Development of bimetallic spinel catalysts for low-temperature decomposition of ammonium dinitramide monopropellants
2023-12-27ShamjithaAnujVargeese
C.Shamjitha,Anuj A.Vargeese
Laboratory for Energetic and Energy Materials Research (LEEMR), Department of Chemistry, National Institute of Technology Calicut, Kozhikode 673601,India
Keywords:ADN monopropellant Mixed metal oxides Doped spinels Catalytic decomposition
ABSTRACT Ammonium dinitramide (ADN) based liquid monopropellants have been identified as environmentally benign substitutes for hydrazine monopropellant.However,new catalysts are to be developed for making ADN monopropellants cold-start capable.In the present study,performance of Co and Ba doped CuCr2O4 nanocatalysts prepared by hydrothermal method was evaluated on the decomposition of aqueous ADN solution and ADN liquid monopropellant (LMP103X).The catalysts were characterized by PXRD (Powder X-ray Diffraction),FTIR (Fourier Transform Infrared spectroscopy),SEM (Scanning Electron Microscopy),TEM(Transmission Electron Microscopy),EDS(Energy Dispersive X-ray Spectroscopy),and XPS (X-ray Photoelectron Spectroscopy).The nanosize was confirmed by SEM and TEM,while the nanoflake morphology was confirmed by the SEM analysis.Further,we obtained the elemental composition from the EDS analysis.We investigated the catalytic activity of the catalysts by thermogravimetric (TG) analysis and the developed catalysts lowered the decomposition temperature of ADN monopropellant by about 55 °C.The XPS analysis confirmed the presence of metal ions with different chemical states.Apparently,increase in the surface area of the catalysts and the mixed active sites as well as the development of oxygen vacancy on the catalyst surface introduced by metal doping are influencing the decomposition temperature of ADN samples.
1.Introduction
Hydrazine (N2H4) has been used as an effective liquid monopropellant for space craft trajectory/orbit control rockets for decades,owing to the high energy density,density impulse and coldstart capability.However,the toxic and carcinogenic nature of hydrazine demands stringent transport and storage conditions,and this necessitates the replacement of hydrazine with a more environment friendly monopropellant [1].Researchers have identified ammonium dinitramide (ADN) based liquid monopropellant as a possible substitute for hydrazine.Many ADN based liquid monopropellant compositions can be prepared owing to the high solubility of ADN in polar solvents.ADN monopropellants possess high energy and offers higher specific impulse and density impulse compared to hydrazine.However,ADN based monopropellants are not cold-start capable and cold-start capability is a necessary requirement for storable liquid monopropellants to find application in trajectory/orbit control rockets.The non-availability of catalysts offering cold-start capabilities has restricted the application of ADN monopropellant in practical propulsion systems.Several countries have been working on the development of new catalysts for ADN liquid monopropellants [2,3] and such a catalyst would eliminate the requirement of separate storage tank for fuel and oxidizer,eventually leading to the reduction in spacecraft size.There are reports in which precious metals (Pt,Rh,Ir) doped hexaaluminate catalysts have been developed and tested[4,5]as catalysts for ADN monopropellants.Some of the commonly used ADN monopropellant compositions and their properties are tabulated as Table 1,and among these,alcohol based ADN monopropellants[4,5] are commonly investigated.

Table 1 Composition and properties of some of the ADN based liquid monopropellants.
Spinel mixed metal oxides are widely exploited for catalytic applications because of their thermal stability and impressive catalytic activity compared to their pure single metal oxide counterparts.The presence of different bivalent and trivalent metal ions in tetrahedral and octahedral sites increases the surface heterogeneity and consequently the catalytic activity of these mixed metal oxides[6].Spinel catalysts have been reported for ammonium perchlorate decomposition [7,8],CO soot decomposition [9,10] and oxidation[9,11],N2O decomposition [12,13],and combustion of organic pollutants[14,15].Ferrite and cobaltites have been reported as efficient catalysts for the decomposition of N2O[13].The enhanced catalytic activity of the mixed metal oxides is generally attributed to the presence of surface exposed partially hydroxylated and coordinatively unsaturated metal ion active sites.The unsaturated metal ions constitute the Lewis acid sites where as the hydroxyl groups adsorbed on the surface act as the Bronsted acid sites.The unsaturated metal ions promote the oxygen vacancy on the surface of the catalysts.Hence,the use of bimetallic catalysts is one of the strategic ways to lower the decomposition temperature of ADN based monopropellants to make them cold-start capable.The tunable physicochemical properties of spinel oxides make them effective bimetallic catalysts for the decomposition of ADN based monopropellants.Even though various studies have been reported on the catalytic properties and applications of chromite-based spinel metal oxides,no studies have been reported on their application as a decomposition catalyst for ADN based monopropellants.
