Synthesis,molecular architecture and characterization of trinuclear Cu(Ⅱ)-Ln(Ⅲ)Schiff base complexes
2023-12-26LIUYiFuMAFangWei
LIU Yi-Fu,MA Fang-Wei
(School of Chemistry and Materials Science,Heilongjiang University,Harbin,150080,China)
Abstract:A series of 3d-4f heterometallic Schiff base complexes of the general formula (CuSaltn)2Ln(H2O)(NO3)3 (Ln = La (1),Ce (2),Pr (3),Nd (4)&Sm (5),where Saltn = N,N′-ethylenebis (salicylideneaminato)was synthesized by reaction of the tetradentate copper(Ⅱ)Schiff base complex (CuSaltn)and Ln(Ⅲ)nitrate. The ligand and complexes were characterized by elemental analysis,thermogravimetric analysis,infrared spectroscopy and ultraviolet spectroscopy. The crystal structure of 5 was determined at room temperature.The Sm (Ⅲ)ion is nine-coordinated with two bidentate Cu(Saltn)molecules,two bidentate nitrate ions and one water molecule. The fluorescence of complexes markedly decreased on forming the trinuclear Cu(Ⅱ)-Ln(Ⅲ)complex.The coordination pattern of comlexes provides valuable information for structural studies of other ralated Schiff base complexes and they are employed in rarious potential fields.
Key words:copper(Ⅱ)-lanthanide(Ⅲ)complexes;Schiff base;crystal structure
0 Intruduction
3d-4f heterometallic complexes have attracted special attention in the last decades due to an interest in the physicochemical properties[1-2]. For example,magnetic properties[3-6],single molecular magnets[7-8]and photophysical properties[9-10]. In order to develop optical properties,heteronuclear lanthanide metals’ complexes have gain massive interest owing to their potential applications as Near-infrared molecular probes in bioimaging and biosensing[11],in light conversion devices[12]and catalytic applications[13]. The NIR luminescence studies of bimetallic Zn-Nd complexes display efficient photo-physical properties because of showing long emission/ luminescent life-times,ligand-induced large stokes shift,narrow emission lines,ligand-dependent luminescence sensitization and high luminescence quantum yields[14]. They are employed in various potential fields like,optical telecommunication[15],organic light emitting diodes (OLED)[16],fluoro-immunoassay and imaging[17].
There has been growing interest on 3d-4f complexes,which exhibit interesting luminescent and magnetic properties. Recently,heteromultinuclear 3d-4f Schiff base complexes have been employed as building blocks to form extended structure. Most of the research attention is placed on the luminescence of terbium (Tb3+)and europium (Eu3+)complexes,which emit in the visible region (green for Tb3+and red for Eu3+)and can be used as pure green or red EL devices.[18-19]There is also currently some growing interest in synthesizing salen based bi-compartmental azomethine ligands that may be exploited for the formation of 3d/4f metals’ complexes,imbibed with photophysical properties[20-23]. In continuation to previous work,we have synthesized five new heterotrinuclear 3d-4f complexes by incorporating Cu2+,La3+,Ce3+,Pr3+,Nd3+and Sm3+ions in order to study their structure and properties.
1 Experimental
1.1 Chemicals and measurements
Solvents and starting materials were purchased commercially and used without further purification. Hydrated Lanthanide(Ⅲ)nitrate were prepared in the usual way by evaporating solution of the corresponding oxide (99.9%) in HNO3. Elemental analyses (C,H and N) were performed by the Perkin-Elmer 2400. Electronic absorption spectra in the UV-vis region were recorded on a UV-2501 UV-visible spectrophotometer. Infrared spectra (KBr pellets)were recorded on a Perkin-Elmer 60000 spectrometer. Fluorescence spectra were measured with a LS-55 fluorospectrophotometer.
1.2 Synthesis of the complexes
[N,N′-ethylenebis(salicylideneaminato)]copper(Ⅱ)(CuSaltn). The copper Schiff base complex was prepared as previously described.[24]Yield:5.857g,90%. M. p. >280 ℃. Anal. Calc for C17H16O2N2Cu:C,59.38;H,4.69;N,8.15. Found:C,60.08;H,4.49;N,8.21. IR (KBr pellets,cm-1):ν(C=N)1 615;ν(ArC-O)1 197. UV-vis (MeOH):λmax:272,360 nm.
(SaltnCu)2La(H2O)(NO3)3(1). The complex was prepared as follows:Addition of Cu(Saltn) (0.346 g,1.0 mmol)in 30 mL chloroform to a 10 mL methanolic solution of La(NO3)3·6H2O (0.216 g,0.50 mmol)induced the formation of a green precipitate,which was filtered off and washed with cold chloroform and diethyl ether. Yield:0.151 g,61%. M.p. >280 ℃. Anal. Calc for C34H34Cu2LaN7O14:C,40.33;H,3.19;N,9.68. Found:C,42.03;H,3.06;N,9.66. IR (KBr pellets,cm-1):ν(C=N)1 617;ν(ArC-O)1 210;νas(NO2)1 476;νs(NO2)1 285;ν(NO)1 034;ν(Ln-O) 449. UV-vis (MeOH):λmax:272,360 nm.
The compounds (SaltnCu)2Ln(H2O)(NO3)3(Ln = Ce (2),Pr (3),Nd (4),Sm (5))were prepared in a similar manner.
