Thrust characteristics of nano-carbon/Al/oxygenated salt nanothermites for micro-energetic applications
2023-12-27AhmFhAlxBrnovskyChrlsDuboisJmlChoukiShrifElbsunyShyShokry
Ahm Fh ,Alx Brnovsky ,Chrls Dubois ,Jml Chouki ,Shrif Elbsuny ,Shy Shokry
a Chemical Engineering Department,′Ecole Polytechnique de Montr′eal, Montr′eal, H3C 3A7, Canada
b Energetic Materials and Nanotechnology Research Center, Technical Research Center, Cairo,11765, Egypt
c Mechanical and Mechatronics Engineering Department, University of Waterloo, Ontario, N2L 3G1, Canada
d Head of Nanotechnology Research Center, Military Technical College, Cairo,11767, Egypt
e Laser and Optoelectronics Research Center, Technical Research Center, Cairo,11765, Egypt
Keywords:Nanothermites Graphene oxide Reduced graphene oxide Carbon nano material Oxygenated salts Laser ignition Computational fluid dynamics
ABSTRACT Combustion within small motors is key in the application-specific development of nanothermite-based micro-energetic systems.This study evaluates the performance of nanothermite mixtures in a converging-diverging nozzle and an open tube.Mixtures were prepared using nano-aluminum (n-Al),potassium perchlorate (KClO4),and different carbon nanomaterials (CNMs) including graphene-oxide(GO),reduced GO,carbon nanotubes (CNTs) and nanofibers (CNFs).The mixtures were packed at different densities and ignited by laser beam.Performance was measured using thrust measurement,high-speed imaging,and computational fluid dynamics modeling,respectively.Thrust,specific impulse(ISP),volumetric impulse (ISV),as well as normalized energy were found to increase notably with CNM content.Two distinctive reaction regimes (fast and slow) were observed in combustion of low and high packing densities (20% and 55%TMD),respectively.Total impulse (IFT) and ISP were maximized in the 5%GO/Al/KClO4 mixture,producing 7.95 mN·s and 135.20 s respectively at 20%TMD,an improvement of 57%compared to a GO-free sample (5.05 mN·s and 85.88 s).CFD analysis of the motors over predicts the thrust generated but trends in nozzle layout and packing density agree with those observed experimentally;peak force was maximized by reducing packing density and using an open tube.The numerical force profiles fit better for the nozzle cases than the open tube scenarios due to the rapid nature of combustion.This study reveals the potential of GO in improving oxygenated salt-based nanothermites,and further demonstrates their applicability for micro-propulsion and micro-energetic applications.
1.Introduction
Demand for improved energetic materials(EMs)is increasing as propulsion technologies strive to produce smaller,efficient,and safer thrusters.Novel EMs include civilian applications such as MEMS(micro-electromechanical systems)lab-on-a-chip devices as well as defence applications such as micro-thrusters and smartprojectiles.Nanothermites are solid-phase mixtures traditionally composed of metal and metal-oxide components at nanometric scale.Compared to traditional micron-scale thermite powders,nanothermites can produce higher reaction rate,heat release,pressure,and thrust output.Additionally nanothermites offer a large specific surface area,a decrease in the required activation energy,a fast energy release,and a high energy density [1,2].Despite their advantages over microthermites,nanothermites still suffer from relatively long ignition delays,slow combustion kinetics,particle agglomeration before ignition,and incomplete combustion which have limited their applicability [2-6].In addition,classical EMs generally suffer from ignition difficulty and propagation within small tubes and slots[7].
A common family of nanothermites is those based on nano aluminum (n-Al) powders,traditionally mixed with metallic oxides.Extensive research has been directed to enhance the performances and combustion characteristics of metallic oxide based nanothermites [8-12].For instance,Dolgoborodov et al.presented the results of Al mixed with metal oxides(CuO,Bi2O3and MoO3) ignited by a pulse of 4 W laser diode.Multichannel pyrometry and high-speed video was employed to record the radiation emission of the reaction products,the ignition delay,minimum ignition energy density,and the average burning rate [8].Bimetal alloy based thermites such as MgAl/CuO demonstrated significant effect on thermo catalytic decomposition of NC,where an NC nanocomposite supplemented with 5%MgAl/CuO increased heat of reaction and energetic performance [9].Novel ternary nanothermite mixtures based on MgAl,Polytetrafluoroethylene (PTFE),and KClO4produced a catalytic effect on thermal decomposition and kinetics of ammonium perchlorate (AP).Thermal analysis of the prepared composites resulted in decomposition of AP in one step instead of two steps without MgAl/PTFE/KClO4and heat released subsequently increased by 86.3% [11].
Recently,novel formulations based-on high electronegative atoms(O,S,F,etc.) have been developed resulting in formulations such as Al/KClO4and Al/K2S2O8,among others [5,13,14].Oxygenated salts may be preferred over metal oxides or oxysalts because of their higher atomic oxygen content,which results in superior burn rates and gas generation capabilities [14,15].Moreover,the lower dissociation energies of non-metal-oxygen bonds (Cl-O,I-O,etc.)in oxygenated salts means higher oxygen mobility,leading to decomposition and oxygen release at lower temperatures when compared to metal-oxygen bonds(Cu-O,Fe-O,Bi-O,etc.)[16,17].Oxygenated salts produce intermediate radicals (e.g.hydroxyl radical,OH·)upon decomposition which in turn can produce other subsequent radicals and oxidants by initiating a chain of degradation reactions.As such,oxygenated salts have higher oxidation ability than oxidizers which undergo an electron capture based oxidation mechanism[18,19].
Oxygenated salt-based nanothermites have a variety of practical uses in the field of solid propulsion and micro energetic systems.For example,ammonium perchlorate(AP,NH4ClO4)and potassium perchlorate(KClO4)are used alone or mixed with Al to improve the ballistic performance of modified double base propellant(MDB)for booster rocket motor applications such as the Tow 2A anti-tank missile [20].Elbasuney et al.used small scale test motors to evaluate the combustion characteristics of oxygenated salt-based thermites;they found that NH4ClO4,KClO4,and a binary mixture of NH4ClO4/Al increased burning rates (5.3%,22% and 12% respectively),and increased the characteristic exhaust velocity (C*)[20,21].Pressure-cell combustion tests of Al mixed with nano periodate salts (50-300 nm) such as sodium periodates (NaIO4)and potassium periodates (KIO4) generated pressurization rates(2.4-2.6 MPa/μs),considerably higher than those obtained with traditional Al/CuO nanothermite(0.06 MPa/μs)[15].In addition,Al/NaIO4and Al/KIO4ignite at 880 K and 950 K,respectively,lower than that of Al/CuO (1040 K) [15].
