Carbon Nitride Quantum Dots: A Novel Fluorescent Probe for Non-Enzymatic Hydrogen Peroxide and Mercury Detection
2023-12-21CHENLeiLIQuanWANGXingWANGWentaiandWANGLisha
CHEN Lei, LI Quan, WANG Xing, WANG Wentai, and WANG Lisha
Carbon Nitride Quantum Dots: A Novel Fluorescent Probe for Non-Enzymatic Hydrogen Peroxide and Mercury Detection
CHEN Lei#, LI Quan#, WANG Xing, WANG Wentai, and WANG Lisha*
The mercury species in the ocean (MeHg, Hg2+) will be enriched in marine organisms and threaten human health through the food chain. While the excessive H2O2in the metabolic process will produce hydroxyl radicals and accelerate the aging of human cells, causing a series of diseases. Hence, the cost-effective and rapid detection of mercury and H2O2is of urgent requirement and significance. Here, we synthesized emerging graphitic carbon nitride quantum dots (g-CNQDs) with high fluorescence quantum yield (FLQY) of 42.69%a bottom-up strategy by a facile one-step hydrothermal method. The g-CNQDs can detect the H2O2and Hg2+through the fluorescence quenching effect between g-CNQDs and detected substances. With the presence of KI, g-CNQDs show concentration-dependent fluorescence toward H2O2, with a wide detection range of 1–1000μmolL−1and a low detection limit of 0.23μmolL−1. The g-CNQDs also show sensitivity toward Hg2+with a detection range of 0–0.1μmolL−1and a detection limit of 0.038μmolL−1. This dual-function detection of g-CNQDs has better practical application capability compared to other quantum dot detection. This study may provide a new strategy for g-CNQDs preparation and construct a fluorescence probe that can be used in various systems involving H2O2and Hg2+, providing better support for future bifunctional or multifunction studies.
carbon nitride; quantum dots; hydrogen peroxide; Hg2+; fluorescence probe
1 Introduction
Polymeric graphitic carbon nitride (g-C3N4) is considered an excellent metal-free photocatalyst for water splitting and pollutant degradation. It has attracted the attention of scientists due to its graphene-like structure, unique physicochemical properties, especially the suitable bandgap, and visible light response (Inagaki., 2019; Wang., 2020). However, the low specific surface area and poor conductivity of g-C3N4limit its wide applications. Hence, various strategies were applied to improve the properties of g-C3N4, including modifications and structural engineering, developing g-C3N4with porous, spherical, nanorods, nanosheets, or nanodots structures (Rajeshwari., 2021). People found that the g-C3N4may exhibit unique fluorescence properties when the particle size is minimized to a certain scale, such as the tiny g-C3N4nanosheets and g-C3N4quantum dots (g-CNQDs), therefore, leading to the broader application of g-C3N4in bioimaging (Zhuang., 2018), QLED displays (He., 2019) and fluorescence sensing (Yin., 2017). Compared with other carbon-based or metal-based quantum dots, g-C3N4quantum dots (QDs) show higher fluorescence quantum yield (FLQY) and better biocompatibility, which makes it has better prospects in the bio-applica- tions (Ahmed., 2019; Castro., 2019).
Top-down methods are the traditional approaches for g-CNQDs synthesis, including chemical exfoliation, soni- cation, hydro/solvothermal, evaporation-condensation, and hydrolysis treatment of bulk g-C3N4(Wang., 2014, 2020). The synthesis procedure is usually costly, and time-consuming, with low yield and low FLQY, and requires strong oxidizing acids in chemical exfoliation. The bottom-up route can achieve the large-scale synthesis of g-CNQDs, using carbon and nitrogen sources as precursors to obtain high-quality g-CNQDs with a smaller particle size that shows higher FLQY (Qian., 2014). The g-CNQDs with stable physical and chemical properties, good biocompatibility, and high quantum yield are effective sensing platforms for biological and environmental detection, such as the ‘off-on’ g-CNQDs fluorescent probe for mercury (II) and sulfide determination and the g-CNQDs with highly blue emission for the detection of trace amounts of ferric ions and imaging biological cells probes (Yin., 2017; Wang., 2018).
Hg2+is one of the toxic heavy metal ions that will cause kidney failure, neurasthenia, and other diseases. Moreover, Hg2+can be methylated to methyl mercu- ry (MeHg) in the ocean through various biochemical pro- cesses, which is more toxic than other heavy metal ions, affecting the ecological balance of the marine environment and enriched in the human body through the food chain (Jinadasa., 2021). Recently, Elumalai. (2023) synthesized highly green fluorescent CQDs via a one-step facile hydrothermal method using a chicken feather as a carbon source, which exhibits a high selectivity reproducibility, and anti-interference ability. The fluorescence probe can detect the Hg2+efficiently, which is more convenient and low-cost than X-ray fluorescence spectroscopy, atomic absorption spectroscopy, inductively coupled plasma mass spectrometry, and electrochemical analysis (Wang., 2021). Similar to various carbon dots, metal-based quantum dots, and fluorescent conjugated polymers (Li., 2013; Wang., 2015; Xi., 2016), the g-C3N4nanosheets and quantum dots also exhibit good sensitivity toward Hg2+(Barman and Sad- hukhan, 2012).
Hydrogen peroxide (H2O2) is a significant chemical substance in the chemical industry, environmental industry, food industry, and life science. The determination of a trace amount of H2O2is of great significance for clinical diagnosis and biological life science. The H2O2not only adjusts the physiological process of the cells but also has a critical relationship with cell oxidative damage and aging, leading to diseases. In the past decades, various methods for H2O2detection have been investigated. For example, the colorimetric method for trace H2O2detection by the sequential addition of H2O2and TMB to Ce(OH)CO3powder (Lian., 2021), the electroche- mical sensor for H2O2detection using transition metal phosphides as cathodes (Wang and Zhang, 2020), and the fluorescence method by quenching the fluorescence system with the substance being detected (Dong., 2020). Moreover, enzymes such as horseradish peroxidase (HRP), and glucose oxidase (GOx) can be applied in the electrochemical or fluorescence detection of H2O2to achieve high sensitivity and selectivity (Lu., 2019; Liu., 2020). However, the drawbacks of high cost and easy inactivation of enzymes greatly restrict the applications of enzymatic sensing of H2O2, which makes non- enzymatic techniques highly desired.
Currently, most studies on non-enzymatic detection of H2O2are using electrochemical strategy (Peng., 2020). The non-enzymatic electrochemical sensing platforms require various electrode materials such as precious metals, metal oxides, and other novel composites (Dong and Zheng, 2020; Lu., 2022). But the non-enzymatic fluorescence sensing systems show the advantages of low costs, good selectivity, sensitivity, and portability. Therefore, the non-enzymatic fluorescence sensing platforms are developed. Sadhukhan. (2014) reported excellent non-enzymatic fluorescence carbon quantum dots for H2O2determination. The carbon quantum dots shows a linear range of 10 to 5mmolL−1and a detection limit of 700nmolL−1, indicating the feasibility of non-enzymatic fluorescence sensing of H2O2. The fluorescence g-C3N4has been widely applied in the sensing of metal ions or biological substances (Bian., 2016; Li., 2019), but its application in non-enzymatic H2O2determination has not been investigated to the best of our knowledge.
Herein, we successfully synthesized graphitic carbon nitride quantum dots (g-CNQDs) through a facile and low-cost bottom-up approach and investigated its performance as a non-enzymatic fluorescence probe for both H2O2and Hg2+determination. The sensing mechanism was also discussed and illustrated. This study may provide a new sensing platform and choice for H2O2detection in various biomedical applications that broaden the application of g-CNQDs.
2 Experiment
2.1 Chemicals and Reagents
Sodium citrate (AR, 500g), urea (AR, 500g), hydrogen peroxide (AR, 30%, 500mL), and Potassium iodide (AR, 500g) were obtained from Sinopharm Chemical Reagent Co., Ltd. The Quinine sulfate fluorescence standard substance (AR, 98.6%, 250mg) was obtained from Shanghai Aladdin Bio-Chem Technology Co., Ltd. and applied as a reference standard with fluorescence quantum yield (FLQY) of 56% to determine the FLQY of as-prepared g-CNQDs. The deionized water (DI) was self-made from laboratory instruments. All chemicals and reagents were used as obtained without further purification.