(SaltnCu)2Ce(H2O)(NO3)3(2). The complexes Ce(NO3)3·6H2O (0.217 g,0.50 mmol)and Cu(Saltn) (0.346 g,1.0 mmol)were used. Yield:0.378 g,76%. M.p. >280 ℃. Anal. Calc for C34H34Cu2CeN7O14:C,40.28;H,3.18;N,9.67. Found:C,41.56;H,3.15;N,9.66. IR (KBr pellets,cm-1):ν(C=N)1 618;ν(ArC-O)1 210;νas(NO2)1 476;νs(NO2)1 284;ν(NO)1 034;ν(Ln-O) 450. UV-vis (MeOH):λmax:273,362 nm.
(SaltnCu)2Pr(H2O)(NO3)3(3). The complexes Pr(NO3)3·6H2O (0.218 g,0.50 mmol)and Cu(Saltn) (0.346 g,1.0 mmol)were used. Yield:0.378 g,76%. M.p. >280 ℃. Anal. Calc for C34H34Cu2PrN7O14:C,40.20;H,3.18;N,9.41. Found:C,41.56;H,3.66;N,9.65. IR (KBr pellets,cm-1):ν(C=N)1 618;ν(ArC-O)1 210;νas(NO2)1 476;νs(NO2)1 284;ν(NO)1 034;ν(Ln-O) 450. UV-vis (MeOH):λmax:273,358 nm.
(SaltnCu)2Nd(H2O)(NO3)3(4). The complexes Nd(NO3)3·6H2O (0.219 g,0.50 mmol)and Cu(Saltn)(0.346 g,1.0 mmol)were used. Yield:0.378 g,76%. M.p. >280 ℃. Anal. Calc for C34H34Cu2NdN7O14:C,40.17;H,3.17;N,9.41. Found:C,41.25;H,3.16;N,9.63. IR (KBr pellets,cm-1):ν(C=N)1 618;ν(ArC-O)1 210;νas(NO2)1 476;νs(NO2)1 284;ν(NO)1 034;ν(Ln-O) 450. UV-vis (MeOH):λmax:272,361 nm.
(SaltnCu)2Sm(H2O)(NO3)3(5). The complexes Sm(NO3)3·6H2O (0.222 g,0.50 mmol)and Cu(Saltn) (0.346 g,1.0 mmol)were used. Yield:0.378 g,76%. M.p. >280 ℃. Anal. Calc for C34H34Cu2SmN7O14:C,39.19;H,3.29;N,9.41. Found:C,41.56;H,3.58;N,9.66. IR (KBr pellets,cm-1):ν(C=N)1 618;ν(ArC-O)1 209;νas(NO2)1 477;νs(NO2)1 283;ν(NO)1 032;ν(Ln-O) 449. UV-vis (MeOH):λmax:272,360 nm.
1.3 X-ray structure determination
Diffraction data were collected on a Rigaku Raxis-Rapid single X-ray diffractometer at 296 K,respectively,in the rotation scan mode using graphite-monochromatized Mo-Kα radiation (λ= 0.071 073 nm). The structures were solved by direct methods with theSHELXS-97package and refined by full-matrix least squares on F2(SHELXL-97).[25]Pertinent crystallographic data of complex5are summarized in Table 1.
2 Results and discussion
2.1 Crystal structure
The crystal structure of5was determined by single-crystal X-ray diffraction analyse. The crystallographic data are summarized in Table 1.crystallizes5in the triclinic system,space group P ī,witha= 1.046 1(2)nm,b= 1.483 8(3)nm,c= 1.503 8(3)nm,α= 104.54(3)°,β= 99.40(3)°,γ= 99.13(3)°,Z= 2. Least-squares refinement of the structure led to a conventional R factor of 0.047 6. The unit cell contains two discrete (CuSaltn)2Sm(H2O)(NO3)3entities (Fig.1). A view of the molecular structure is shown in Fig.2. This structure consists of two Cu(Saltn)moieties bound to the Sm(Ⅲ)ion via the two phenolic oxygen atoms of saltn. The bridging network SmO1O2Cu has a butterfly shape:taking O1O2 as the hinge,the SmO1O2 and CuO1O2 planes make a dihedral angle of 146.5(2)° (the SmO3O4 and CuO3O4 planes make a dihedral angle of 149.9(2)°). In the trinuclear species the Sm(Ⅲ) ion is nine-coordination. The Sm environment is composed of nine oxygen atoms,four belonging to saltn,four belonging to the two nitrate anions in bidentate coordination and one coming from one water molecule. The third nitrate ion is coordinated to one of the two copper atoms of Cu(Saltn). The Cu(Ⅱ)ion has square planar environment with the N2O2 donor atoms of the ligand. The Sm coordination sphere can be viewed as a tricapped trigonal prism (Fig.3). Selected bond lengths and angles are given in table 2. The intramolecular metal-metal separations are 0.339 4(4) nm for Cu1-Sm,0.335 4(4)nm for Cu2-Sm and 0.380 7(5)nm for Cu1-Cu2. The angle Cu1-Sm-Cu2 is equal to 68.68(17)°.