In this study,the performance of chromite based non-precious metal doped spinel bimetallic catalyst for the decomposition of aqueous ADN solution and ADN liquid monopropellant is reported.The catalytic effect of Co and Ba doped CuCr2O4on the decomposition of ADN was investigated.Thermogravimetric (TG) analysis was used for understanding the effect of the developed catalysts on the decomposition temperature of aq.ADN and ADN monopropellant.
2.Experimental methods
2.1.Preparation of catalysts
The nanocatalysts,spinel Co doped CuCr2O4and Ba doped CuCr2O4were prepared via hydrothermal method [7,16].Aqueous solution of Cu(NO3)2·3H2O and Cr(NO3)3·9H2O (Loba Chemie Pvt.Ltd.),as well as Co(NO3)2·6H2O and Ba(NO3)2(NICE Chemicals Pvt.Ltd.) in equimolar ratio were used for the catalyst preparation.
2.2.Preparation of aqueous ADN solution and ADN monopropellant(LMP103X)
The ADN required for the study was synthesized through a reported procedure [17].To prepare the required aq.ADN sample,approximately 375 mg of ADN was dissolved in 125 mg of deionized water.The solution was thoroughly mixed for 30 min,stored in an air-tight container at ambient conditions and used for further studies.
The ADN monopropellant was prepared by dissolving 70 mg of ADN in a mixture of 26 mg of water and 12 mg of methanol(11 wt%)as fuel.The monopropellant prepared was immediately used for catalytic activity evaluation.
2.3.Characterization of catalysts
The crystal structure of the synthesized catalyst samples was analyzed by powder X-ray diffraction (PANalytical X'Pert3Powder X-ray Diffractometer)using Cu Kβ radiation.The diffractogram was recorded for a 2θ range of 10°-70°with a step size of 0.026°.FTIR spectra of the catalysts were recorded by using JASCO FT/IR-4700 Spectrometer.The microscopic analysis for understanding the catalyst's morphology and particle distribution was performed using JEOL JSM-7600F Field Emission Gun Scanning Electron Microscope (SEM) and JEOL/JEM-2100 High Resolution Transmission Electron Microscope (HRTEM).The particle size and interplanar distance (d) were calculated by using ImageJ software and Gatan Microscopy Suite software respectively.The specific surface area of catalysts was determined by the Brunauer-Emmet-Teller (BET)method using Belsorp-max,BEL MicrotracBEL Corp.Equipment by N2adsorption at 77 K at a saturated vapour pressure of 106.2 kPa.We conducted X-ray Photoelectron Spectroscopy (XPS) analysis of both catalysts by using a PHI 5000 VersaProbe II XPS instrument equipped with micro-focused (100 μm,15 kV) monochromatic Al-Kβ X-Ray source(hν=1486.6 eV).We recorded both survey spectra and narrow scan (high-resolution spectra) using the instrument.Survey scans were recorded with an X-ray source power of 50 W and pass energy of 187.85 eV.High-resolution spectra of the major elements were recorded at 46.95 eV pass energy.XPS data were processed using PHI's Multipak software.We referenced the binding energy to the C 1s peak at 284.8 eV.
2.4.Thermogravimetric (TG) analysis
The catalytic activity of the prepared catalysts was investigated by Perkinelmer STA6000 thermogravimetric (TG) analyser in N2atmosphere.For this analysis,about 4 mg of the catalyst was placed on an alumina crucible and 5 μL of aq.ADN or LMP103X monopropellant was added onto the catalyst in the crucible.Thermal analysis was carried out from 30°C to 240°C at a heating rate of 5°C/min.During the analysis,the N2flow rate was maintained at 20 mL/min for the sample purge gas.The same method was pursued for the analysis of other samples.