2.2 Instruments
The morphology of g-CNQDs was captured by transmission electron microscope (TEM) on the Tenchi G2 F20 S-TWIN transmission electronic microscope (FEI, USA). The D8-Advance X-ray diffractometer (Bruker Co., Germany) was applied for the X-ray diffraction (XRD) analysis. The X-ray photoelectron spectroscopy spectra (XPS) were obtained by Escalab 250 X X-ray photoelectron spectrometer (VG Scientific Co., USA). Fourier tran- sform infrared (FT-IR) spectra were collected by Avatar 360 FT-IR spectroscope (Nicolet Instrument Co., USA). The UV-vis spectra and FL spectra were collected by UV- 2450 spectrophotometer (Shimazu Co., Japan) and F-4600 FL spectrophotometer (Hitachi Co., Japan), respectively.
2.3 Synthesis of g-CNQDs
The g-CNQDs were prepared by hydrothermal method using sodium citrate and urea as precursors. Typically, 1.5 g of sodium citrate and 1.2g of urea were mixed and dissolved in 25mL deionized water, followed by the hydrothermal treatment in a 50mL Teflon lined stainless steel autoclave for 4h at 180℃. Afterward, the suspension was centrifuged to remove the particles with a larger size, and the supernatant was further purified through dialysis for 48h. Finally, the g-CNQD solution was concentrated and then stored in the refrigerator at 4℃for further characterization and applications. The fabrication process of the g-CNQDs was shown in Fig.1.
Fig.1 The fabrication process for g-CNQDs.
2.4 H2O2 Sensing
The typical procedure of the H2O2detection is as follows: 1mL of as-prepared g-CNQDs and 0.5mL of KI solution (1mmolL−1) were dispersed in 8mL of phosphate buffer solution (0.02molL−1, pH=5.0), followed by adding 0.5mL of different concentrations of H2O2. The resultant solution was mixed and incubated for 30min at room temperature. Then the fluorescence spectra were recorded under the excitation wavelength of 355nm with an emission peak of 440nm.
尽管各个高校财务部门想尽种种办法,但因每天财务部门所能处理的总体业务量有限,只能在排队时间和人数总量上稍微有所限制。财务预约报销 “排队时间长、手续繁琐、下班时仍有师生不愿离去”导致报账人员办理报销业务时和财务人员的矛盾冲突频发,探究其根本原因:
2.5 Hg2+ Sensing
The Hg2+determination tests were performed at room temperature. Theg-CNQDs solution with a given concentration was prepared before measurement. 2mL of g- CNQDs solution was transferred into a quartz cuvette followed by the addition of a calculated amount of Hg2+solution. After mixing uniformly and incubating for 30min, the PL emission spectra were collected.
3 Results and Discussion
3.1 Morphologies and Structures of g-CNQDs
The TEM images of as-prepared g-CNQDs were shown in Fig.2. It can be observed that the g-CNQDs have good monodispersity (Fig.2A) with a uniform size ranging from 1.5nm to 3.5nm and an average particle size of about 2.6nm (Fig.2C). The HRTEM image of g-CNQDs in Fig.2B shows low lattice fringes, indicating the amorphous nature of g-CNQDs. The inset of Fig.2B shows a lattice distance of 0.21nm, which is consistent with the (100) diffraction planes of graphitic carbon. As shown in Fig.2D, a broad diffraction peak was observed, indicating the low crystallinity graphitic nature of g-CNQDs, which is in accordance with the HRTEM result. A small peak at 11.2˚ corresponds to the planar structure of the nitrogen-linked triazine unit. The 2value of the broad diffraction peak is at about 27.1˚, which could be ascribed to the stacking of the conjugated aromatic layers, which corresponds to the (002) plane of graphite (Shorie., 2019).
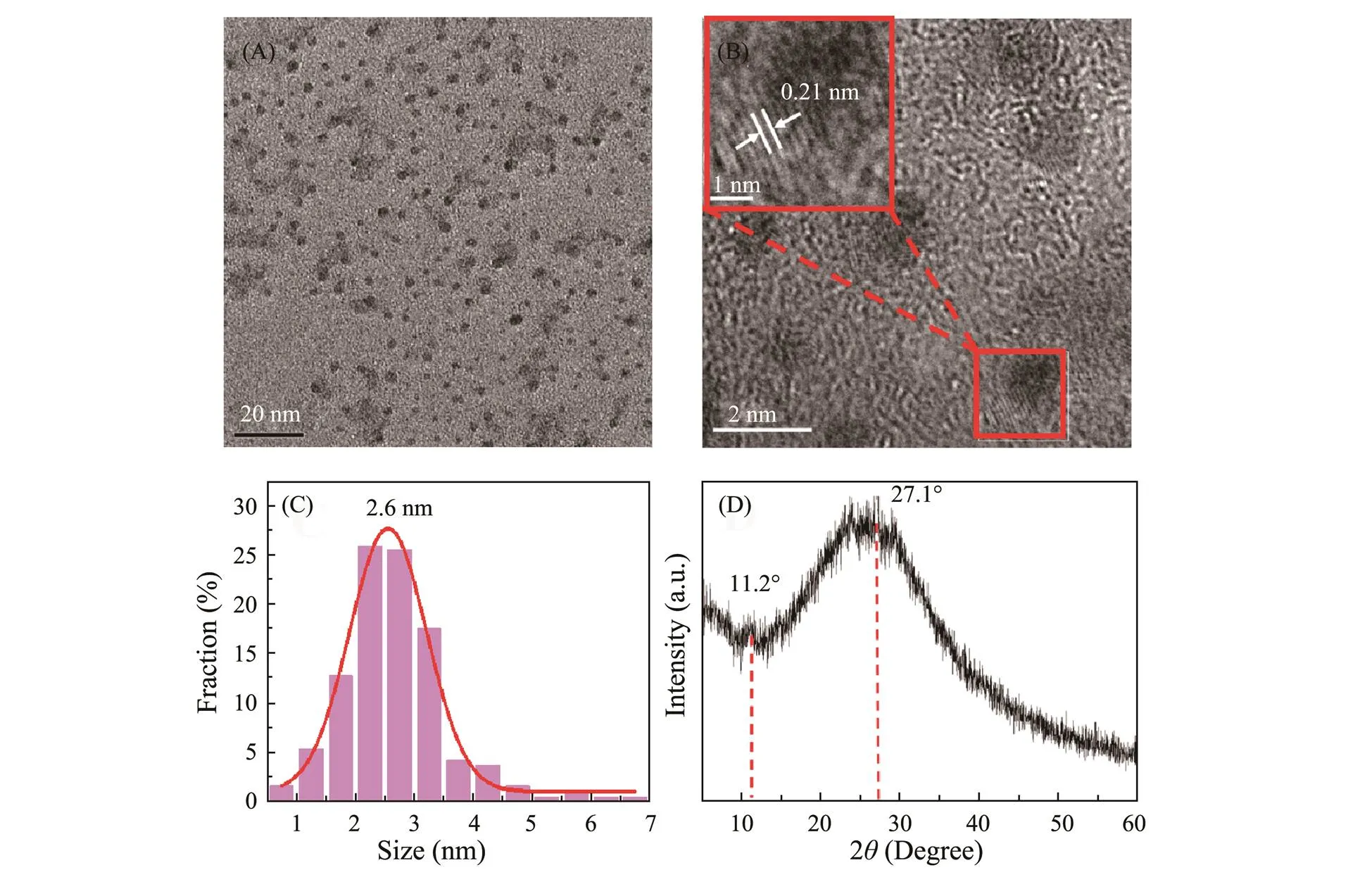
Fig.2 (A) The TEM image, (B) HRTEM image (inset: lattice fringe), (C) size distribution, and (D) XRD pattern of as-prepared g-CNQDs.
The structure of as-prepared g-CNQDs was analyzed by FTIR and XPS, respectively. The surface functional groups of g-CNQDs were characterized by FTIR and shown in Fig.3A. The stretching bonds of O-H and N-Hat the absorption bands of 3600cm−1and 3150cm−1lead to the excellent hydrophilicity of g-CNQDs. The stretching vibration of C-N is confirmed by the absorption bands centered at 1415cm−1and 1590cm−1. The peak at 770cm−1originated from the stretching vibration of the C-H group. The observed N-H and C-N bonds indicate the successful reaction between sodium citrate and urea. Noticeably, the characteristic absorption peak of the triazine ring appears at the absorption peak of 835cm−1, which is evidence of the formation of a graphitic carbon nitride stru- cture.
