and liquid temperatures forWe=20-75:
The comparison of the findings obtained for the collisions between pyrolyzing particles and coal substrates with the findings for the interaction of droplets with heated metal[53,54]and inorganic[55] surfaces indicates that the gaseous volatile production has no significant effect on the regimes and characteristics of the droplet destruction.The main factors affecting the droplet impact outcome regime are the surface temperature,roughness and porosity.Changing the volatile gas release rate and density in the temperature ranges under study are not sufficient for the formation of a gas cushion between the solid surface and the fuel that could lead to the bounce off the surface.It is possible that at temperatures above 550°C,the emerging gas-vapor mixture increases the pressure in the pores of the rough coal surface and is another cause of the droplet destruction with the formation of a great number of child droplets.For fire and explosion safety reasons,the temperature is not raised to such high levels in the fuel component mixing systems.In the collision with an unheated surface,the air in the pores also affects the water droplet destruction at highWe.Therefore,it is difficult to estimate separately the effect of pyrolysis on the droplet destruction.In the temperature range of 20-700°C,the Leidenfrost regime was not recorded.Meanwhile,it was recorded on metal and inorganic surfaces,e.g.,atTs=340-380°C[54] and on glass atTs≈300°C [55].The thermophysical properties,high porosity and roughness of coal shift the Leidenfrost regime boundary towards higher temperatures.
The research findings are fundamental for the development of low-grade coal and coal flotation waste gasification technologies.Such fuel types have a low calorific value and high ash content,which complicates their direct combustion in boiler units.However,equipment corrosion caused by aggressive liquid waste formation and low combustion temperature of low-grade coal and coal flotation waste prevent their wide application.This difficulty can be resolved by using specialized additives.Gasification can also help to partly overcome the above drawbacks.One of the most effective ways to reduce harmful emissions is by adding water to pyrolyzing or gasifying fuels [56-58].The solid fragment temperature in gasifier reactors ranges from 400 to 1,100°C depending on the type of reactor and fuel fed.Thus,the injection of water into the environment with soaring coal fragments leads to the droplet atomization not only as a result of impact but also due to rapid boiling of liquid on contact with superheated soaring particles.Dry gasification of high-grade coals produces not only combustible syngas,but also sulfur and nitrogen oxides that cause acid rains and deteriorate the quality of the air around the plant.Spraying water in the reactor reduces the amount of harmful gas emissions,while cleaning the syngas produced.Direct injection of additional water into the reactor has a positive effect on the environmental aspects of the process causing insignificant energy losses from the evaporation of excess moisture.Considering the available gasifier types,the proposed technology can be utilized in entrained flow gasifiers and in fluidized bed gasifiers.The reactors are shown schematically in Fig.14.

Fig.14.Schematic image of (a) entrained flow and (b) fluidized bed gasifiers.
Entrained flow gasifiers use milled solid industrial waste,pulverized coal and other similar materials as feedstock.Chlorides and sulfur contained in the gas are refined out by spraying liquid.In fluidized bed gasifiers,the particle size is in the range of 20-60 mm.The steam-air mixture supplied to the bottom part of the gasifier leads to a continuous movement of the bed and the gasified material.Thus,the temperature throughout the bed levels out,the reaction area increases and unwanted impurities are removed from the gas.These effects significantly increase the facility performance.It appears promising to adapt the findings of the present research to power plants of different sizes.
4.Conclusions
(i) The interaction of water droplets with solid particles and substrates of coal was experimentally studied,when varying their temperature from 20 to 700°C.The droplet diameter was varied from 0.7 to 4 mm,and the velocities ranged between 0.5 m/s and 4 m/s.This corresponded to the Weber number range from 7 to 830.Differences in the characteristics of the interaction of water droplets with coal particles at varying temperatures were identified.The obtained findings are fundamental for developing the technologies of separate injection of composite fuel components into boiler units,flotation facilities,dust and gas collecting systems,as well as technologies of secondary atomization of liquids and agglomeration of particles and droplets.
(ii) Following the conducted experiments,agglomeration and separation were distinguished in the collisions of droplets with coal particles during chemical reactions and phase transitions.Differences in the characteristics of interaction of water droplets with coal particles were identified at varying surface temperatures (Ts=20-700°C),droplet sizes(Rd=1.2-1.8 mm) and velocities (Ud=1-3.5 m/s).The gaseous volatile production in coal pyrolysis was found to have a modest effect on the regimes and characteristics of the droplet destruction in the temperature range under consideration.Changing the density,concentration and velocity of volatile gases atTs=20-700°C is insufficient to form a strong gas cushion between the solid surface and the fuel that could affect the collision outcome.The main factors affecting the droplet impact outcome regime are the surface temperature and roughness.
(iii) Collision regime maps for coal particles interacting with water droplets were plotted in the В=f(We),We=f(Oh)andWe=f(Ca) coordinates.With a coal particle temperature increase,the boundary between agglomeration and separation shifts towards higher Weber numbers.The regime boundaries in the В=f(We)coordinates were approximated by a function likeB=a·e-b·We.Those in theWe=f(Oh)andWe=f(Ca) coordinates were approximated by power functionsWe=a·OhbandWe=a·Cab.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Research was supported by the Russian Science Foundation(project 18-71-10002-π,https://rscf.ru/en/project/21-71-03001/).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.03.013.
杂志排行
Defence Technology的其它文章
- Multifunctional characteristics of 3D printed polymer nanocomposites under monotonic and cyclic compression
- The concept of sUAS/DL-based system for detecting and classifying abandoned small firearms
- Modelling and predicting the dynamic response of an axially graded viscoelastic core sandwich beam
- Development of bimetallic spinel catalysts for low-temperature decomposition of ammonium dinitramide monopropellants
- Thrust characteristics of nano-carbon/Al/oxygenated salt nanothermites for micro-energetic applications
- Ballistic response of skin simulant against fragment simulating projectiles