An active field of nanothermite research is the development of miniature thrusters for small attitude control,spacecraft station keeping,drag compensation,orbital transformation,and course correction of high-velocity projectiles [22,23].Dai and colleagues used a 10×10 microthruster array with varying quantities of AP within an Al/CuO nanothermite base matrix.Their results showed that addition of 7.5 wt% AP to Al/CuO substantially improved specific impulse and heat energy(61 s and 1113 J/g)compared to their reference sample without AP(22 s and 792 J/g)[24].Nanothermites can also be used in pyrotechnical micro electro mechanical systems(PyroMEMS) such as pyroswitches or circuit breakers in electrical systems,actuators,and safe-and-arm devices[25,26].Nicollet et al.exploited the reaction of Al/CuO nanothermite to fabricate a miniature nanothermites-based circuit breaker for over-current protection.The developed circuit breaker resulted in 0.57 ms response time with a 59 μs ignition delay,notably quicker than the 1 ms response time of traditional mechanical circuit breakers.In addition,the response time was demonstrated to be adjustable by varying the mass of nanothermite.The proposed circuit breakers can be used in energy storage,aerospace manufacturing,and parachute deployment systems [26].
Recently,CNMs and their derivatives have drawn much attention as additives for nanothermites due to their large specific areas and unique thermal,electrical,and mechanical properties[27-30].In particular,GO is an appropriate candidate for nanothermites due to its catalytic properties and relatively simple preparation method[31].GO itself can readily undergo violent exothermic decomposition,upon heating,resulting from oxygenic functional groups on the basal plane (phenol,hydroxyl,and epoxide) and along sheet edges (carboxylic)[32].
Studies on novel nanothermite compositions and the effects of CNMs on combustion performance are contributing to a growing body of literature [33].When the effects of CNTs on the CuO/Al thermite reaction were assessed,it was found that the enthalpy of CuO/Al/CNT thermite reaction increased with the addition of CNTs(300% at 15 wt% CNTs),and the ignition temperature was reduced[34].In Ref.[35],the authors developed high performance nanoenergetic composites based on bismuth trioxide (Bi2O3) and Al using functionalized graphene sheets (FGS) and investigated the effect of GO on their combustion properties.For the FGS selfassembled nanocomposites,energy release increased from 739±18 to 1421±12 J/g,as observed in DSC results.
The most critical requirements for any propulsion system design are: minimized overall system mass,and maximized performance parameters such as thrust and impulse [36,37].By producing improved performance without incurring any associated mass increase,the overall propulsion design is improved.The use of oxygenated salt nanothermites is one way to improve performance without introducing a mass penalty,but the novelty of these compositions means that propulsion-specific characterization is limited in existing literature.
The goal of this work is to propose new nanothermite mixtures with controllable combustion and tailored thrust performance for micro-propulsion and micro-energetic applications.This will be realized by assessment of thrust production and dynamic performance of the nanothermite mixtures within a small-scale test rocket motor (STM) with two exhaust configurations: open-tube and converging-diverging (CD) nozzle.The effects of chemical composition and packing density on the performance (IFT,ISP,ISVand normalized energy output) of nanothermites are investigated first using n-Al,potassium perchlorate (KClO4) and varying quantities of GO.Then,other carbon nano materials are examined as additives to the base thermite mixture,including reduced graphene oxide (RGO),multi-walled carbon nano tubes (MWCNTs) and carbon nano fiber (CNF) to substitute GO.Thrust performance of GOenhanced nanothermites is compared to those with alternative oxidizers,such as potassium periodate (KIO4),potassium persulphate(K2S2O8),potassium permanganate(KMnO4)and ammonium nitrate(NH4NO3).Computational fluid dynamics(CFD)modeling is used to reveal the evolving velocity and temperature profiles of the nozzle plume,and to evaluate how the thrust is dependent on geometry and packing density for the n-Al and KClO4composite.In doing so,the usefulness of a model-based design tool can be determined.
2.Materials and methods
2.1.Materials
Graphite flakes,phosphoric acid (H3PO4),sulphuric acid(H2SO4),hydrogen peroxide (H2O2),and acetone used in the preparation of GO were purchased from Sigma-Aldrich.KClO4and NH4NO3,were obtained from Defence Research and Development Canada.All other oxidizers,namely KIO4,K2S2O8and KMnO4were purchased from Sigma-Aldrich.The as-purchased Al nanoparticles from US Research Nanomaterials Inc.Have a spherical shape with an average particle size of 40 nm,a purity of more than 99.9%metal basis,an active Al content of 80wt%(mass fraction),a surface area of 30-50 m2/g and bulk density of 2.7 g/cm3as specified by the manufacturer.MWCNT and CNF were purchased from Sigma-Aldrich.MWCNT has >98% carbon basis,O.D.× L 6-13 nm×2.5-20 μm and specific surface area of 216 m2/g.CNF is graphitized platelets (conical) and has >98% carbon basis,and dimensions D×L 100 nm×20-200 μm.
2.2.Synthesis of GO and RGO
Graphene oxide was synthesized using an improved Hummers'method with graphite powder as the starting material [38].The detailed procedures of the GO and RGO preparation can be found in our previously published article [39].The composition and heat of combustion of CNMs used in the experiment was checked by a CHNSO Elemental Analyzer EA3000(EuroVector)and oxygen bomb calorimetry.The mass fraction(wt%)of C,H,N,S and O and heat of combustion of each CNMs is summarized in Table 1.
Table 1 Elemental analysis and heat of combustion of CNMs.
2.3.Preparation of nanothermite compositions
Nanothermite compositions were prepared by weighing the fuel(n-Al) and oxidizers,individually,based on the stoichiometric ratios presented in Table 2.The dispersion of n-Al with each oxidizer and GO (2.5 wt%,5.0 wt%,7.5 wt%,and 10 wt%) was conducted in acetone.The samples were then put in an ultrasonic bath for 30 min to ensure proper dispersion.Acetone was evaporated at 60°C for 1 h with simultaneous mechanical stirring to prevent the reagglomeration of nanoparticles.Wet particles were then fully dried in a vacuum desiccator for 12 h to drive off any remaining acetone.Finally,the dried powder was gently broken up with a spatula to remove any large clumps and until the consistency was that of a loose powder.For comparison purposes,other CNMs(RGO,MWCNTs and CNFs) and oxidizers (KIO4,K2S2O8,KMnO4and NH4NO3) were used as alternatives to GO and KClO4respectively.Different nanothermite compositions and their reactions are shown in Table 2.

Table 2 Chemical composition and reaction of different nanothermite samples.
2.4.Instrumentation
Laser ignition and high-speed imaging was used to evaluate the combustion performance of the prepared composites;a schematic is shown in Fig.1(a).Schematic dimensions of the STMs used are illustrated in Figs.1(b) and 1(c) and 1(d) showing the nozzle and no-nozzle configurations;the nozzle used is a generic convergingdiverging (CD) design intended to accelerate exhaust gasses to a supersonic state [40].
A 5 W continuous wave 532 nm laser was used to ignite samples.The combustion of the samples was recorded at 180,000 fps with 600 ns exposure time using a Phantom v2012 high-speed camera.A Dytran (1051V2) force transducer with a reference sensitivity of 22.43 mV/N was fixed underneath the sample to measure the thrust force.Two photodiodes (Thorlabs DET102A)were used to capture the light intensity output from the nanothermite sample.A neutral density filter (Thorlabs NDUV06B) was installed on one photodiode unit and a 488 nm wavelength filter(Thorlabs FLA488-10) on the other;483 nm being a characteristic wavelength for the AlO thermite reaction intermediary [41].A Tektronix MSO56 oscilloscope was used to capture force and photodiode data at a sampling rate of 1 MHz.Data recording and laser triggering was performed by a Tektronix AFG1022 waveform generator using a standard TTL signal.All tests were carried out at normal atmospheric conditions.