3.Results and discussions
The powder x-ray diffraction peaks(Fig.1)for Co and Ba doped CuCr2O4catalyst samples were indexed with the help of International Centre for Diffraction Data (ICDD) database available in HighScore Plus software provided with the PANalytical X'Pert3Powder X-ray Diffractometer.In the PXRD patterns of Co doped CuCr2O4,the high intense peak observed at 36.12°was assigned to the diffraction of(311)plane of Co3O4(ICDD number:01-080-1540)having a d-spacing of 2.48 Å.In the case of Ba doped CuCr2O4,the high intense peak (100%) observed at 2θ value 35.15°corresponds to the (211) plane of CuCr2O4(ICDD number: 01-088-0110) with a d-spacing of 2.55 Å.The composition of the catalysts has been determined from the PXRD analysis using HighScore Plus software and is shown in Table 2.The detailed comparison of PXRD stick patterns of the compounds with ICDD data is given as supporting information (Figs.S1 and S2).The PXRD analysis of Co doped CuCr2O4sample indicated the presence of CuO(ICDD number:00-045-0937) and CuCr2O4(ICDD number: 00-026-0509) besides the presence of Co3O4.Meanwhile,the diffraction pattern of Ba doped CuCr2O4consists of peaks corresponding to BaCr2O4(ICDD number:00-016-0322).Both the doped catalysts showed the signature of CuO(ICDD number:00-045-0937 or 01-089-5898)possibly formed during the high temperature calcination in air.

Fig.1.PXRD patterns of Co and Ba doped CuCr2O4.

Table 2 Analysis of composition of CuCr2O4 and doped CuCr2O4 from PXRD.
The FTIR spectra (Fig.S3) showed the presence of absorption bands in the 800-400 cm-1region,usually attributed to different M -O bonds in crystalline materials.These absorption bands indicate the presence of Co-O,Ba-O,Cu-O,and Cr-O bonds in the catalysts.Elemental mapping was used to analyse the distribution of Co,Ba,Cu,and Cr in the catalysts and confirm the uniformity of dopant across the region in the sample (Figs.S5 and S7).The uniform spread of different colours indicates a homogeneous distribution of the elements in the samples.The CoKβ,BaLβ,CuKβ and CrKβ were used to map the distribution of corresponding elements.Further analysis showed that in Co doped sample the Cu:Cr:Co ratio was 0.27:1.32:1,and in Ba doped sample the Cu:Cr:Ba ratio was 1:1.45:0.02 by atomic weight percentage.The lower presence of Ba than the expected value can be attributed to the lower solubility of the Ba salt used in the experiment.Though the values obtained by the EDS method are semi quantitative,they corroborate with the results obtained from the PXRD analysis.
The SEM images(Fig.2)of Co and Ba doped catalysts confirmed the morphology that the particles are having distorted oval shape in nanometer dimensions.The high resolution TEM images are shown in Fig.3.The particles are distributed uniformly in both the CuCr2O4samples,while the particle size is relatively large for Co doped CuCr2O4compared to Ba doped CuCr2O4.The average particle size was determined from TEM images by ImageJ software by taking 35 particles for each of the catalyst samples.The histogram(Fig.4) shows that the average size for Co doped and Ba doped CuCr2O4as 47 nm and 20 nm respectively,indicating smaller particles in both the catalyst samples.Also,the histogram shows that the Ba doped CuCr2O4has a very narrow particle size distribution in 10-40 nm region.The selected area electron diffraction patterns confirmed the polycrystalline nature of both the catalyst samples(Fig.S8).
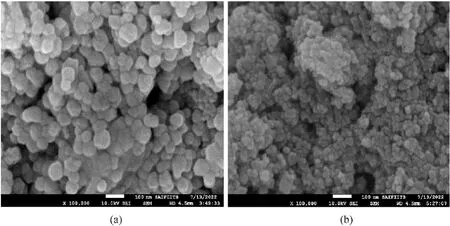
Fig.2.The SEM images of (a) Co doped CuCr2O4 and (b) Ba doped CuCr2O4 catalysts.

Fig.3.The HR-TEM images of (a) Co doped CuCr2O4 and (b) Ba doped CuCr2O4 catalysts.

Fig.4.Particle size distribution of Co and Ba doped CuCr2O4 catalysts.