The XPS spectra can give more information about the surface elemental composition and chemical bond state of g-CNQDs, which can be used to confirm the formation of graphitic carbon nitride structure. In Fig.3B, the four peaks at 284.8, 399.9, 531.6, and 1027.6eV are attributed to C, O, N, and Na elements, with an elemental ratio of C: N:O:Na = 53:8:35:4, respectively. The C1s spectrum in Fig.3C shows four peaks at 284.6, 286, 287.6, and 288.6eV, which could be corresponded to the C–C, C–N, C=O, and O–C=O groups, respectively. The fitting peaks at 531.6 eV and 532.9 eV in Fig.3D are related to the C=O and O–C=O bonds, indicating that the surface of g-CNQDs contains oxygen-rich groups. Fig.3E shows three distinct peaks of N 1s spectrum at 399.7, 400.4, and 401.5eV, attributed to the C–N=C, N–(C)3and N–H groups, respectively (Zhuang., 2018). The XPS results also confirm the presence of triazine heterocyclic ring in the g-CNQDs, which is consistent with the FTIR results. Hence, the above characterization indicates the successful synthesis of g-CNQDs through the hydrothermal treatment of sodium citrate and urea.
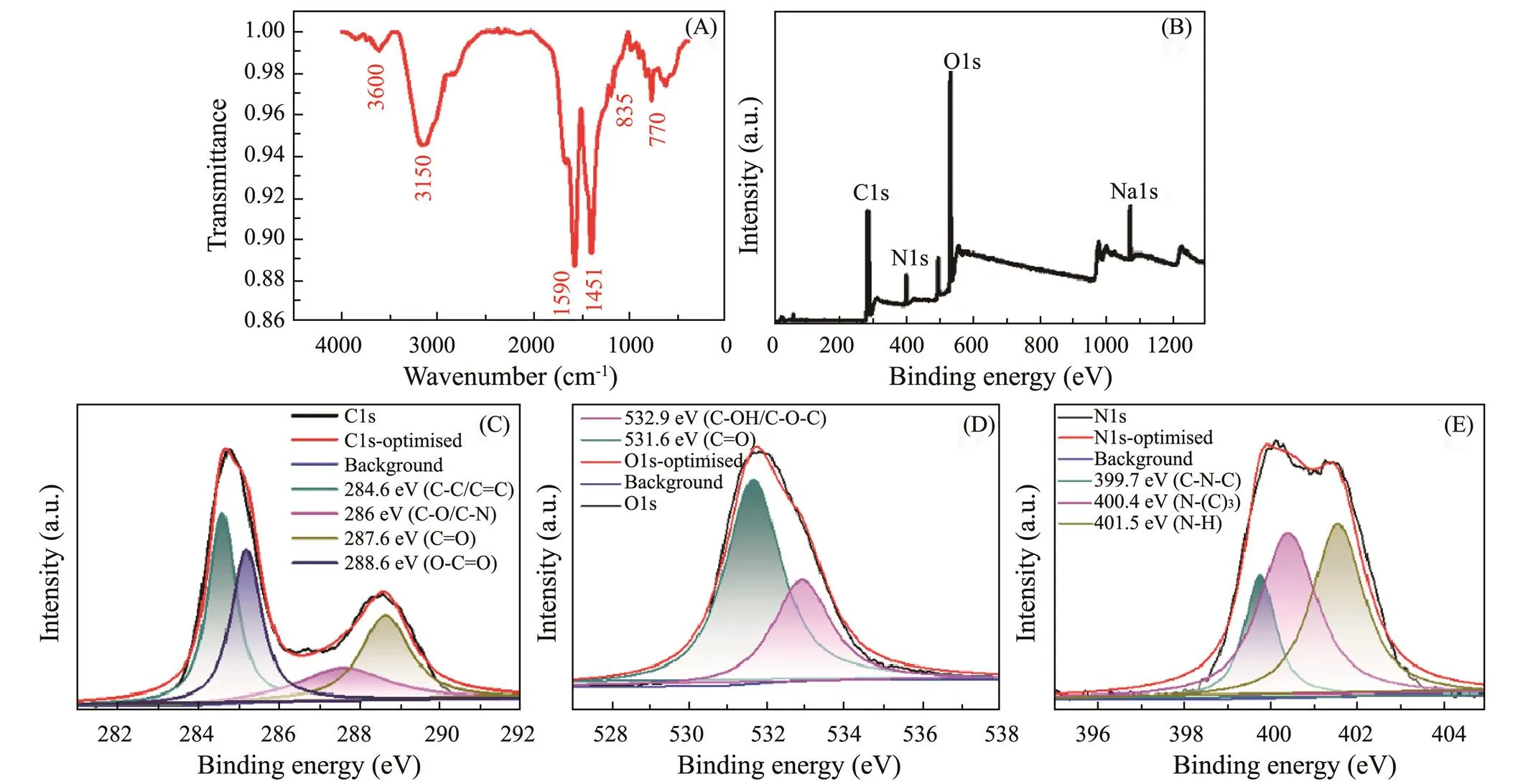
Fig.3 (A) FTIR spectrum, (B) XPS survey scan spectrum, (C) C 1s spectrum, (D) O1s spectrum and (E) N1s spectrum of as-prepared g-CNQDs.
3.2 Optical Properties of g-CNQDs
The UV-vis spectrometer and FL spectroscopy was applied to characterize the optical properties of g-CNQDs. In Fig.4A, the two distinct absorption peaks at 235nm and 340nm corresponds to the–π* electron transition and π–π* electron transition, respectively, owing to the conjugated structures of g-CNQDs, which is consistent with the results of FTIR and XPS. The inset of Fig.4A shows the bright blue light-emitting form g-CNQDs under the illumination of 365nm UV light. The inset in Fig.4A (left) shows the emission spectrum (red) at an excitation wavelength of 345nm UV irradiation and the excitation spectrum (black) at an emission wavelength of 440nm with a red shift in the emission wavelength. Fig.4B shows the 3D fluorescence scanning spectra of g-CNQDs showing the optimal excitation wavelength of 345nm and the maximum emission wavelength of 440nm. The fluorescence emission of g-CNQDs ranges from 400nm to 600nm.
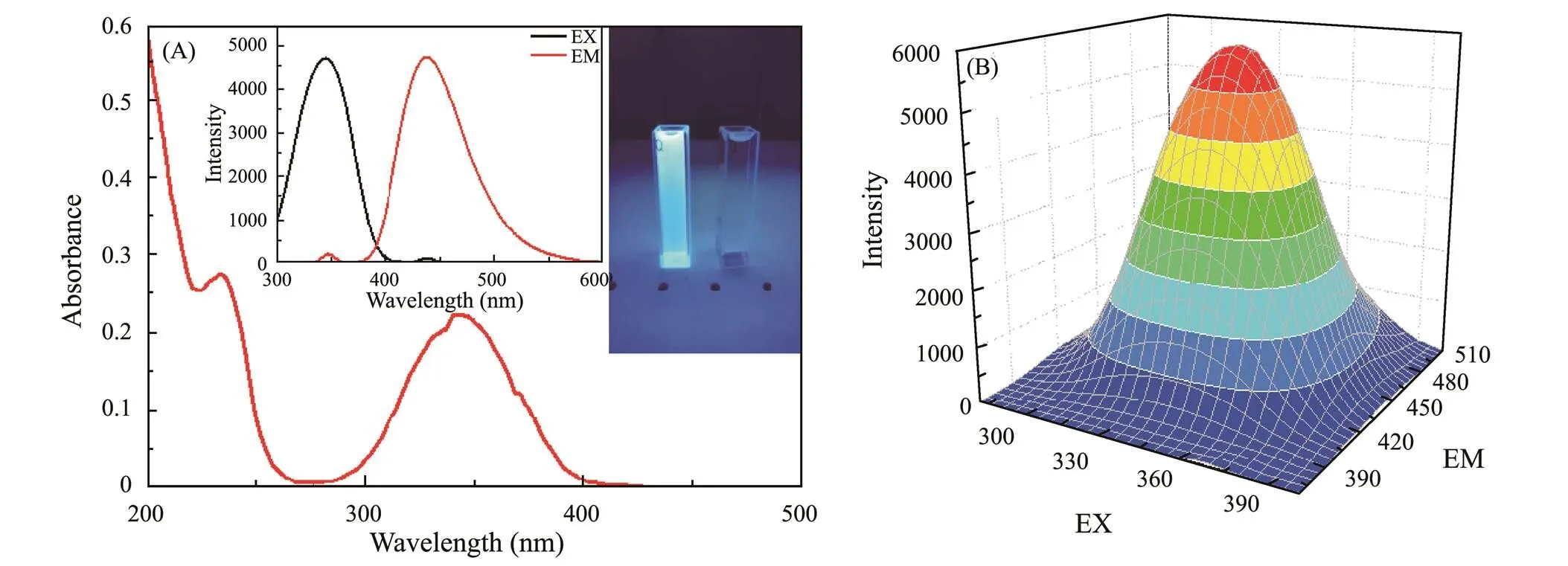
Fig.4 (A) UV-vis absorption spectra, inset: 365nm UV light exposure photo, (B) 3D fluorescent scanning spectra.