2.5.Numerical modelling
Combustion of the reference sample (Al/KClO4) was modeled using the ICT code [42,43] under the assumption that reactants were perfectly mixed.This code solves the adiabatic combustion of a heterogeneous mixture and simulates the final product composition,temperature,and pressure.Note that with the stoichiometric ratio of one,the reactants produce gaseous products which include 19 mol%of oxygen containing gases,11 mol%of AlO and 23 mol%of KCl.Due to the complexity of gas mixture during the combustion process,the working fluid was modeled as an ideal gas.Table 3 lists the values of the specified parameters at the nozzle inlet.
The ICT code cannot account for the effects of the packing density on the combustion products and their thermodynamic properties.To investigate the qualitative trend of the transient thrust production with reduced energy release from a burning pellet with varying packing density,three simulation cases were run on the CD nozzle and two simulation cases were run on the straight open tube.This largely simplified approach uses the estimated mass flow rate at the inlet based on the following assumptions: i) the reference sample has high oxygen content,resulting in a reduced amount of solid phase residual and negligible effects of gas diffusion through the condense phase and ii) the combustion behavior of the pellet is similar to a porous propellant grain,with the generation of gas regressing linearly with time and increasing linearly with porosity.
A compressible Navier-Stokes CFD model was used to evaluate the flow within the nozzle and straight-tube test motor configurations,with turbulence modeled using the Spalart-Allmaras method.The nozzle geometry shown in Fig.1(d) was used directly and,to keep the back pressure constant,a large reservoir surrounding the nozzle and open tube outlets was added to the computational domain.The domain geometry is presented in Fig.2,along with an outline of the boundary and initial conditions.
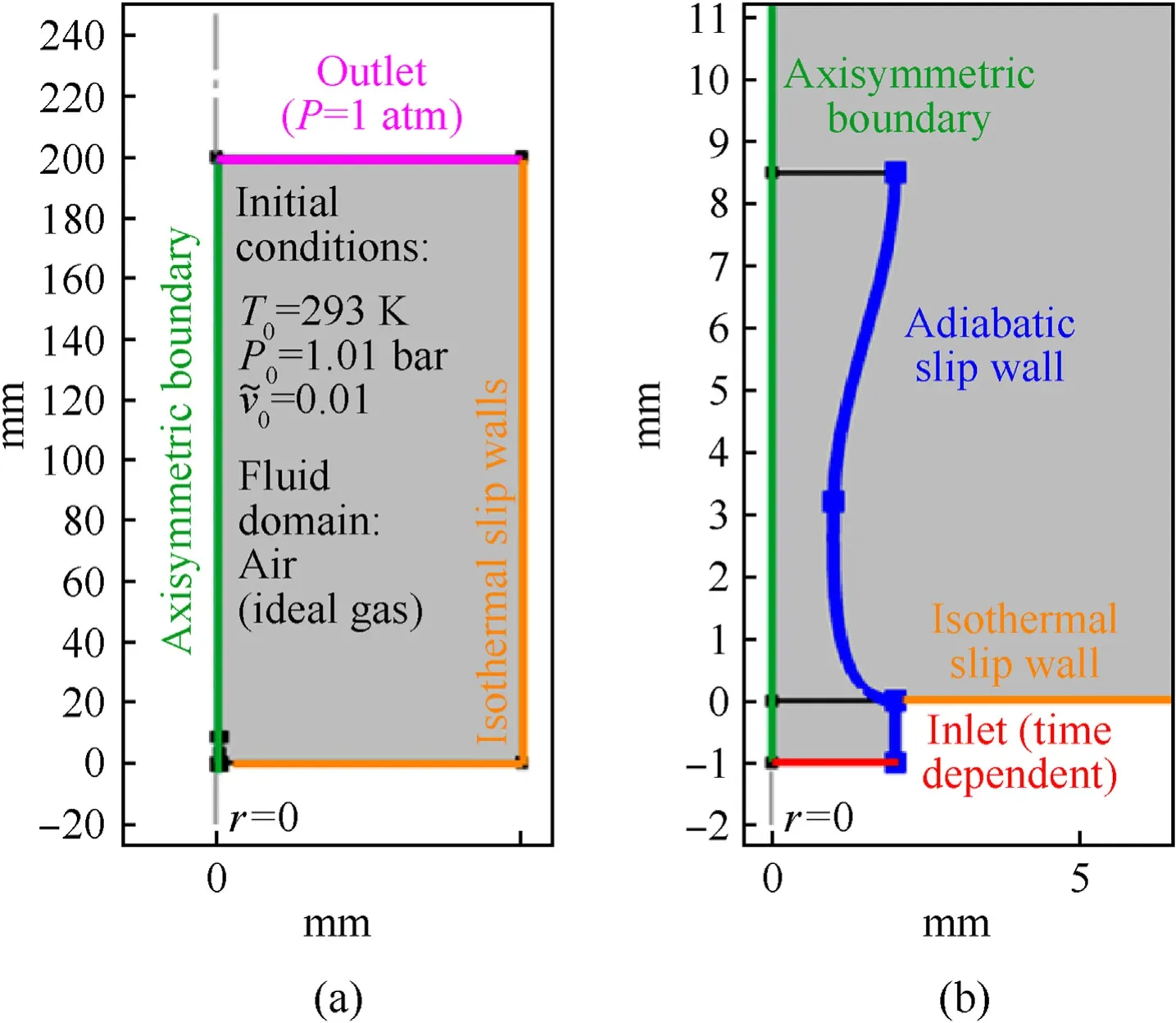
Fig.2.Schematic diagram of 2D-axisymmetric domain used for modeling showing: (a) Reservoir boundary conditions;(b) Nozzle-area boundary and initial conditions.
Dependence of the solution on the mesh size was evaluated through a grid convergence study.Three meshes were considered;a coarse mesh consisting of 165,263 elements,a medium density mesh with 206,329 elements,and a fine mesh with 256,022 elements.The Mach number,temperature,and thrust force results were considered in the evaluation of grid independence.The convergence study found the medium density mesh (206,329 elements) to be sufficient to remove grid effects from the results.Details of the numerical model can be found in the supplemental information section.
3.Results and discussions
3.1.Effect of chemical composition
The combustion characteristics,particularly thrust output and specific impulse,are the most important parameters that need to be measured to evaluate the propellant performance of a nanothermite mixture.They are highly dependent on the chemical composition of mixtures and allow us to predict performance,thereby determining the appropriate application for the prepared samples.Static thrust data was collected from different samples in the open-tube test engine shown in Fig.1(b).The comparative performance of various CNM and oxidizer combinations is shown in Fig.3.