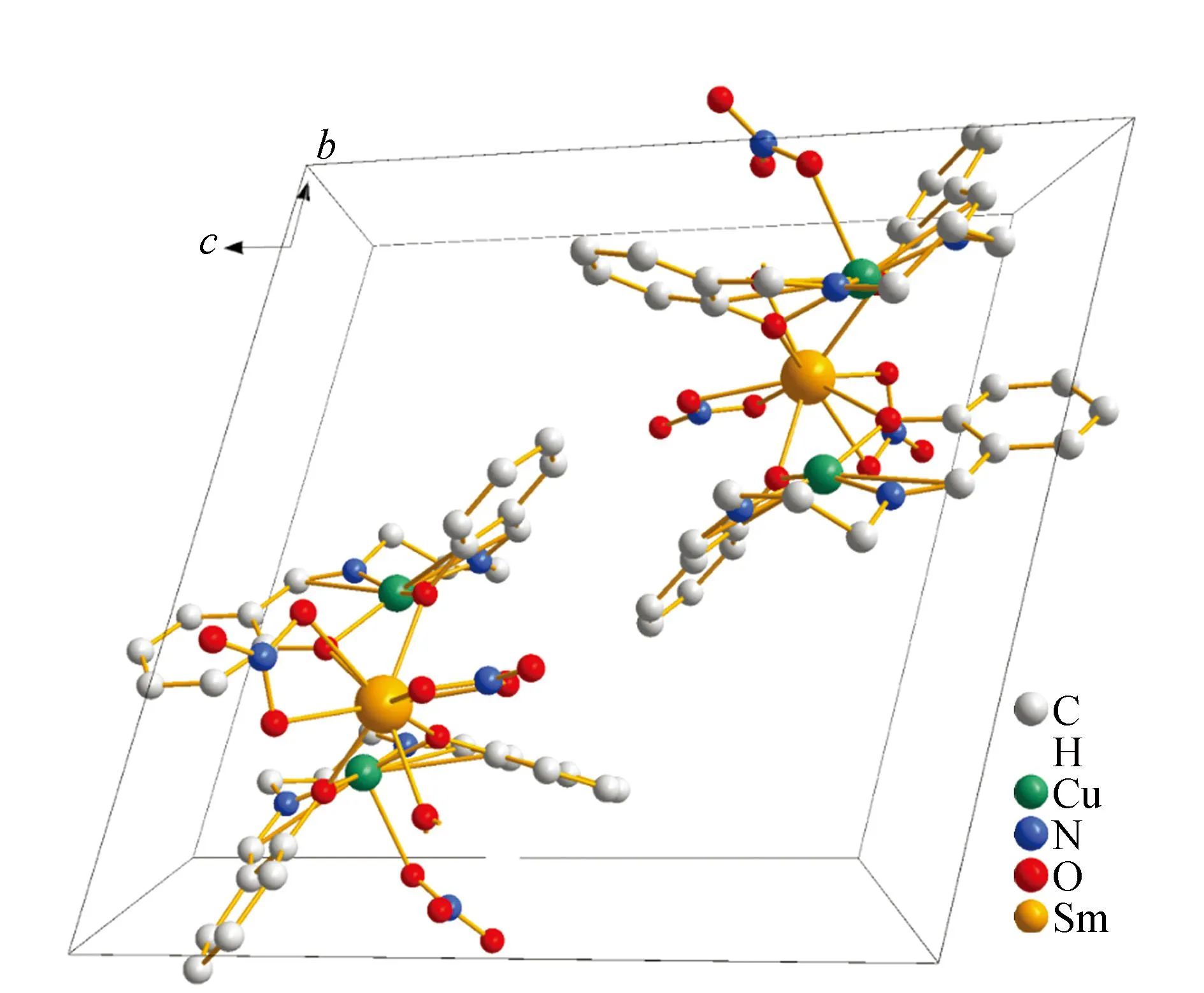
Fig.1 Packing diagram of crystal structure of 5
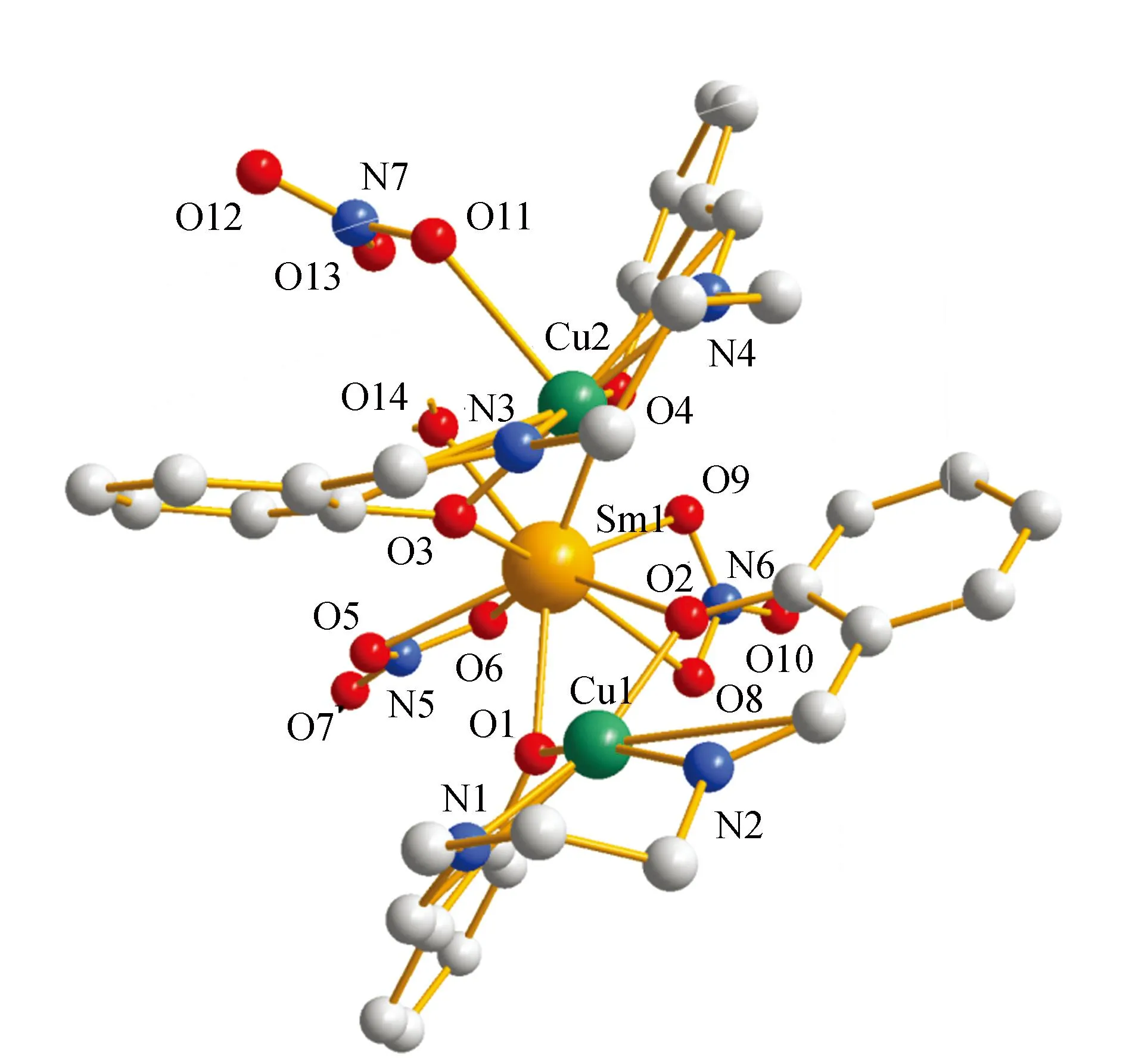
Fig.2 Molecular structure of complex 5
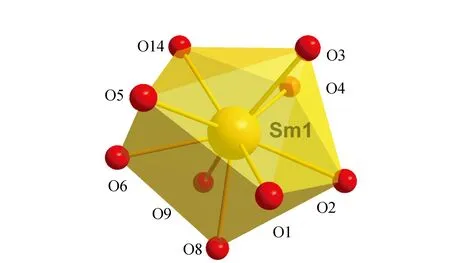
Fig.3 Tricapped trigonal prism coordination of Sm(Ⅲ)ion in 5

Table 1 Crystal datas and structure refinement
To the best of knowledge,analogous compound with isolated Cu(Ⅱ)-Gd(Ⅲ)pairs has already been reported.[22]That is (CuSaltn)2Gd(H2O)(NO3)3,where Nine Gd-O bonds vary from 0.238(1)to 0.255(5) nm,while the Sm-O bonds are larger [0.238 1(4)to 0.258 2(5)nm],as expected.
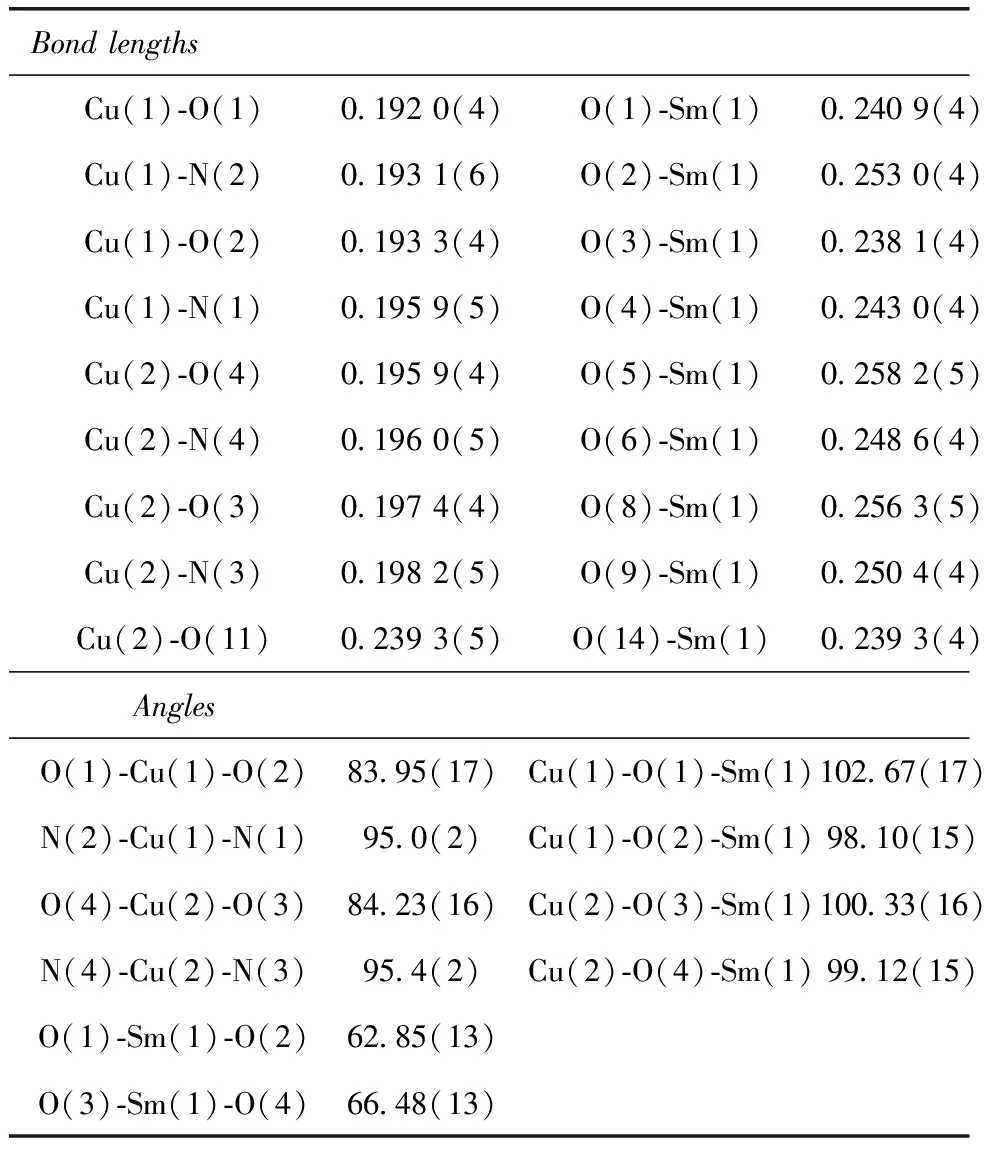