3.3 The Sensing of H2O2
Fig.5A shows the results of a series of comparative experiments to confirm the feasibility of H2O2detection with g-CNQDs. There is no fluorescence observed when only adding KI and H2O2without the presence of g- CNQDs. Adding KI or H2O2alone into the g-CNQDs solutions will not cause fluorescence quenching, indicating that KI or trace H2O2could not interact with g- CNQDs. The fluorescence of g-CNQDs can only be quenched by the simultaneous presence of KI and H2O2, confirming the feasibility of g-CNQDs for H2O2determination. Fig.5B shows concentration-dependent fluore- scence curves. The fluorescence intensity weakened gra- dually with the increase of H2O2concentration from 0 to 2.5mmolL−1. Fig.5C shows the curve of (0−)/0H2O2concentration, exhibiting a continuous growth of the fluorescence quenching efficiency with H2O2concentration and remains constant when concentrations are higher than 1.5mmolL−1. The inset of Fig.5C shows a linear response range of 1–1000μmolL−1, where the (0−)/0increases linearly with the concentration of H2O2. The linear relationship is described by the following equation of=0.0009+0.0163, with a correlation coefficient of2=0.999, and the standard error of the slope is 0.00001. The detection limit (LOD) is calculated as low as 0.23μmolL−1at a signal-to-noise ratio of 3 (The LOD is three times the standard deviation of blank solution divided by the slope of the concentration calibration curve).
The selectivity of this H2O2sensing system was investigated by the fluorescence response of various common interfering substances in the human body with a concentration of 1mmolL−1. As shown in Fig.5D, the ascorbic acid (AA), dopamine (DA), uric acid (UA), acetaminophen (AP), glucose, fructose, and maltose have negligible quenching on the fluorescence intensity, while the H2O2shows obvious fluorescence quenching, indicating the good selectivity of g-CNQDs for H2O2. These results indicate that the g-CNQDs have a high accuracy and selectivity in the sensing of trace H2O2. The detection performance of g-CNQDs toward H2O2was compared with other reported fluorescent probes as shown in Table 1. The non-enzymatic probes have a comparable linear range with the enzymatic probes, but the enzymatic probe shows a lower detection limit, which could be due to the high sensitivity of various enzymes. However, the g- CNQDs also show advantages in the comparable sensing performances, low cost, and facile preparation process, which makes it a promising H2O2probe.
3.4 Sensing of Hg2+
The sensing performances of as-prepared g-CNQDs toward Hg2+with different concentrations were investigated as well. Figs.6A and B shows the fluorescence sensitivity of g-CNQDs toward various metal cations with a concentration of 50μmolL−1, including Ag+, Ba2+, Ca2+, Cd2+, Co2+, Cu2+, Al3+, Fe3+, Fe2+, Hg2+, K+, Mg2+, Mn2+, Zn2+, and Na+, to illustrate the selectivity of as-prepared g-CNQDs. Most of the metal cations have a slight effect on the fluorescence intensity, but Hg2+can significantly quench the fluorescence of g-CNQDs, indicating the sensitivity of g-CNQDs toward the Hg2+. The selectivity experiments result in Figs.6C and D show the effect of Hg2+on the fluorescence in the presence of mixed metal cations, which exhibits a significant fluorescence quen- ching. While only a slight change in the fluorescence in- tensity of g-CNQDs when adding the mixed metal cations without Hg2+. These results confirm the high sensitivity and selectivity of g-CNQDs toward Hg2+, which makes g-CNQDs a probe for detecting Hg2+.
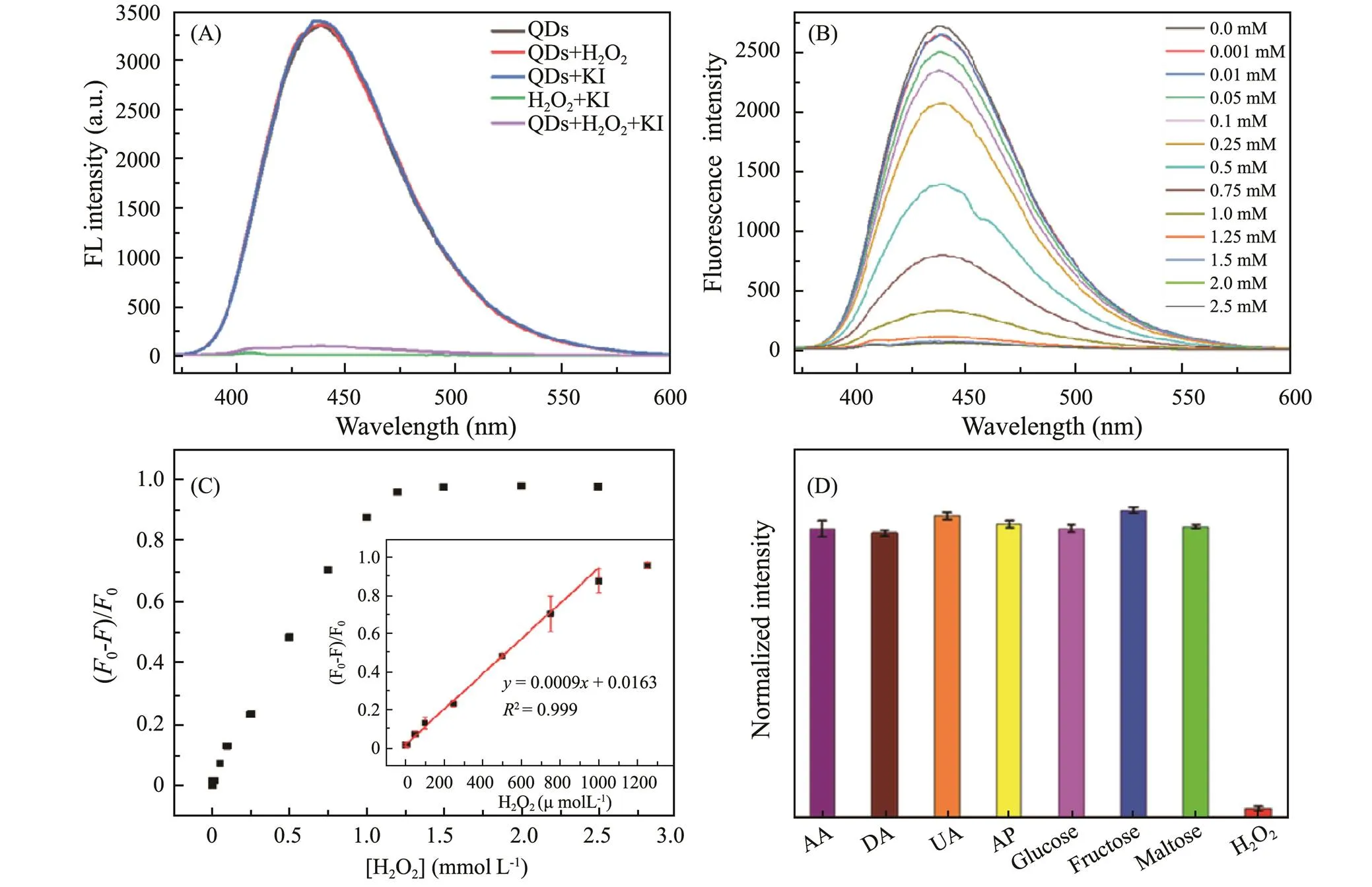
Fig.5 (A) Fluorescence spectra of g-CNQDs with the addition of KI and H2O2, (B) Changes of fluorescence intensity upon the addition of different concentrations of H2O2 (0−2.5mmolL−1), (C) (F0−F)/F0 corresponding to the different concentrations of H2O2 (inset: the linear plot between the (F0 −F)/F0 and the H2O2 concentration from 1 to 1000μmolL−1), (D) Selectivity of the H2O2 detection system against AA, DA, UA, AP, glucose, fructose, maltose and H2O2 with a concentration of 1mmolL−1.