The BET results(Table 3)showed that the specific surface area of Ba doped CuCr2O4is moderately higher than that of Co doped CuCr2O4catalyst sample,concurring with their lower particle size observed during the TEM analysis.

Table 3 Surface area,pore diameter and pore volume determined by BET analysis of the catalyst samples.
X-ray Photoelectron Spectroscopy(XPS)was used to investigate the elemental composition and chemical states on the surface of the catalysts.The chemical states of all metal ions present in both the catalyst samples were confirmed by the XPS analysis.Fig.5 shows the XPS spectra of Cu 2p and Cr 2p for both the catalysts.Furthermore,the XPS spectra of Co 2p and Ba 3d are also shown in Fig.5 for Co doped CuCr2O4and Ba doped CuCr2O4respectively.The XPS Cr 2p spectrum for Co doped CuCr2O4showed the binding energy values of 588.44 eV and 586.44 eV corresponding to Cr(VI)and Cr(III)respectively due to Cr 2p1/2.The binding energy values of 576.90 eV and 578.69 eV were assigned to Cr (VI) and Cr (III)respectively due to Cr 2p3/2[18].The XPS Cu 2p spectrum showed the presence of two main peaks due to Cu 2p1/2and Cu 2p3/2.The peak corresponding to Cu 2p3/2was deconvoluted into two main peaks at 933.29 eV which emerged from the ligand to metal charge transfer between the O 2p orbital and Cu 3d orbital(O 2p →Cu 3d)corresponding to Cu(II)[19].The peak at 936.04 eV was assigned to Cu (0) states of Cu.The presence of Cu (II) ions was further confirmed by the associated satellite peaks at 940.92 eV and 943.22 eV.The deconvolution of Cu 2p1/2peak presented three satellite peaks at 955.09 eV,958.60 eV,and 962.49 eV indicating the presence of Cu(II)ion.The Co 2p XPS spectrum showed two main peaks corresponding to Co 2p1/2and Co 2p3/2.The Co 2p1/2peak could be deconvoluted into two peaks at high binding energies,796.35 eV and 798.49 eV corresponding to Co(III)and Co(II)states respectively present at tetrahedral sites.The satellite peaks due to Co(II)ions were observed at 787.38 eV and 804.22 eV for Co 2p3/2and Co 2p1/2respectively.The deconvolution of Co 2p3/2gave rise to a high intense peak at 781.07 eV corresponding to Co(III)ions and a peak at 783.26 eV corresponding to Co(II)ions at octahedral sites.A very less intense peak at 776.74 eV corresponding to metallic cobalt(Co(0)) was also observed.The presence of such metal ion active sites might have enhanced the catalytic activity of the mixed metal oxides.

Fig.5.The XPS spectra of (a)-(c) Co doped CuCr2O4 and (d)-(f) Ba doped CuCr2O4 catalysts.
The XPS Ba 2p spectrum for Ba doped CuCr2O4showed two main peaks at 794.22 eV and 780.12 eV due to Ba 3d3/2and Ba 3d5/2respectively indicating the presence of Ba2+ions on the catalyst surface.The satellite peaks at 786.67 eV and 798.99 eV confirmed the presence of Ba2+ions.The Cu 2p spectrum displayed two main peaks corresponding to Cu 2p1/2and Cu 2p3/2.The Cu2p1/2peak was deconvoluted into a peak at 953.80 eV indicating the presence of Cu(II) ions accompanied by two satellite peaks at 958.48 eV and 961.42 eV which are due to the presence of Cu(II) state.The deconvolution of Cu2p3/2peak resulted in two peaks at 933.22 eV and 935.13 eV corresponding to Cu(II) and Cu(OH)2respectively.The two satellite peaks observed at 940.36 eV and 942.57 eV were raised due to the Cu(II) state.A peak at 585.97 eV and a peak at 575.70 eV were obtained through the deconvolution of Cr 2p peaks corresponding to Cr2p1/2and Cr2p3/2,respectively.The XPS Cr 2p spectrum for Ba doped CuCr2O4produced two mains peaks due to Cr2p1/2and Cr2p3/2.The Cr2p1/2peak could be deconvoluted into two peaks at 585.97 eV and 587.73 eV attributing to the surface Cr(III)and Cr(VI)ions.The intensity of the reduced metal ion,Cr(III)is relatively higher than that of Cr(VI)ions in both the catalysts.This leads to the presence of higher oxygen vacancies on the catalyst surface to maintain the electrical neutrality in catalysts.