Fig.3.Comparative plots for nanothermites based on: (a) Al/KClO4 with various CNM additives;(b) Al based mixtures with different oxidizers;all enriched with 5% GO content.
Fig.3(a)shows that addition of CNMs improves performance of nanothermite mixtures in terms of total and specific impulses.Adding CNT,RGO,and CNF to Al/KClO4nanothermite increased the total and specific impulses by 31%,22% and 11% respectively.This enhancement can be attributed to the highly conductive thermoelectric properties and large specific areas of CNMs which facilitate the uniform mixing of n-Al and KClO4[31].Furthermore,CNMs may act as secondary fuels which add energy to the system,help in the completion of n-Al combustion,and compensate for the reduction of active Al content due to the Al2O3shell.These effects combine to increase the total and specific impulses compared to the base thermite mixture.
Fig.3(b)shows the combustion characteristics of nanothermite composites based on n-Al and various types of oxygenated salts.These results show that nanothermites based on oxygenated salts have higherISPthan those with metallic oxides such as Al/CuO(26.7 s)and Al/Bi2O3(59.4 s)as previously reported in literature[44,45].Oxygenated salt-based nanothermites produce largeIspdue to the high oxidizing power,high reaction rate,lower bond energy,and low oxygen release temperature of nonmetal-oxygen pairs compared to the metal-oxygen bonds in metal oxides[14-17].GO/Al/KClO4nanothermite displayed the highestIFTandISPdue to the high enthalpy of the C-KClO4(154 kJ/mol) and C-KIO4(72 kJ/mol)side reactions [46].The reduced performance of the GO/Al/K2S2O8mixture with respect to the others may stem from the oxidation of nano-C by K2S2O8in the mixed suspension before ignition [47,48].Therefore,the GO/Al/K2S2O8nanothermite does not have the same proposed high energy output reaction paths,like C-O and C-KClO4in GO/Al/KClO4,which may aid in completion of the main thermite reaction.
For the highest performing mixture,GO/Al/KClO4,the GO quantity was varied to observe compositional effects on the thrust performance.The resultant thrust profiles are presented in Fig.4.
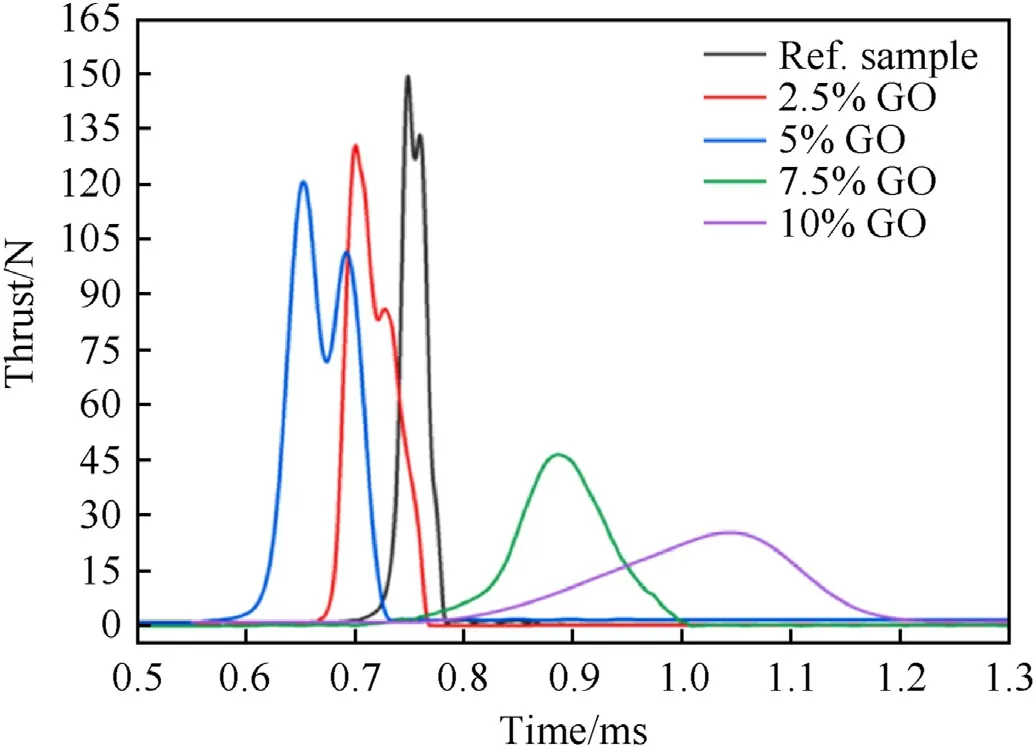
Fig.4.Thrust profiles for different GO/nanothermite samples at 20%TMD in the opentube engine configuration.
The reference sample (Al/KClO4) exhibits a relatively narrow thrusting duration (~0.1 ms) which can be correlated to the high oxidizing power and exothermic decomposition nature of KClO4.Moreover,the reference sample shows two peaks,the first of which may be caused by either the shock release from the exit of the STM or heterogeneous combustion of the sample.
Adding GO into the mixture brings about three major effects.First,the peak thrust value is decreased(15%reduction for 2.5%GO sample,80%for 10%GO sample),largely due to the reduced energy release which is caused by the separation of the oxidant and the metal as well as potential GO-oxidizer side reactions that detract from the main thermite reaction.Second,the thrust delay time decreases with 2.5%and 5%GO samples,while the 7.5%and 10%GO samples show a similar or marginally increased delay compared to the reference sample.Thirdly,the thrusting duration increases with the GO content from 0.1 ms for the reference sample to about 0.5 ms for the 10% GO sample.It is apparent from this figure that addition of up to 5% GO yields two peaks;the first peak was from the exothermic decomposition of GO,KClO4and preignition reactions between GO decomposition products and nanothermite reactants,while the second came from the main thermite reaction[39].Addition of up to 5% GO content produces slightly decreased thrust peaks compared to the reference sample but enhances the total performance by improving the contact between reactive particles and the heat transfer pathway.Though increasing the amount of GO over 7.5% significantly reduces the peak thrust and slightly increased the ignition delay time,it helped in sustaining a more uniform burning process.To analyze the combined effects of thrust forces and combustion duration,impulse is quantified through total impulse (IFT),specific impulse (ISP),and volumetric impulse (ISV).These are calculated as follows:
where:Wt,Vt,tiandteare the nanothermite weight(N),volume of the nanothermite charge (mm3),initial combustion time (s),and ending combustion time(s)respectively.The effect of GO quantity on the sample propulsion characteristics (IFT,ISPandISV) are presented in Table 4.

Table 4 Combustion characteristics of nanothermite samples at 20%TMD.