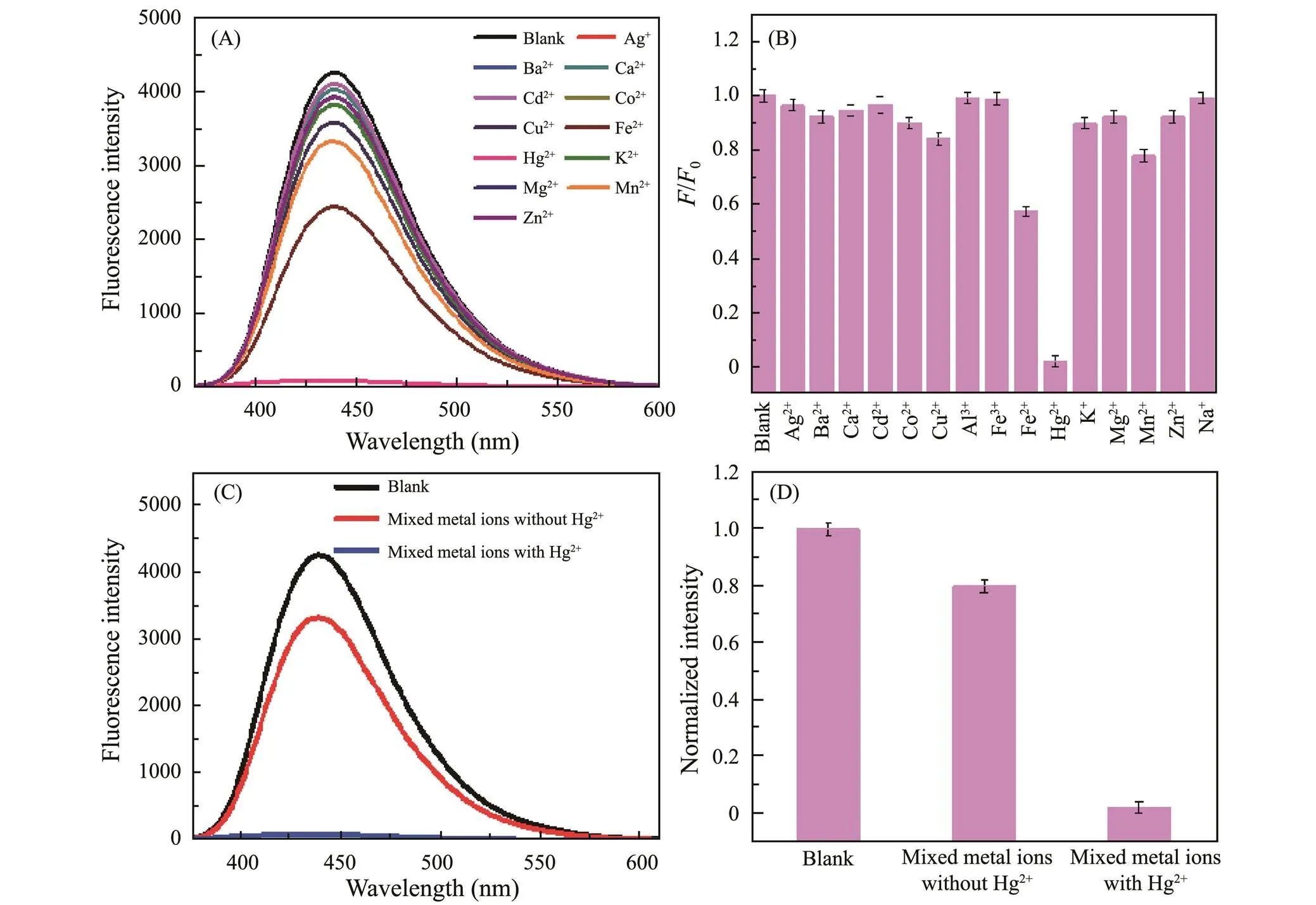
Fig.6 (A) Fluorescence spectra ofg-CNQDs after adding different metal cations, (B) Relative fluorescence intensity of g-CNQDs after addition of cations, (C) Fluorescence spectra for the selectivity experiments of mixed metal cations to Hg2+, (D) Normalized fluorescence intensity for the selectivity experiments.
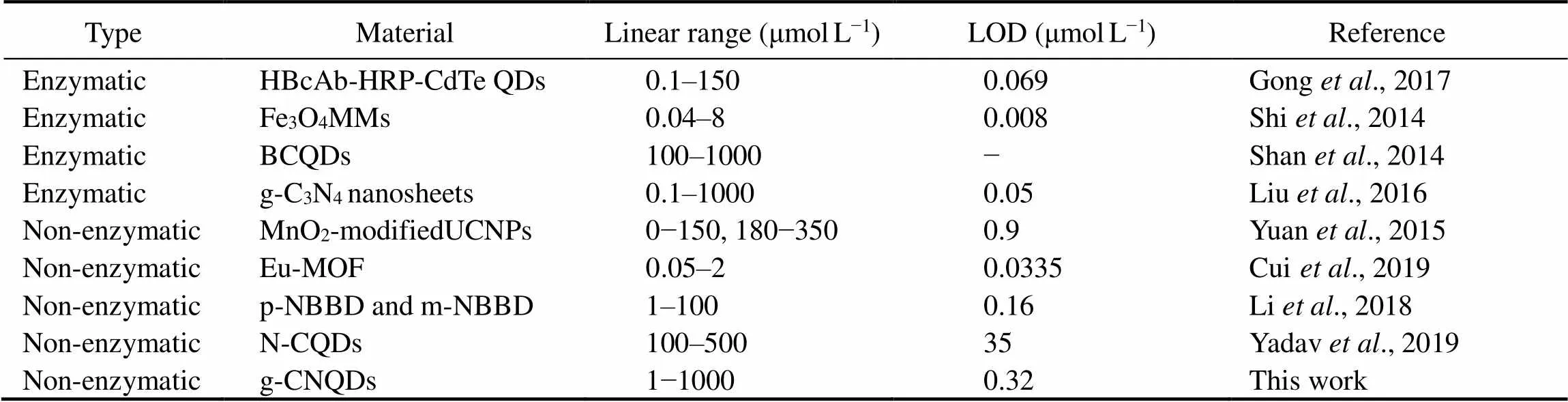
Table 1 Performance comparison of as-prepared g-CNQDs with other H2O2 fluorescent probes
The fluorescence intensities of g-CNQDs with various Hg2+concentrations from 0μmolL−1to 50μmolL−1were recorded in Figs.7A and B shows the enlarged details in lower Hg2+concentrations. The fluorescence intensity of g-CNQDs gradually decreases as the Hg2+concentration increase. Fig.7C shows the relationship between the0/valueHg2+concentration. And the0/valueHg2+concentration does not follow a linear relationship in the Hg2+concentration range of 0–1μmolL−1. However, Fig.7D shows a linear relationship in a lower concentration range of 0–0.10μmolL−1. The data were fitted by the Stern-Volmer equation0/=sv[]+1, to get the following equation:=0.48+1, with a correlation coefficient of2=0.98. The slope of the fitted equation is 0.48μmolL−1, which is thesvin the Stern-Volmer equation. The detection limit is determined to be 0.038μmolL−1by the equation, LOD=3/sv(Zhang., 2015), whereis the standard deviation of 10 blank g- CNQDs (=0.61%), andsvis the slope of the fitted equation.
Fig.7 (A) Fluorescence spectra of g-CNQDs containing different concentrations of Hg2+, (B) Enlarged image of fluorescence spectra at lower Hg2+ concentration, (C) Relative fluorescence intensity F0 /F, (D) The linear relationship between the F0 /F and Hg2+ concentration.
Stability is one of the important parameters of detection performance. The fluorescence intensity of g-CNQD in different pH and concentration of NaCl solutions is also measured. The pH of domestic wastewater and factory wastewater is around the range of 6.5–9. Fluorescence emission wavelength and fluorescence intensity of g-CNQD with different pH values can be obtained from Figs.8A and B, the pH value is from 6.5 to 12 and can maintain a stable state, so it is largely undisturbed by pH. The optimal fluorescence emission wavelength and fluorescence intensity of g-CNQD in an aqueous solution did not change significantly with increasing NaCl concentration, from Figs.8C and D, indicating that g-CNQD can still maintain its fluorescence stability in the environment of high concentration of saline solutions, indicating that these g-CNQD have good stability under different conditions and have good potential for application.
Several approaches have been developed for the detection of Hg2+, including fluorescent, colorimetric, and elec- trochemical methods,. These methods can be applied for both qualitative and quantitative analysis of mercury and offer high sensitivity and selectivity with distinct ad- vantages. For example, the fluorescence method offers several advantages, such as the ability to detect the trace amounts with high sensitivity and specificity, low sample consumption, compatibility with various sample types, and environmentally friendly properties, making it a va- luable tool for chemical and biological analysis. Colori- metry, on the other hand, is a popular choice for on-site measurement of various analysis due to its simplicity, visual display, and the minimal instrument requirements. Electrochemical detection offers fast and accurate results, low cost, high sensitivity, and the ability for direct on-site and continuous detection. Table 2 summarizes the comparison of different Hg2+detection methods.