More information about the oxygen vacancy was derived from O1s XPS spectra(Fig.6)for both the catalysts.The binding energies for different states of the metal ions and different types of oxygen present on the surface of the catalysts were confirmed from literature [20-22].The XPS O1s spectrum of Co doped CuCr2O4exhibited three peaks corresponding to three types of oxygen.The high intense peak at 531.48 eV(O1)indicated the adsorbed oxygen on the oxygen vacancy.The peak at 533.41 eV (O2) suggested the adsorbed hydroxyl oxygen.The peak at a binding energy of 535 eV(O3) confirmed the third type of oxygen which indicated the presence of gaseous water molecule adsorbed on the surface.The Ba doped CuCr2O4catalyst also displayed three different types of oxygen given by three peaks in the XPS O1s spectrum.The slightly lower binding energy value from 530 eV (529.93 eV,O1) revealed the lattice oxygen (O2-) in the crystal frame work or the metaloxygen (M -O) bond in the crystal lattice.The peak at a binding energy of 531.96 eV(O2)corresponded to the oxygen vacancies on the catalyst surface and the peak at 533.35 eV(O3)designated the adsorbed hydroxyl species or dissociatively adsorbed water molecule.The intensity of the peaks corresponding to Cr(III)in both the catalysts and the intensity of Co(II)metal ions in Co doped CuCr2O4catalyst is higher than the intensity of the peaks corresponding to Cr (VI) and Co (III) ions.This implied a reduction in the oxidation state of the metal ions which may promote the oxygen vacancies on the catalyst suface to maintain the electrical neutrality in the crystal.

Fig.6.The XPS O1s spectra of (a) Co doped CuCr2O4 and (b) Ba doped CuCr2O4 catalysts.
In literature,the mechanism of the ADN decomposition has been studied by employing different techniques such as TGA[23,24],TGA-FTIR[23,25]and fixed bed reactors[4].In the absence of any catalyst,solid ADN decomposition starts at 135°C and completes the decomposition before 210°C in differential thermal analysis[23].Further,an endothermic peak at 92°C corresponding to the melting of solid ADN and an exothermic peak corresponding to decomposition around 185°C are observed.The TG analysis data and differential scanning calorimetry (DSC) data of solid ADN,aq.ADN and ADN monopropellant are shown in Fig.7.The TG plot of aq.ADN sample shows an initial mass loss of about 32% corresponding to the removal of water content and a second weight loss corresponding to the ADN decomposition.The analysis showed that the aq.ADN decomposes in the temperature range of 175°C-196°C in the absence of any catalyst.In the DSC plots shown in Figs.7-9,all the samples exhibited an exothermic decomposition.The onset of aq.ADN decomposition was observed at about 150°C with peak decomposition at 185°C while the catalyzed decomposition started around 125°C and the peak decomposition was observed at around 136°C.

Fig.7.TG-DSC of solid ADN,aq.ADN and LMP103X.
The TG curve for non-catalyzed decomposition of ADN liquid monopropellant LMP103X showed a similar behaviour as that of aq.ADN decomposition.The monopropellant decomposition occurred in the temperature range of 150°C-200°C with the peak decomposition temperature at 191°C.For the catalyst evaluation,5 μL of aq.ADN or LMP103X monopropellant was dropped onto~4 mg of solid catalyst and heated in the TG analyzer.The aq.ADN decomposed explosively in the presence of Co doped CuCr2O4at 136°C(Fig.8)and at 137°C in the presence of Ba doped CuCr2O4.Both the bimetallic spinel oxide catalysts exhibited similar effect on the thermal decomposition of aq.ADN.We identified the quick decomposition by analysing the sudden weight loss observed in the TG data.The DSC analysis also substantiated the behaviour observed during the TG analysis.

Fig.8.TG-DSC of aq.ADN over Co doped CuCr2O4 and Ba doped CuCr2O4 catalysts(the insets shows the peaks corresponding to decomposition).