Table 2 Bond lengths [nm]and angles [deg]for 5
2.2 Characterization and spectroscopic studies
To further characterize the structures of the free ligand (Fig.S1)and its five d-f complexes1~5,the IR spectroscopic data (Fig.4)were studied. Free ligand shows three characteristic bands at 1 615,1 197 and 1 483 cm-1owing to vibration of ν(C=N),ν(Ar-O) and the carbon skeleton of benzene ring,respectively. The complexes showed an Ln-O stretching frequency around 450 cm-1. Coordination of the phenolic oxygen was indicated by a significant decrease in the C-O stretching frequency (Δν ~ 70 cm-1).[26]Large and strong bands at 1 476 cm-1for complexes (νasNO2)confirm the presence of nitrate ions. Their νsNO2counterparts appear at 1 284 cm-1,respectively. The difference between the two vibrations (Δν ~ 195 cm-1)is consistent with an η2 bidentate chelation of the nitrate ion and indirectly supports their coordination to the lanthanide ion.[27]UV spectrum of the complexes showed two new absorption bands in methanol about 272 and 360 nm,which were absent for free ligand (Fig.5). Apparently these new bands were attributed to the electron transfer between the metal center and the coordinated ligand.
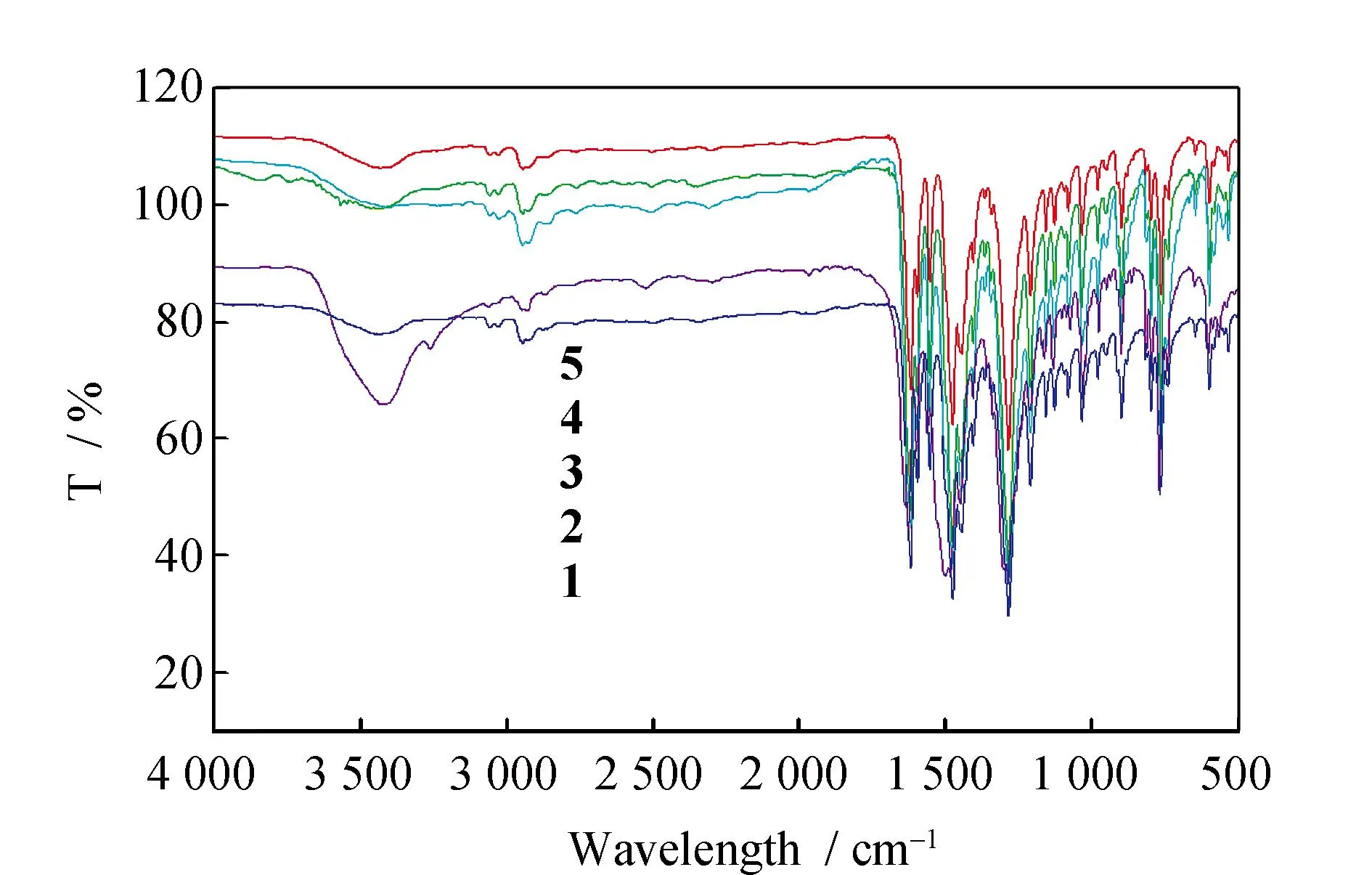
Fig.4 IR spectrum of complexes 1~5
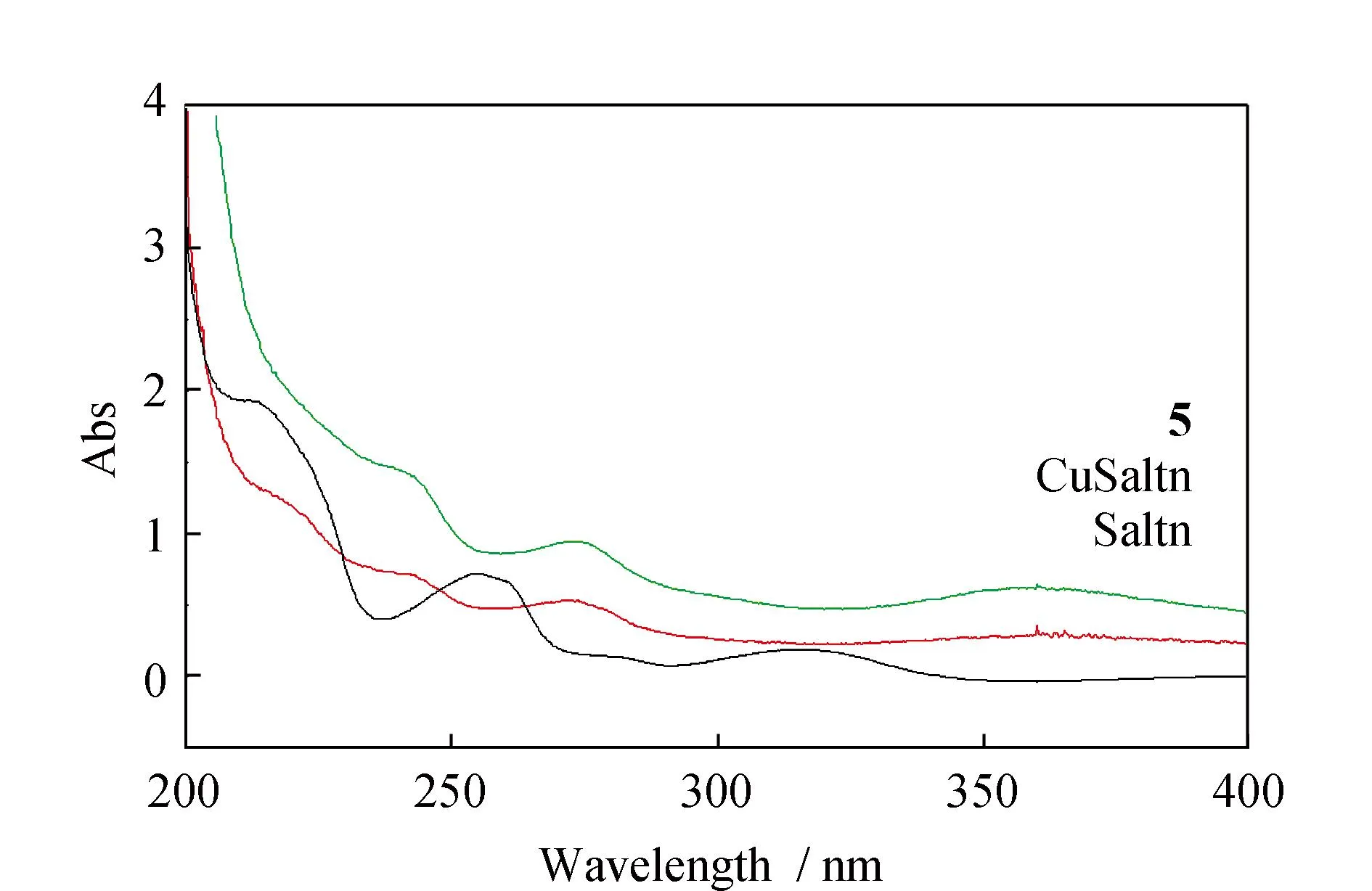
Fig.5 UV-vis spectrum of Saltn,CuSaltn and complex 5
2.3 Thermal properties
In the investigation of the thermal stabilities (Fig.6),it is found complexes1~5remain high thermal stability at 280 ℃. These complexes explosively decomposes when heated to around 300 ℃,resulting in a weight loss of about 35%,which could be ascribed to the removal of the organic joint of the Schiff base ligands. The further increase of temperature causes the decomposition of the remaining solid organic parts. The weight loss did not stop after reaching 650 ℃,indicating that the final products (CuO and Ln2O3)had not been completely formed.