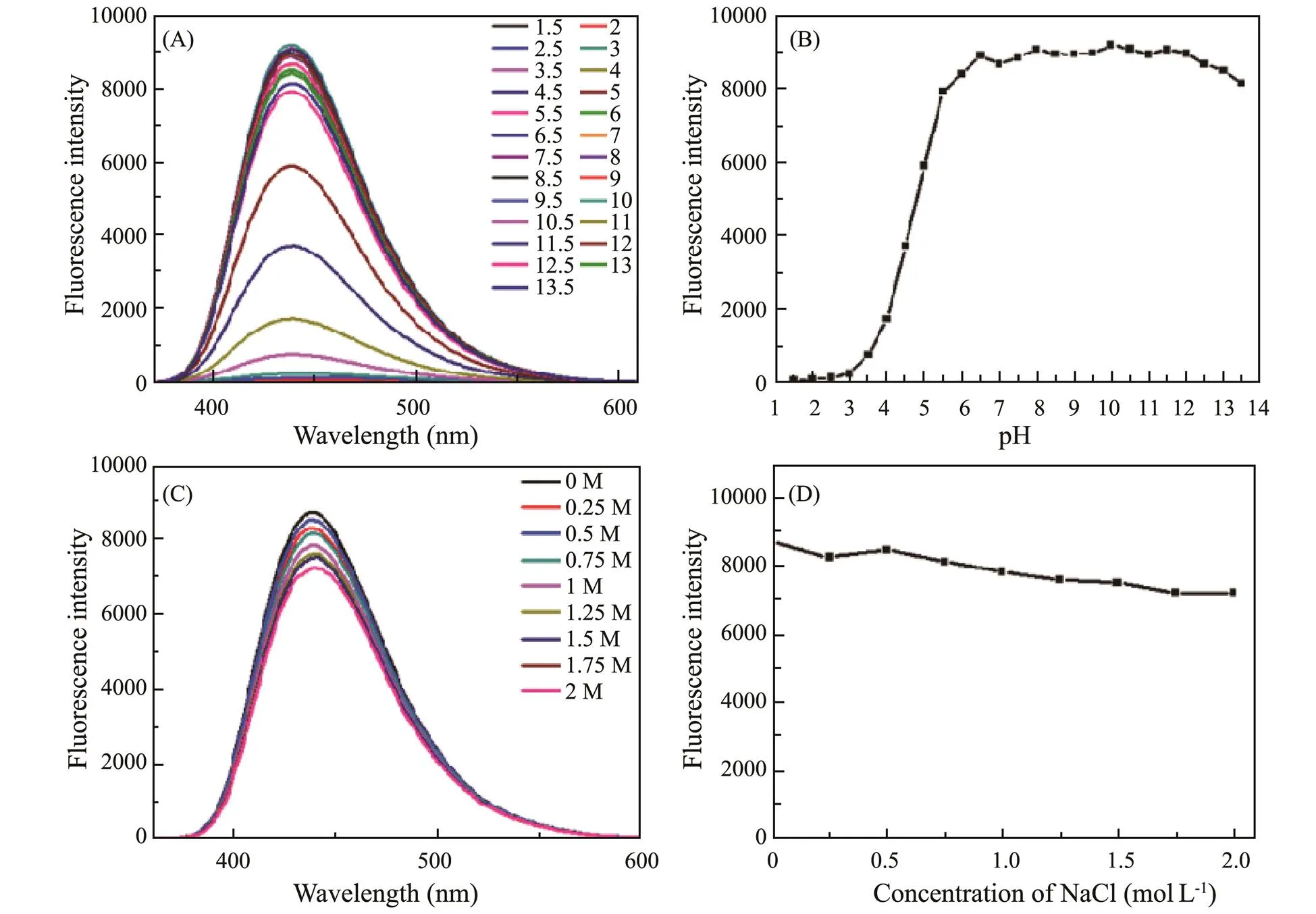
Fig.8 (A) Fluorescence spectra of g-CNQDs at different pH values, (B) Relative fluorescence intensity of g-CNQDs with different pH values, (C) Fluorescence spectra for the different NaCl concentrations experiment, (D) Relative fluorescence intensity of g-CNQDs with different NaCl concentrations.
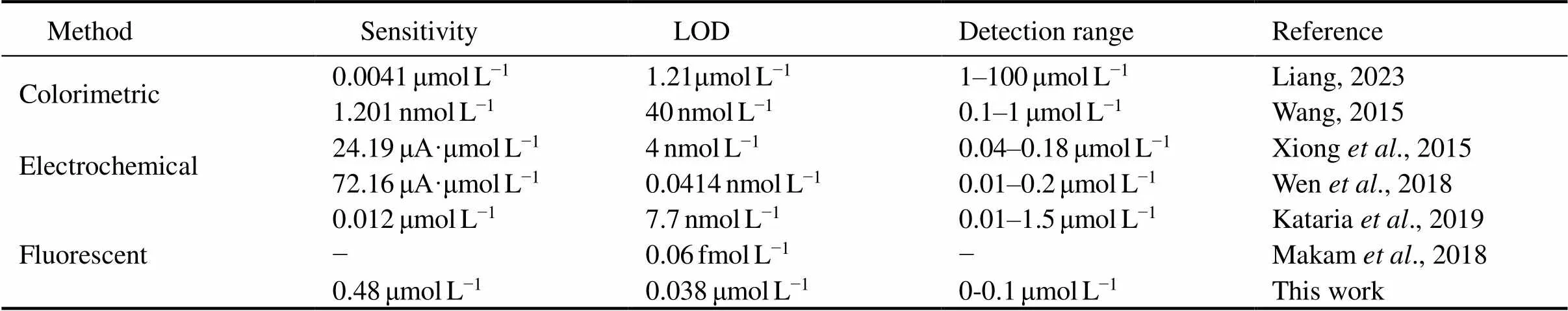
Table 2 Comparison of the performance of different methods for Hg2+ detection
3.5 Sensing Mechanism of g-CNQDs TowardH2O2 and Hg2+
The schematic sensing process of the g-CNQDs is shown in Fig.9. The fluorescence quenching of g-CNQDs follows the photo induced electron transfer (PET) mechanism in the determination of H2O2and Hg2+. The H2O2is an oxidizing substance that will react with the KI to form I3−through the oxidation-reduction reaction. The I3−will be adsorbed on the surface of g-CNQDs through the electrostatic interaction, generating a new complex with the g-CNQDs. When illuminated by the UV light, the photogenerated electrons are transferred from the g-CNQDs to I3−instead of returning to the original ground state of g-CNQDs to emit fluorescence, leading to a certain degree of fluorescence quenching which can be utilized for the detection of H2O2. The fluorescence quenching of g- CNQDs by Hg2+also follows the electron transfer mecha- nism. The Hg2+is adsorbed on the surface of g-CNQDs and chelated with the N of g-CNQDs, leading to the tran- sfer of electrons in the excited state to the unfilled Hg2+orbital, causing the non-radiative electron/hole recombination and the fluorescence quenching of g-CNQDs (Wang., 2015, 2021).