Further,we explored the activity of catalysts on the decomposition of ADN liquid monopropellant LMP103X (Fig.9).A similar method used for aq.ADN was adopted for understanding the catalytic decomposition behaviour of LMP103X.The monopropellant exhibited a two-stage decomposition with peaks at 157°C and 191°C.However,in presence of the catalyst decomposition started below 125°C with peak decomposition around 135°C.The sudden decomposition has possibly disturbed the sensitive microbalance in the TGA instrument and due to this disturbance some discrepancy was observed in the residual mass.The TG-DTG plots of aq.ADN (Figs.S11 -S13) and ADN monopropellant (Figs.S14 -S16) over the catalyst are given as supporting information.In the presence of Co doped CuCr2O4,the decomposition of monopropellant occurred explosively at 135°C.LMP103X monopropellant over Co doped CuCr2O4and Ba doped CuCr2O4also explosively decomposed at 135°C and 136°C respectively.The catalytic effect of doped CuCr2O4on the decomposition temperature of ADN and ADN monopropellant are compiled in Table 4.

Fig.9.TG-DSC of LMP103X over Co doped CuCr2O4 and Ba doped CuCr2O4 catalysts(the inset shows the peaks corresponding to decomposition).
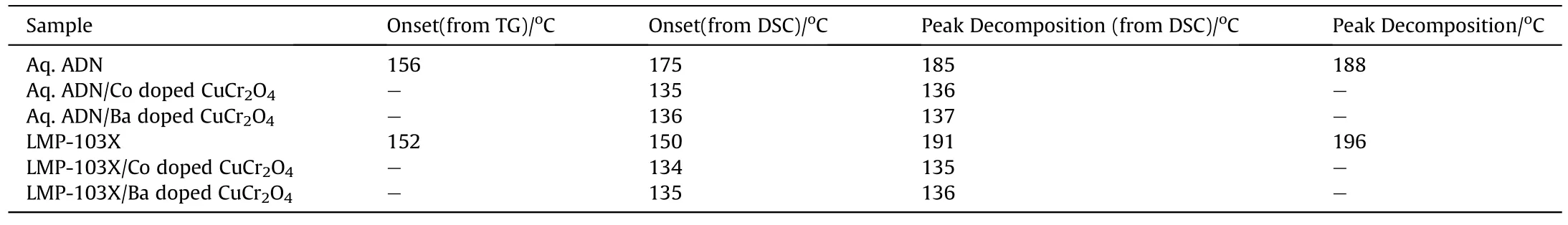
Table 4 Evaluation of catalytic performance of CuCr2O4 and doped CuCr2O4 on the decomposition of ADN and ADN monopropellant.
The observations show that Co doped CuCr2O4and Ba doped CuCr2O4are good thermal decomposition catalysts for aq.ADN as well as LMP103X monopropellant.The exothermic decomposition of ADN mainly leads to the formation of NO2,N2O,H2O and ammonium nitrate(AN),and the AN formed further decomposes to NO2and water[26].Earlier studies showed that the decomposition products of aq.ADN mainly contain H2O,N2O,NO2,and NH3and the addition of CuO catalyst accelerates the decomposition rate and alters the NO2to N2O ratio[23].The overall decomposition possibly follows the reaction path R1 shown in [23,27],though the NH3formation is contested by later investigators [26].According to an earlier mass spectrometry (MS) study,the ADN decomposes by an ionic mechanism,forming a condensed phase of ammonium salt[26].In the present study,the active metal sites present in the catalyst can interact with the ADN as well as ADN decomposition products.In literature different spinels have been identified for the catalytic decomposition of N2O [28,29],one of the major decomposition products of ADN and aq.ADN.It is generally accepted that on the active surface of the metals,either N2O is reversibly decomposed into N2and adsorbed surface oxygen(M -O),or the subsequent recombination of the adsorbed oxygen atom with another oxygen atom to form gas phase O2[30,31].This could be the leading mechanism that enhances the overall decomposition rate of ADN samples.Another study reported that condensed phase thermal decomposition of ADN proceeds via two pathways,in which below 130°C,ADN is converted to AN by elimination of N2O and at higher temperatures,evidence of N-N bond scission forming NO2has been observed [26].