The measurements summarized in Table 4 show that GO addition has a positive impact in bothISPandISV,up to a point.Impulse values reach a maximum at 5%GO composition,where specific and volumetric impulses show over 50%improvement compared to the reference sample.The increase in ISPandISVvalues is attributed to the role of GO in improving both the fuel-oxidizer contact and mass-diffusion of the reactants.GO acts as a substrate,increasing the dispersion of n-Al and effectively reducing its aggregation within the KClO4matrix.Consequently,uniform mixing of the n-Al metal fuel and the KClO4oxidant occurs,and more complete combustion is expected [49-51].Furthermore,the decomposition of GO gives rise to reactions between C and nanothermite reactants(C-O,C-KClO4and C-Al) which are likely prominent reaction pathways in the early stages of combustion [35].These promote completion of the main thermite reactions (Al-KClO4and Al-O)and this is a proposed explanation for the observed increase in the combustion time [39].Also,the exothermic decomposition nature of both KClO4and GO introduce pre-ignition reactions which may help in cracking the inert alumina(Al2O3)shell layer and improving the diffusion process of both free oxygen and the molten Al core in a melt-dispersion mechanism [48].However,excessive GO content(more than 5%) in nanothermites gives KClO4opportunity to grow directly on GO nano sheets,thereby causing separation of the oxidizer and metal fuel and a reduction of thrust from the thermite reaction [35,51,52].Interestingly,the reduction in thrust with the addition of GO (exceeding 5%) is compensated by sustained,but lower,thrust over a longer combustion time such that impulse is still increased compared to the reference sample.
Ignition delay of nanothermite mixtures is determined using light signals recorded via photodiodes during sample combustion,measuring the delay between laser spot activation(t=0 s)and the first maxima of the photodiode signal.In addition,high-speed video is used to support ignition delay results by measuring the time difference between the first frame where the laser spot is visible and the first frame where a distinct flame front develops.Variation of the light emission of nanothermite mixtures with different GO content is shown in Fig.5.

Fig.5.Optical emissions of different GO/nanothermite samples at 20%TMD in the open-tube engine configuration.
Addition of GO to the nanothermite mixtures yields two distinct effects on ignition delay;reduction for samples below 5% GO and increase for samples above 5% GO.Addition of up to 5% GO decreases the ignition delay time by approximately 11% and 40% for 2.5%and 5%GO respectively.This is because GO absorbs laser light upon illumination and undergoes exothermic reactions,generating heat and radicals from GO decomposition reactions which facilitate the ignition and combustion of nanothermite[53].The presence of GO causes fast local ignition of the nanothermite mixture by the photo-thermal effect.Increasing the amount of GO above 5% increases the ignition delay from the reference sample by up to~50 ms (for 10% GO sample).This suggests that increasing the quantity of GO more than 5% in the GO/nanothermite matrix is detrimental to the photo-thermal effect due to environmental heat dissipation through the rapid heat transfer within aggregated GO.
Table 5 collects values of the minimum laser power required to ignite loose-packed GO/nanothermite samples,their onset time,ignition delay,and specific energy release.Specific energy release of the sample combustion reaction can be evaluated by calculating the normalized time-averaged photodiode signal (integrating area under the curve) according to the following equation:

Table 5 Ignition properties of nanothermite composites.
whereE,ti,te,m are the photodiode signal(mV),initial combustion time (s),ending combustion time (s) and nanothermite mass charge (mg) respectively.
Specific energy release (normalized photodiode signal) increases with the amount of GO up to 5% composition;further addition of GO results in reduced normalized photodiode signal.This again supports the observation that the 5% GO mixture performs better than the other samples tested.Improving the specific energy of the samples can be accredited to the catalytic effect of GO on the dissociation of oxygen molecules,providing a direct diffusion pathway for the dissociated oxygen atoms to reach Al,thus accelerating the oxidation of Al during the combustion process[54].The catalytic effect of GO occurs when heat and gaseous products are released at relatively low temperature (200°C) by decomposition and oxidation reactions [55,56].Additionally the GO decomposition gases can enhance the convective heat transfer during combustion of the composites and hinder the aggregation of nanothermite reactants [57].Finally,the observed trends are confirmed by the AlO light signals captured by photodiode with a 488 nm wavelength filter (Thorlabs FLA488-10),shown in Fig.6.
Fig.6.AlO photodiode signal of different nanothermite samples.
The intensity of the AlO photodiode signal increases with the addition of GO,with the largest intensity occurring at 5% GO,supporting the findings observed in Figs.3 and 4.AlO is the main gaseous reaction intermediate in the oxidation of Al,occurring in the range of 1200-3300°C.Thus,correlation between the amount of AlO formed during the reaction,as measured by the intensity of a photodiode signal filtered at 488 nm,can be used as indication of the completion of combustion[58].The agreement of the AlO data signal and values in Table 5,therefore suggests that the 5% GO sample has greatest impulse performance due to a maximization of combustion completion [41].
3.2.Effect of nanothermites packing density
To describe the bulk density for powder samples,the theoretical maximum density(TMD)is defined as the theoretical density of the powdered materials if they could be combined into a single,monolithic solid.Comparison between the measured sample density and TMD is expressed as a percentage,%TMD,and calculated according to the following equation:
Here,ρmeasuredis the density of the nanothermite sample based on the corresponding sample mass and volume,as measured upon loading into the STM,whereas ρcalculatedis the density calculated by the summation of weighted average densities of each reactant in the composite[59].Variation ofISPwith different TMD percentages for all GO/nanothermite samples is shown in Fig.7.

Fig.7.Specific impulse plotted against powdered sample density(%TMD)for Al/KClO4 based nanothermites.
Fig.7 shows that the bulk density does not have a significant influence on ISPand that no distinguishable correlation can be observed between the specific impulse and%TMD.TheISPvalues for all GO/nanothermite composites indicate that mass-based thrust efficiency is almost constant at different packing densities.This finding agrees with other works that have studied the effects of packing pressure on specific impulse in energetic nanothermite mixtures [40].
More interestingly,two distinct reaction regimes were detected while varying the packing density as illustrated in Fig.8.

Fig.8.Peak thrust and combustion duration of 5% GO/Al/KClO4 at different %TMD,showing fast reaction regime (yellow),transitional regime (green),and slow reaction regime (gray).
Fig.8 shows that low-density samples produced high peak thrust for short combustion durations whereas high-density samples produced low peak thrust but for long combustion times.In between the extremes,at 42.5%TMD,the sample thrust and combustion time were between high and low packing density results.It is apparent in Fig.8 that there are three distinct regimes: a fast reaction regime at low packing density less than 32.5%TMD(yellow region),a slow reaction regime at high packing density exceeding 55%TMD (gray region),and a transitional regime (green region) in between.It is likely that the low-density samples undergo supersonic combustion: this behaviour of low-density nanothermites has been observed in other studies using open-ended combustion tubes [44,60].So,the fast reaction behaviour may be correlated to the rapid discharge of combustion products caused by reflected wave of the combustion front (supersonic combustion performance).Additionally,in open STMs,combustion is controlled by convective heat transfer so,at low packing density,pores within the sample allow hot gas propagation into the fuel grain and thus heat transfer into the sample from the flame front via convection.Removing these pores by compressing thermite into a pellet(reducing volume but keeping constant mass) reduces the convective pathways,so the role of convective mechanisms on the flame propagation is reduced and conduction becomes dominant.