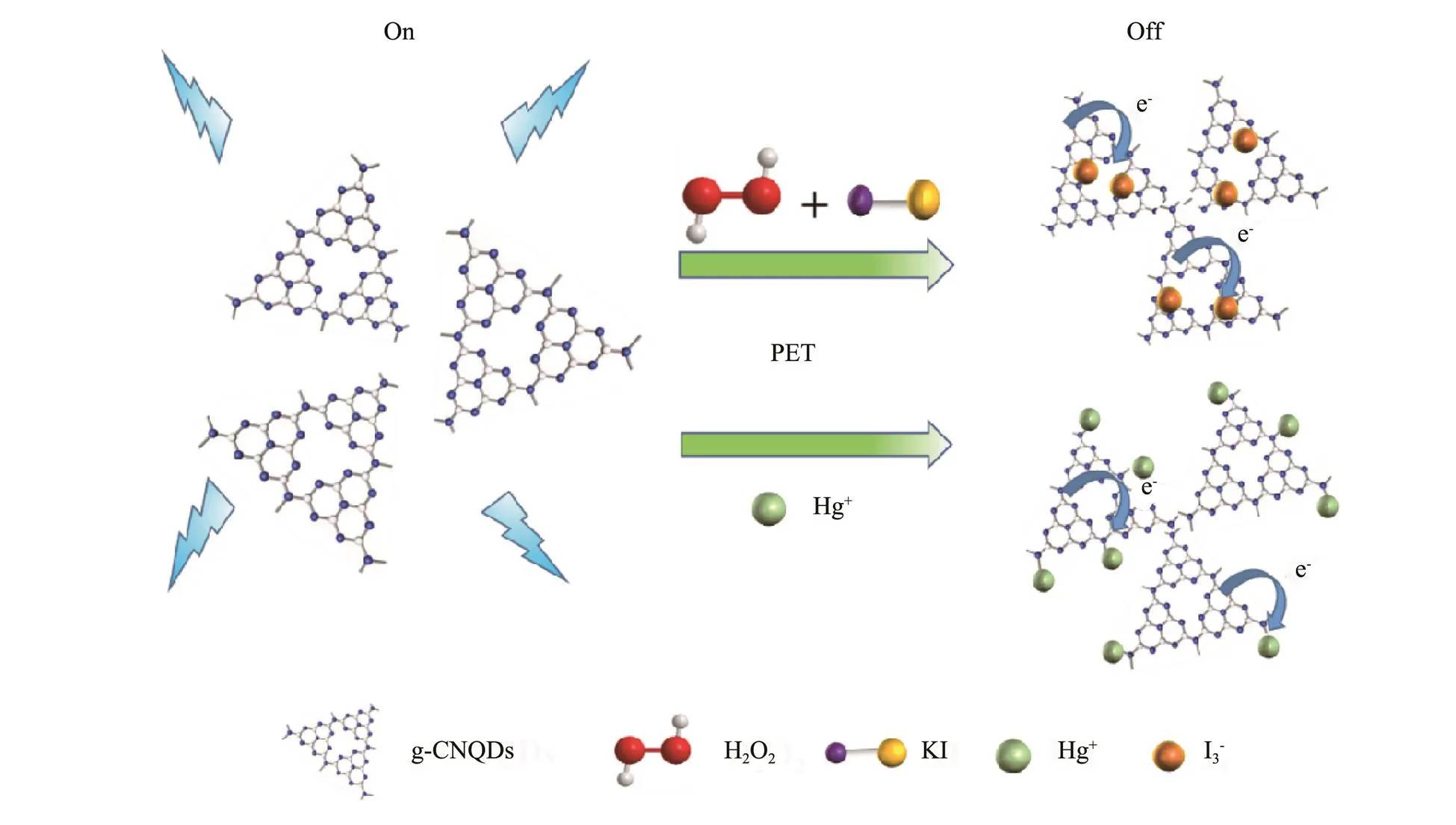
Fig.9 Sensing principle of the g-CNQDs toward H2O2 and Hg2+.
4 Conclusions
In summary, we successfully synthesized graphitic car- bon nitride quantum dots (g-CNQDs) with a high FLQY of 42.69% by a facile one-step hydrothermal method. The fluorescence can be quenched by the H2O2in the presence of KI and can be directly quenched by the Hg2+. The g- CNQDs probe shows a wide detection range of 1–1000 μmolL−1and a low detection limit of 0.23μmolL−1toward H2O2, as well as high sensitivity toward Hg2+with a detection range of 0–0.10μmolL−1and a detection limit of 0.038μmolL−1. This study may provide a facile bottom-up approach for the low-cost, non-toxic, and environmentally friendly preparation of g-CNQDs than the previous top-down methods. Additionally, the investigation may broaden the application of g-CNQDs as a fluorescence H2O2probe, providing a reference for the development and use of multifunctional fluorescent probes.
Acknowledgements
The authors acknowledge the support from the Natural Science Foundation of Shandong Province (No. ZR2021 MB075), National Natural Science Foundation of China (No. 51602297), and the Opening Fund of State Key La- boratory of High-Efficiency Utilization of Coal and Green Chemical Engineering (No. 2021-K53).
Ahmed, S. R., Cirone, J., and Chen, A. C., 2019. Fluorescent Fe3O4quantum dots for H2O2detection., 2 (4): 2076-2085, DOI: 10.1021/acsanm.9b00071.
Barman, S., and Sadhukhan, M., 2012. Facile bulk production of highly blue fluorescent graphitic carbon nitride quantum dots and their application as highly selective and sensitive sensors for the detection of mercuric and iodide ions in aqueous media.,22 (41): 218-232, DOI: 10.1039/c2jm35501a.
Bian, W., Zhang, H., Yu, Q., Shi, M. J., Shuang, S. M., Cai, Z. W.,., 2016. Detection of Ag+using graphite carbon nitride nanosheets based on fluorescence quenching., 169: 122-127, DOI: 10.1016/j.saa.2016.06.024.
Castro, R. C., Soares, J. X., Ribeiro, D. S. M., and Santos, J. L. M., 2019. Dual-emission ratio metric probe combining carbon dots and Cd Te quantum dots for fluoro metric and visual determination of H2O2., 296: 126665, DOI: 10.1016/j.snb.2019.126665.
Cui, Y., Chen, F., and Yin, X. B., 2019. A ratiometric fluorescence platform based on boric-acid-functional Eu-MOF for sensitive detection of H2O2and glucose., 135: 208-215, DOI: 10.1016/j.bios.2019.04.008.
Dong, R. Y., Yao, Y. Y., Li, D. N., Zhang, H. R., Li, W., Molokee, M.,., 2020. Ratio fluorescent hybrid probe for visualized fluorescence detection of H2O2and., 321: 128643, DOI: 10.1016/j.snb. 2020.128643
Dong, Y., H., and Zheng, J. B., 2020. Tremella-like ZIF-67/rGO as electrode material for hydrogen peroxide and dopamine sensing applications., 311: 127918, DOI: 10.1016/j.snb.2020.127918.
Elumalai, D., Maadeswaran, P., Mohan, R., Vairamuthu, R., Kandiah, K., and Duraisamy, N., 2023. A potential forecast of carbon quantum dots (CQDs) as an ultrasensitive and selective fluorescence probe for Hg (II) ions sensing.,287: 116098, DOI: 10.1016/j. mseb.2022.116098.
Gong, T. T., Liu, J. F., Wu, Y. W., Xiao, Y., Wang, X. H., and Yuan, S. Q., 2017. Fluorescence enhancement of CdTe quantum dots by HBcAb-HRP for sensitive detection of H2O2in human serum., 92: 16-20, DOI: 10.1016/j.bios.2017.01.048.
He, L. R., Fei, M., Chen, J., Tian, Y. F., Jiang, Y., Huang, Y.,., 2019. Graphitic C3N4quantum dots for next-generation QLED displays., 22: 76-84, DOI: 10.1016/j. mattod.2018.06.008.
Inagaki, M., Tsumura, T., Kinumoto, T., and Toyoda, M., 2019. Graphitic carbon nitrides (g-C3N4) with comparative discussion to carbon materials., 141: 580-607, DOI: 10. 1016/j.carbon.2018.09.082.
Jinadasa, B., Jayasinghe, G., Pohl, P., and Fowler, S. W., 2021. Mitigating the impact of mercury contaminants in fish and other seafood–A review., 171: 112710, DOI: 10.1016/j.marpolbul.2021.112710.
Kataria, R., Sethuraman, K., Vashisht, D., Vashisht, A., Mehta S. K., and Gupta, A., 2019. Colorimetric detection of mercury ions based on anti-aggregation of gold nanoparticles using 3, 5-dimethyl-1-thiocarboxamidepyrazole., 148, 299-305, DOI: 10.1016/j.microc.2019.04.068.
Li, B. S., Chen, J. B., Xiong, Y., Yang, X., Zhao, C. D., and Sun, J., 2018. Development of turn-on fluorescent probes for the detection of H2O2vapor with high selectivity and sensitivity., 268: 475-484, DOI: 10. 1016/j.snb.2018.04.140.
Li, M., Wang, B., An, X. F., Li, Z. Y., Zhu, H. T., Mao, B. Y.,., 2019. A practical graphitic carbon nitride (g-C3N4) based fluorescence sensor for the competitive detection of trithiocyanuric acid and mercury ions., 170: 107476, DOI: 10.1016/j.dyepig.2019.04.021.
Li, Y. N., Huang, H., Li, Y., and Su, X. G., 2013. Highly sensitive fluorescent sensor for mercury (II) ion based on layer- by-layer self-assembled films fabricated with water-soluble fluorescent conjugated polymer., 188: 772-777, DOI: 10.1016/j.snb.2013.07.093.