Eventhough the Co doped CuCr2O4showed a variation in particle size and thus in surface area from that of Ba doped CuCr2O4,it exhibited similar catalytic activity as that of Ba doped catalyst towards the aq.ADN and LMP103X decomposition.This may be attributed to the variable oxidation states of Co,Cu and Cr transition metal ions that can generate a significant amount of oxygen vacancies on the surface of the catalyst.The variable oxidation states of metal ions introduced through doping in the catalysts boost the oxygen vacancy and thus increase the number of active sites on the surface of the catalyst enhancing the catalytic activity.In other words,even though the exact active sites that are responsible for the catalytic activity of the mixed metal oxides are not predictable,the presence of different active sites,Co(II),Co(III),Cu(II),Cr(III),Cr (VI) and Ba (II) ions from different metal oxide phases may be attributed to the catalytic activity of the doped catalysts along with the high surface area of these catalysts.
The high temperature thermal treatment in air during the hydrothermal synthesis of the catalysts facilitates the oxygen vacancy generation on the catalysts’ surface [32].The presence of oxygen vacancies and adsorbed hydroxyl groups which are confirmed by XPS analysis indicated the presence of Lewis acid sites and the Bronsted acid sites respectively.The Cr(VI)and Co(III)in the metal oxide lattices undergo reduction and form reduced metal states,Cr(III) and Co (II) respectively.This is confirmed by the enhanced intensity of the peaks corresponding to these metal ions in their XPS spectra compared to that of their corresponding oxidized metal states.The presence of oxygen vacancy increases the number of unsaturated coordinated metal cations in the lattice.This will enhance the affinity of the catalyst towards ADN and its decomposition products and thereby improves the catalytic activity.In addition,the basicity due to the low charge density on the large alkaline earth metal ion,Ba (II) also is attributed to the catalytic activity of the Ba doped CuCr2O4.Thus,it is revealed that the synergistic effect of different types of metal ion active sites,oxygen vacancies,and higher surface area could be enhancing the catalytic activity of the prepared Co and Ba doped CuCr2O4mixed metal oxides.
Lowering of the preheating temperature of LMP103X using doped bimetallic spinel ternary metal oxides promises the possibilities of applying more energetic and environmental friendly ADN monopropellant in spacecraft trajector/orbit control rockets.
4.Conclusions
Two different non-precious bimetallic nanocatalysts,Co and Ba doped CuCr2O4were prepared by hydrothermal method.During the catalytic activity evaluation,the Ba doped CuCr2O4catalyst showed a slightly better performance over the Co doped catalyst on ADN monopropellant's thermal decomposition.Aq.ADN decomposition temperature was lowered to 136-137°C in the presence of doped catalysts while LMP103X decomposed at 135-136°C.The catalytic activity of the doped CuCr2O4can be attributed to the increase in the number of active metal sites and oxygen vacancy on the surface of the catalyst along with the increase in the surface area.The presence of more active sites on surface of doped samples is due to the presence of Cu(II),Cr(III),Cr(VI),Co(III),and Co(II)or Ba (II) from different metal oxide phases confirmed by the XPS analysis that promotes the decomposition of the ADN degradation products,convincingly the major decomposition product N2O.The lowering of ADN monopropellant decomposition temperature over the doped bimetallic catalysts indicates the possibility of lowering preheating requirements for orbit control thrusters.However,the catalyst's long-term stability and possible loading onto catalyst supports such as hexaaluminate need to be evaluated.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Anuj A Vargeese reports financial support was provided by Defence Research and Development Organisation(DRDO).Anuj A Vargeese reports financial support was provided by Science and Engineering Research Board (SERB).
Acknowledgments
The authors thank NIT Calicut for the facilities and support provided.The financial support by DST-SERB(Grant No.SRG/2021/001182) and DRDO (Grant No.ARMREB/HEM/2021/241) is gratefully acknowledged.
Appendix A.Supporting information
The PXRD data comparison with ICDD (number and pattern),FTIR spectra,BET data and TG-DTG curves of aq.ADN and ADN monopropellant decomposition are given as Supplementary material.
杂志排行
Defence Technology的其它文章
- Interaction of water droplets with pyrolyzing coal particles and tablets
- Multifunctional characteristics of 3D printed polymer nanocomposites under monotonic and cyclic compression
- The concept of sUAS/DL-based system for detecting and classifying abandoned small firearms
- Modelling and predicting the dynamic response of an axially graded viscoelastic core sandwich beam
- Thrust characteristics of nano-carbon/Al/oxygenated salt nanothermites for micro-energetic applications
- Ballistic response of skin simulant against fragment simulating projectiles