To observe the time-resolved thrust behaviour of samples in the slow reaction regime,thrust traces are plotted for Al/KClO4samples with varying GO quantities at 55%TMD in Fig.9.
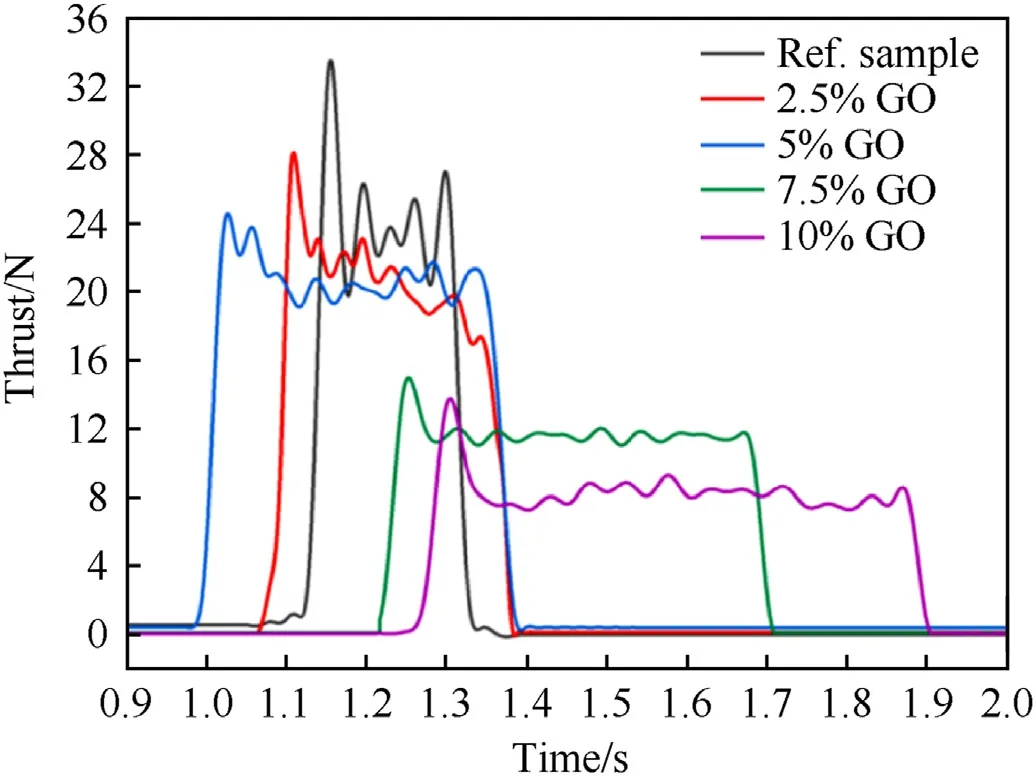
Fig.9.Thrust profiles for different GO/nanothermite samples at 55%TMD in open-tube engine configuration.
For combustion of highly porous media(low-density),unsteady reaction rates can lead to unstable energy release and gas production.However,in the slow reaction regime (high packing density),the behaviour is analogous to a stable burning stage of a typical propellant grain where thrust force and combustion rates are relatively constant throughout the combustion period,albeit over a very short period [61].The shift in the flame propagation mode can be another possible explanation for the fast and slow reaction regimes observed in Fig.8[62].Combustion characteristics for GO/nanothermites reacting in the slow regime (55%TMD) are summarized in Table 6.

Table 6 Combustion characteristics of nanothermite samples at 55%TMD.
3.3.Effect of convergent-divergent nozzle
Limited data is available for these nanothermite compositions in small scale test engines,so an STM with a generic convergingdiverging (CD) nozzle,as shown in Fig.1(c),was used to evaluate the in-device performance of the prepared nanothermites.It is intended that the captured thrust data serves as a baseline for future propulsion system designs and refinements.Time resolved thrust-force data for different GO/nanothermite composites for slow and fast reaction regimes(20%and 55%TMD)in CD nozzle and no-nozzle STM configurations are presented in Figs.10(a)and 10(b)respectively.
Introducing a CD nozzle has an opposite effect on the performance of GO/nanothermite samples depending on the packing density.Comparison with open-tube configurations shows that the CD nozzle significantly increased the thrust amplitude and reduced the combustion duration for slow reaction regime samples (55%TMD);contrary effects were observed for all low-density(20%TMD)samples,though to a lesser degree.To observe the quantitative effects of the CD nozzle,combustion characteristics for GO/nanocomposites within the CD nozzle STM at high and low packing densities are presented in Table 7.

Table 7 Thrust performance of GO/nanothermites within the CD nozzle STM at 20 and 55%TMD.
The effect of a CD nozzle on the thruster performance is mixed,depending primarily on packing density.As can be seen in Fig.10(a)and Table 7,peak thrust is reduced and combustion time increases for 20%TMD samples with the introduction of a CD nozzle.This trend is true for all low-density samples,regardless of GO content.For low packing density,the total,specific,and volumetric impulses are increased with the addition of the CD nozzle.This indicates a propulsion efficiency gain in the low-density samples,although performance is contrary to what is expected from a CD nozzle(reduced thrust,increased combustion duration).Meanwhile,the results in Fig.10(b)show a trend that is expected for the addition of a CD nozzle (increased thrust,reduced duration),although unlike the low-density samples,the net effect is a reduced specific impulse compared to 55% samples in Tables 6 and 7.

Fig.10.Thrust-time curves for different GO/nanothermite samples at (a) 20%TMD,(b) 55%TMD with and without CD nozzle.
To investigate the effect of the CD nozzle at different packing densities,we need to consider the definition of thrust.Thrust is defined by Eq.(6) [63].
The first term is the momentum force,corresponding to the gaseous product mass flow rate () and its exhaust velocity (V2)relative to the STM.The second term represents the pressure thrust;it consists of the product of the cross-sectional area at the nozzle exit (A2) and the difference between the exhaust gas pressure at the exit and the ambient fluid pressure (P2-Pamb) [63,64].Most nozzle designs aim to achieve fully expanded nozzle flow,so that the pressure at nozzle exit is equal to ambient pressure thereby making momentum force the dominant component of overall thrust.
In the slow reaction regime,the increase in thrust force is expected since the nozzle is implemented to convert energy from the combustion process into momentum force by increasing exhaust velocity.The reduction in combustion time is a result of increased burn rate due to geometric confinement of the solid propellant[44,63].A high-density sample in the open tube configuration burns without geometric acceleration of the exhaust,so the total thrust will have a larger pressure force component but the pressurization rate is low due to slow burn rate.However,when a low-density sample(fast reaction regime)is used in the open-tube configuration,the rapid burn rate should result in high pressurization and a large,rapid impulse of pressure-induced thrust.This form of combustion is not suited well to a nozzle-configuration engine.