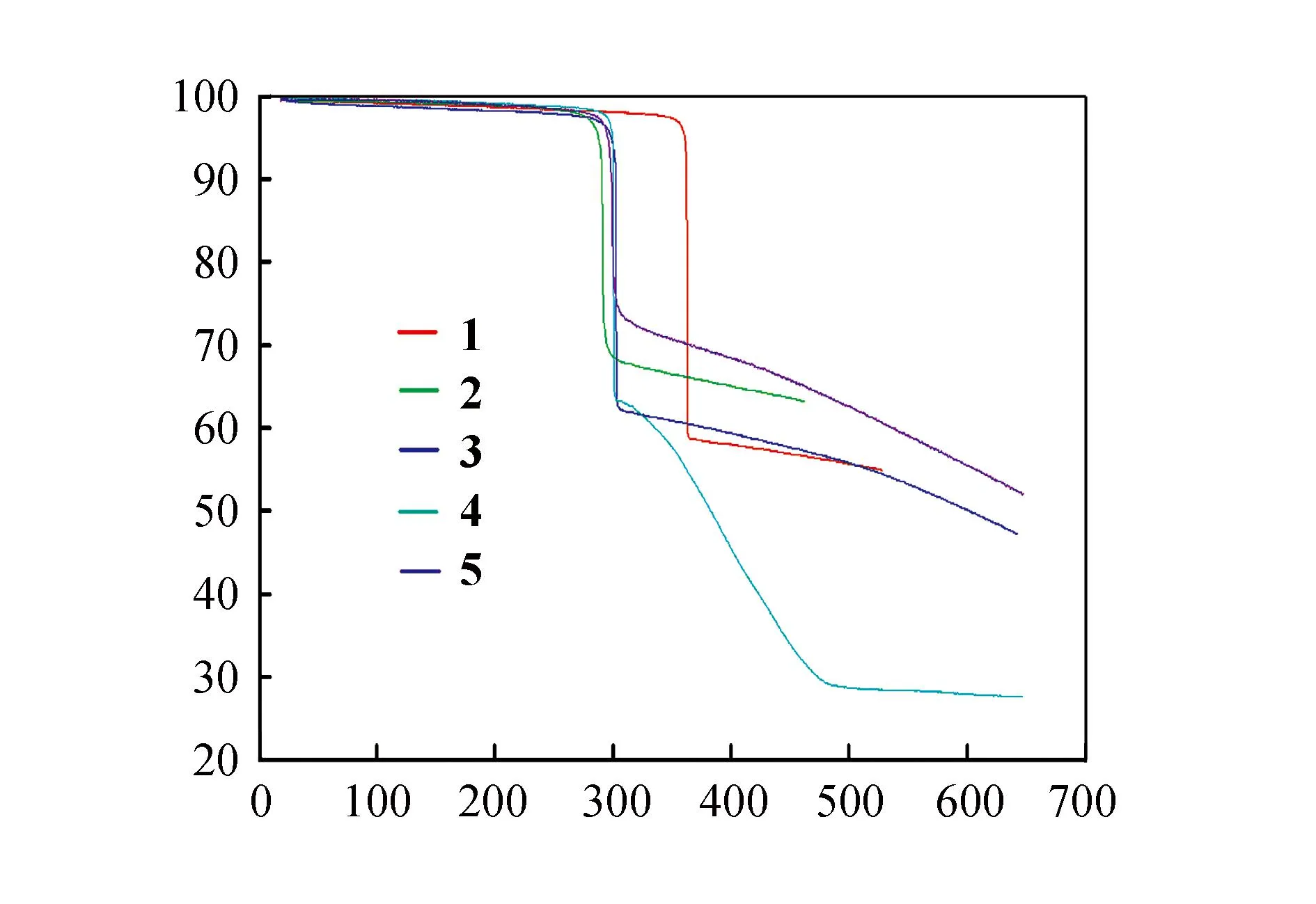
Fig.6 TGA curves of complexes 1~5
2.4 PXRD analysis
Powder X-ray diffraction (PXRD)analysis reveal that patterns of complexes1~5are in agreement with the simulated ones (Fig.7)and there are different crystalline solvent molecules in complexes1~5. PXRD analysis further demonstrates that the crystal structures of complexes1~5are truly representative of the bulk materials. The differences in intensity are due to the preferred orientation of the powder samples.

Fig.7 Powder X-ray diffraction patterns and simulated patterns of complexes 1~5
2.5 Fluorescence spectra
The fluorescence intensity of complexes markedly decreased on forming the trinuclear Cu(Ⅱ)-Ln(Ⅲ)complexes. At room temperature,the fluorescence spectra of5was compared with that of the mononuclear Cu(Saltn)complex,in order to investigate the effect of an adjacent Cu(Ⅱ)ion on the fluorescence of Sm(Ⅲ) (Fig.8). The mononuclear Sm(Ⅲ)complex should show the fluorescence bands attributable to the4G5/2→6H5/2,4G5/2→6H7/2,4G5/2→6H9/2,4G5/2→6H11/2transitions at 565,601,645,712 nm respectively. The intensities of these bands are sognificantly decreased in the Cu(Ⅱ)-Sm(Ⅲ)complex. It was well known that the luminescent intensity decreases by radiationless energy loss through the excited state of other molecules which are near the exiting species[28]. From these considerations,we presume that the fluorescence intensity of the present Cu(Ⅱ)-Ln(Ⅲ)complex is effectively quenched by the enery transfer from the excited Ln(Ⅲ)to the Cu(Ⅱ) centre through the bridging oxygen atoms,although the fluorescence decrease resulting from the absorption of exciting light by the Cu(Ⅱ)-Ln(Ⅲ)complex itself also cannot be ruled out[29].