The ideal nozzle,as stated by Sutton,assumes that combustible materials and reaction products are homogenous,combustion products are in gaseous state with adiabatic,steady,and continuous flow with no friction forces and neglected boundary effects.We can certainly say these conditions are not met with our sample behaviour.Due to the small dimensions of the STM used in this work,friction forces and boundary effects cannot be neglected[7,44].Also,low-density nanothermites can generate shock waves and so uniformity and continuity cannot be assumed with GO/nanothermites samples,especially as it is likely that a shock wave travels through the nozzle as combustion evolves[60,65].It can be seen from the force data in Figs.4 and 10(a) that the low-density sample never reaches a steady state,reducing the usefulness of the CD nozzle.For the low-density samples,where reaction rates are increased,the deviations from ideal conditions are likely amplified and so attenuation in thrust force in the fast reaction regime is expected within the CD nozzle STM.
The effect of GO on the ignition and flame evolution of oxygenated-salt nanothermite composites is presented in Fig.11,as obtained from high-speed imaging.

Fig.11.Snapshots of different GO/nanothermite composites in the CD STM at 20%TMD.
All the prepared samples readily ignited by laser heating and sustained combustion.All samples were accompanied by bright flames (as seen in Fig.11) indicating high combustion capability[53,66].The images shown in Fig.11 support a trend first observed in Table 4;the improvement in nanothermite thrust performance with the addition of GO up to 5%.This trend is observed in Fig.11 as the 5%GO sample can be seen to have the largest plume at 720 μs.The enhancement of the GO/nanothermite sample is attributed to the energetic nature of GO and its ability to increase the intimate mixing between fuel and oxidizer through its cross-linked porous framework structure[53].Further addition of GO shows a reduced plume size and intensity,as observed in Fig.11,once again supporting the observed trend in Table 7.
We can observe solid and liquid combustion products ejected within the exhaust plume in Fig.11,such as at 830 μs for the 5%GO sample.Therefore,frictional force occurs between solid and liquid products and along thruster walls,introducing significant two phase losses[67].Meanwhile,the CD nozzle likely moves the STMs away from ideal nozzle conditions.This is yet another possible contribution to the reduced thrust observed for low-density samples in Fig.10 and Table 7 when used in the CD nozzle STM.Similarly,this is likely a contributing factor to the reduction of Ispin high-density samples in Table 7.
The shape change of the flame from 80 to 200 is visible in the experimental images(730-810)shown in Fig.11.The exit speed of product gasses reached between 1800 and 2000 m/s which is double that of metal oxide-based nanothermites,which previous literature has reported around 1000 m/s [68-70].This result supports the higher impulse of the prepared ternary nanothermite and highlights the advantage of using oxygenated salts as alternatives of metallic oxides in propulsion applications.
3.4.Revealing velocity and temperature fields by CFD modeling
The high-speed flow through the CD nozzle and resultant plume were modeled using CFD code.Three mass flow rates at the inlet of the CD nozzle were used:61.55 mg/ms for the ICT calculated value,42.41 mg/ms for the 20%TMD sample,and 30.37 mg/ms for the 55%TMD sample.Fig.12 shows the simulated profile of the Mach number magnitude within and above the CD nozzle,which is produced by the 20%TMD sample.
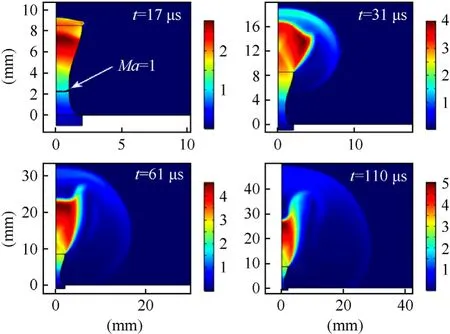
Fig.12.Numerical Mach number evolution for the reference sample at 20%TMD in the CD nozzle domain.
Three key observations about the nozzle plume can be made in Fig.12.First,a sonic interface(Ma=1)is formed at the throat of the CD nozzle as expected (17 μs profile),and its location remains unchanged during the simulation time.Secondly,the design of the CD nozzle is not optimal and thus leads to the formation of an under-expanded plume.So,for the environmental conditions used in testing,the thrust produced by this nozzle can be improved by increasing the degree of expansion at the outlet.Thirdly,transition of the plume shape is visible,analogous to the experimental results presented in Fig.11.At 17 μs,the high-speed flow reaches the nozzle outlet,accompanied with the rapid increase of the thrust.At 31 μs,the shape of the plume is bell-like,characterized by the maximum value at the center line of the nozzle.At 61 μs the shape of the plume evolves into an inversed-bell with the minimum value of the velocity magnitude existing at the center line.At 110 μs,the inversed-bell shape can be seen to transition into a typical jetplume.Eventually the width of the plume in the radial direction decreases and leads to the extinction of the flame.
The temperature profiles of these high-speed flows were also simulated.Fig.13 shows the simulated profile of the temperature field within and above the CD nozzle,which is produced by the 20%TMD sample.While the peak temperature of the combustion exhaust remains about 3800 K near the nozzle inlet,the plume temperature still reaches about 1500-2000 K in all these images.The transition of the plume shape is clearly visible with increasing time,changing from a bell shape to an inverse bell,as observed in Fig.12.
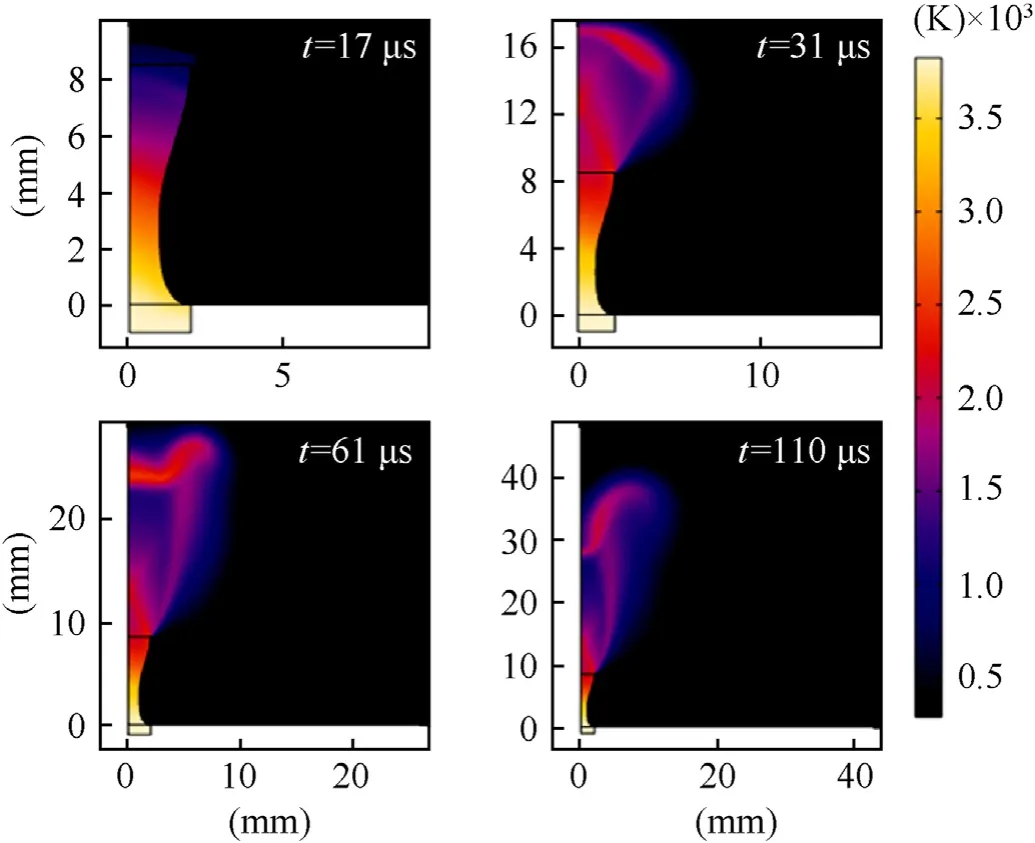
Fig.13.Numerical temperature evolution for the reference sample at 20%TMD in the CD nozzle domain.