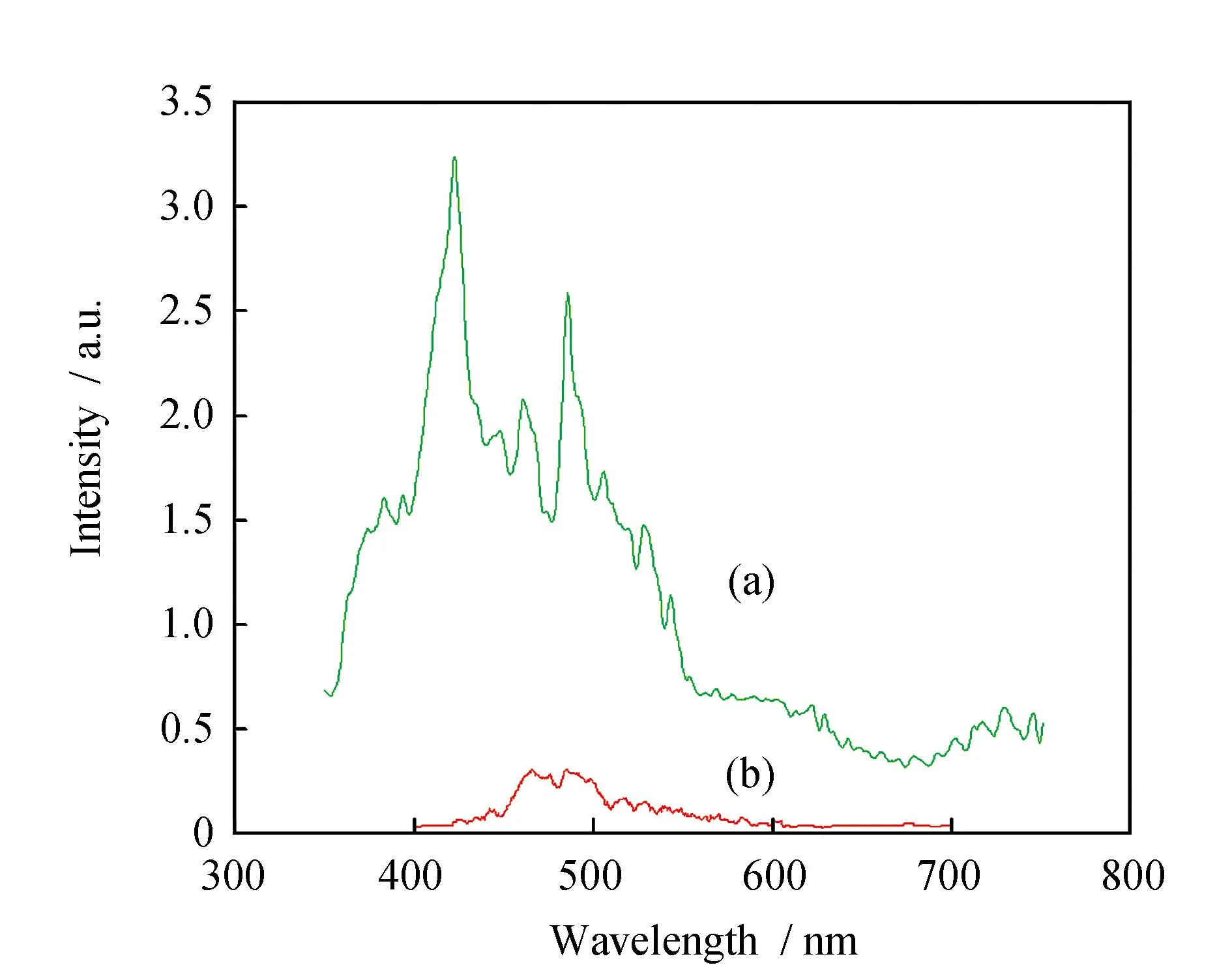
Fig.8 Fluorescence spectra of (a)Cu(Saltn)and (b)coordination of the Sm(Ⅲ)ion in 5
The NIR luminescence of the complex4has been investigated in CH3CN and CH3OH. The emission spectrum of complex (Fig.9)presents three bands at 896,1 060 and 1 312 nm,which can be assigned to4F3/2→4I9/2,4F3/2→4I11/2and4F3/2→4I13/2transitions of the Nd(Ⅲ)ion. The lifetime cannot be obtained due to the weakness of the signal and the limitations of our equipment. In addition,The results reveal again the enhanced energy transfer from the ligand to the Ln(Ⅲ)ion in the solid state compared to that in solution. The NIR luminescence intensity of complexes4in CH3CN is stronger than that in CH3OH. This is attributed to the O-H oscillators in CH3OH,coupling of overtones and the excited state of Nd(Ⅲ)ion,resulting in the nonradiative transition,which quench the NIR luminescence.
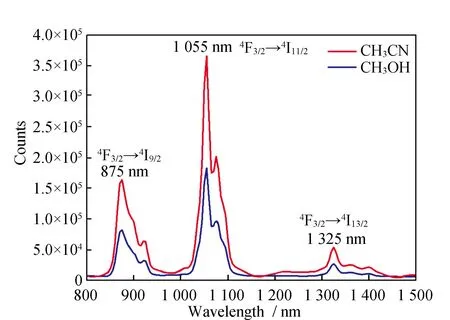
Fig.9 The NIR emission spectrum of complexe 4 in CH3CN and CH3OH
3 Conclusions
In summary,a series of 3d-4f heterobimetallic Cu(Ⅱ)-Ln(Ⅲ)(Ln = La,Ce,Pr,Nd,Sm)Schiff base complexes have been prepared. Elemental analysis,thermal analyses,IR,UV and fluorescence Spectra data of complexes show that their structures agree with their solid-state structures. The fluorescence of Saltn ligand markedly decreased on forming the Cu(Ⅱ)-Ln(Ⅲ)complexes. The coordination pattern of complex5revealed by X-ray analysis provides valuable information for structural studies of other related lanthanide complexes.