Two mass flow rates at the inlet of the open tube were used to calculate the velocity and temperature fields: 42.41 mg/ms for the 20%TMD sample and 30.37 mg/ms for the 55%TMD sample.Fig.14 shows the simulated temperature and velocity profiles from the open tube for the reference sample at 20%TMD.Flow within the tube is supersonic throughout,but the Mach number at the tube outlet decreases substantially with time.When the flow first reaches the outlet at 43 μs,the Mach number at the outlet is 2.8.At 50 μs,the outlet Mach number drops to 1.4,and then to 1.3 as the plume evolves at 80 μs.These results suggest that momentum thrust should maximize when the flow first reaches the tube exit and stabilize as the exhaust plume develops.

Fig.14.(a) Numerical Mach number and (b) temperature profile time evolution for the reference sample at 20%TMD in the straight-tube configuration.
By comparing the numerical results for the open-tube and CD cases we can see that the Mach number at the outlet is generally higher for the CD case throughout.Temperature at the outlet is lower in the CD case than in the open-tube case.These observations suggest that pressure forces contribute to thrust production in the open tube case more than in the CD case.Performing a force balance at the nozzle outlet,the momentum and pressure forces are summed throughout the simulation time to obtain force profiles for the given simulation cases as shown in Fig.15.

Fig.15.Simulated thrust-time profiles of the Al/KClO4 nanothermite(reference)sample with different nozzle geometries:(a)20%TMD;(b)55%TMD;(c)The CD nozzle with varying TMDs.
All profiles in Fig.15 show the same characteristic shape with a large peak followed by a period of steady force.In both 20 and 50%TMD cases,the open tube test showed greater delay in force production because the length of the open tube is greater than that of the CD nozzle,as can be seen in Fig.1.The momentum component of thrust dominates in the CD cases shown in Figs.15(a)and 15(b),whereas pressure force contribution remains substantial in the open tube tests.This result is expected,as the CD nozzle is implemented specifically to convert heat and pressure into thrust.The results also suggest that,regardless of packing density,pressure force in the open-tube case makes up 25-30% of thrust,and that the peak force observed is produced mostly by momentum force.
The force is generally over-predicted in the model when compared to experimental results,but there is agreement in order of magnitude.Like the experimental results shown in Fig.10,the open tube cases seen in Figs.15(a)and 15(b)generate higher peak forces than the CD nozzle cases.In the 20%TMD case,the steadystate force output is greater in the open tube configuration;the opposite is true for the 50%TMD case.This observation agrees with the experimental data presented for 55%TMD samples in Fig.10(b),where the quasi-steady force is increased with the addition of a CD nozzle.The similarity between experimental and numerical results for the high-density (~50%TMD) cases also extends to the magnitude of steady-state forces as well as general profile shape,albeit experimental results do not show the same peaks that are observed in the model.These similarities are not observed with the lowdensity (~20%TMD) cases,since there is no steady force production in the experimental results seen in Fig.10(a).This is likely a limitation of the idealized assumptions used to model the flow domain and mass-flow resulting from combustion.
Considering only the CD nozzle case shown in Fig.15(c),it is seen that the highest forces are produced when mass flow is maximized;mass flow increases with decreasing %TMD.This agrees with the results presented in Table 7,where average force is higher for 20%TMD samples.This suggests that the nozzle is not mass-flow choked and,for the given nozzle geometry,packing density should be minimized if output force is to be maximized.
The simulation supports the experimental investigations for the 20%TMD,open-tube reference (Al/KClO4) sample with results of 86.3 N,0.06 ms,5.2 mN·s,105.6 s,and 0.38 mN·s/mm3for average thrust,combustion time,IFT,ISPandISVrespectively.These results agree with the experiments within approximately 25%error.So,the model can be used with an understanding of its limitations for further investigation of the important physical characteristics of the developed ternary nanothermites.This will subsequently help develop engineering applications for these new composites.
4.Conclusions
In this study,we have examined experimentally the effect of CNMs on the ignition and thrust performance of various types of oxygenated-salts/nanothermite mixtures and demonstrated nonmetallic oxidizers as good alternative energetic oxidants for thermite mixtures with high combustion performance.CNMs were successfully integrated into nanothermite compositions using a simple ultrasonic mixing method.Prepared nanothermites were ignited in both an open tube and CD nozzle STMs at different packing densities to assess the practicality of these mixtures for propulsion applications.All prepared nanothermites ignited successfully and combusted self-sufficiently inside the STMs.The CNMs,especially GO,were shown to be capable of improving sample combustion performance.Compared to the reference sample,significant improvements inIFT,ISP,ISV,and reduction in ignition delay were observed with the addition of up to 5%GO.The improved combustion properties of the GO/Al/KClO4compositions are proposed to be resultant of the catalytic nature of GO in facilitating uniform mixing of n-Al and KClO4,in addition to its effect as an energetic reactant acting as a secondary fuel and adding energy to the system.Additionally,two different reaction regimes(fast and slow) are recognized as result of the sample packing density.The introduction of a CD nozzle is shown to have two opposing effects on thrust in fast and slow reaction regimes where it reduces thrust output and increased impulse in fast regime samples but increases thrust and reduced impulse in slow regime samples;this is observed numerically and experimentally.Deviations from ideal nozzle design conditions suggest that a nozzle may hinder performance in fast reaction regimes.This study shows that energetic composites with tailored and controlled thrust performance can be developed via CNMs/n-Al/oxygenated-salt nanothermites and enables development of more focused future propulsion applications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Ahmed Fahd acknowledges the financial funding from the Egyptian government.Charles Dubois acknowledges the financial funding from the NSERC Discovery grant.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.03.009.
杂志排行
Defence Technology的其它文章
- Interaction of water droplets with pyrolyzing coal particles and tablets
- Multifunctional characteristics of 3D printed polymer nanocomposites under monotonic and cyclic compression
- The concept of sUAS/DL-based system for detecting and classifying abandoned small firearms
- Modelling and predicting the dynamic response of an axially graded viscoelastic core sandwich beam
- Development of bimetallic spinel catalysts for low-temperature decomposition of ammonium dinitramide monopropellants
- Ballistic response of skin simulant against fragment simulating projectiles