Lian, M. L., Liu, M. H., Zhang, X., Zhang, W., Zhao, J. B., Zhou, X. M.,., 2021. Template-regulated bimetallic sulfide nanozymes with high specificity and activity for visual colorimetric detection of cellular H2O2., 13 (45): 53599-609, DOI: 10.1021/acsami. 1c15839.
Liang, X. Y., Du, X., Liu, A., Cai, Z. X., Li, J. W., Zhang, M. S.,., 2023. Au/Ag2S dimeric nanostructures for highly specific plasmonic sensing of mercury (II)., 34: 107491-107497, DOI: 10.1016/j.cclet.2022.05. 005.
Liu, H. J., Lv, X. T., Qian, J. C., Li, H., Qian, Y., Wang, X. Y.,., 2020. Graphitic carbon nitride quantum dots embedded in carbon nanosheets for near-infrared imaging-guided combined photo-chemotherapy., 14 (10): 13304-13315, DOI: 10.1021/acsnano.0c05143.
Liu, J. W., Luo, Y., Wang, Y. M., Duan, L. Y., Jiang, J. H., and Yu, R. Q., 2016. Graphitic carbon nitride nanosheets-based ratiometric fluorescent probe for highly sensitive detection of H2O2and glucose., 8 (49): 33439-45, DOI: 10.1021/acsami.6b11207.
Liu, T., Zhang, S. X., Liu, W., Zhao, S., Lu, Z. W., Wang, Y. Y.,., 2020. Smartphone based platform for ratiometric fluo- rometric and colorimetric determination H2O2and glucose.,305: 127524, DOI: 10. 1016/j.snb.2019.127524.
Lu, D. X., Li, J. H., Wu, Z., Yuan, L., Fang, W. H., Zou, P.,., 2022. High-activity daisy-like zeolitic imidazolate framework-67/reduced grapheme oxide-based colorimetric biosensor for sensitive detection of hydrogen peroxide., 608: 3069-3078, DOI: 10. 1016/j.jcis.2021.11.034.
Lu, H. Z., Yu, C. W., Zhang, Y., and Xu, S. F., 2019. Efficient core shell structured dual response ratiometric fluorescence probe for determination of H2O2and glucoseetching of silver nanoprisms., 1048: 178-185, DOI: 10.1016/j.aca.2018.10.025.
Makam, P., Shilpa, R., Kandjani, A. E., Periasamy, S. R., Sabri, Y. M., Madhu, C.,., 2018. SERS and fluorescence-based ultrasensitive detection of mercury in water., 100, 556-564, DOI: 10.1016/j.bios.2017.09. 051.
Peng, R. Q., Andreas, O., Ermolenko, Y., and Mourzina, Y., 2020. Biomimetic sensor based on Mn(III) meso-tetra (N- methyl-4-pyridyl) porphyrin for non-enzymatic electrocatalytic determination of hydrogen peroxide and as an electrochemical transducer in oxidase biosensor for analysis of biological media., 321: 128437, DOI: 10.1016/j.snb.2020.128437.
Qian, Z. S., Ma, J. J., Shan, X. Y., Feng, H., Shao, L. X., and Chen, J. R., 2014. Highly luminescent N-doped carbon quantum dots as an effective multifunctional fluorescence sensing platform., 20 (8): 2254-2263, DOI: 10.1002/chem.201304374.
Rajeshwari, M., Raaja, S. K., and Khan, S. S., 2021. Recent de- velopments in architecturing the g-C3N4based nanostructured photocatalysts: Synthesis, modifications and applications in water treatment., 291: 132735, DOI: 10. 1016/j. chemosphere.2021.132735.
Sadhukhan, M., Bhowmik, T., Kundu, M. K., and Barman, S., 2014. Facile synthesis of carbon quantum dots and thin graphene sheets for non-enzymatic sensing of hydrogen peroxide., 4 (10): 4998-5005, DOI: 10.1039/C3RA4605 0A.
Shan, X. Y., Chai, L. J., Ma, J. J., Qian, Z. S., Chen, J. R., and Feng, H., 2014. B-doped carbon quantum dots as a sensitive fluorescence probe for hydrogen peroxide and glucose detection., 139 (10): 2322-2325, DOI: 10.1039/c3an02222f.
Shi, Y., Su, P., Wang, Y. Y., and Yang, Y., 2014. Fe3O4peroxidase mimetics as a general strategy for the fluorescent detection of H2O2-involved systems., 130: 259-264, DOI: 10.1016/j. talanta.2014.06.053.
Shorie, M., Kaur, H., Chadha, G., Singh, K., and Sabherwal, P., 2019. Graphitic carbon nitride QDs impregnated biocompatible agarose cartridge for removal of heavy metals from contaminated water samples., 367: 629-638, DOI: 10.1016/j.jhazmat.2018.12.115.
Wang, C. F., and Zhang, Z. H., 2020. Transition-metal phos- phide/sulfide nanocomposites for effective electrochemical non-enzymatic detection of hydrogen peroxide., 7 (16): 3416-3419, DOI: 10.1002/celc.202000867.
Wang, T., Nie, C. Y., Ao, Z. M., Wang, S. B., and An, T. C., 2020. Recent progress in g-C3N4quantum dots: Synthesis, properties and applications in photocatalytic degradation of organic pollutants., 8 (2): 485-502, DOI: 10.1039/C9TA11368A.
Wang, W. J., Jimmy, C. Y., Shen, Z. R., Chan, D. K., and Gu, T., 2014. g-C3N4quantum dots: Direct synthesis, upconversion properties and photocatalytic application., 50 (70): 10148-10150, DOI: 10.1039/C4CC02543A.
Wang, Q. R., Li, Y. R., Li, M., Wen, C. Y., Liu, R. S., Subhan, F.,., 2015. A colorimetric approach for measuring mercuric ions with high selectivity using label-free gold nanoparticles and thiourea., (7): 6837-6841, DOI: 10. 1039/c5ay01328c.
Wang, W. T., Tak, K., Yan, Z. F., Zhu, G. S., Ivan, C., Nguyen, N. T.,., 2015. Carbon dots functionalized by organosilane with double-sided anchoring for nanomolar Hg2+detection., 437: 28-34, DOI: 10.1016/j.jcis.2014.09.013.
Wang, X., Yang, X. F., Wang, N., Lv, J. J., Wang, H. J., Martin, M.,., 2018. Graphitic carbon nitride quantum dots as an ‘off-on’ fluorescent switch for determination of mercury(II) and sulfide., 185 (10): 1-8, DOI: 10.1007/ s00604-018-2994-0.
Wen, G. L., Zhao, W., Chen, X., Liu, J. Q., Wang, Y., Zhang, Y.,., 2018. N-doped reduced graphene oxide/MnO2nanocomposite for electrochemical detection of Hg2+by square wave stripping voltammetry., 291: 95- 102, DOI: 10.1016/j.electacta.2018.08.121.
Wang, Y., Zhang, L., Han, X. Y., Zhang, L. W., Wang, X. Y., and Chen, L. X., 2021. Fluorescent probe for mercury ion imaging analysis: Strategies and applications., 406: 127166, DOI: 10.1016/j.cej.2020.127166.
Xi, L. L., Ma, H. B., and Tao, G. H., 2016. Thiourea functionalized CdSe/CdS quantum dots as a fluorescent sensor for mer- cury ion detection., 27 (9): 1531- 1536, DOI: 10.1016/j.cclet.2016.03.002.
Xiong, S. Q., Yang, B. Y., Cai, D. Q., Qiu, G. N., and Wu, Z. Y., 2015. Individual and simultaneous stripping voltammetric and mutual interference analysis of Cd2+, Pb2+and Hg2+with reduced graphene oxide-Fe3O4nanocomposites., 185: 52-61, DOI: 10.1016/j.electacta.2015.10.114.
Yadav, P. K., Singh, V. K., Chandra, S., Bano, D., Kumar, V., Talat, M.,., 2019. Green synthesis of fluorescent carbon quantum dots from azadirachtaindica leaves and their peroxidase-mimetic activity for the detection of H2O2and ascorbic acid in common fresh fruits., 5 (2): 623-632, DOI: 10.1021/acsbiomaterials.8b 01528.
Yin, Y., Zhang, Y. M., Gao, T. L., Yao, T., Han, J. C., Han, Z. B.,., 2017. One-pot evaporation–condensation strategy for green synthesis of carbon nitride quantum dots: An efficient fluorescent probe for ion detection and bioimaging., 194: 293-301, DOI: 10.1016/j.mat chemphys.2017.03.054.
Yuan, J., Cen, Y., Kong, X. J., Wu, S., Liu, C., Chen, L. W.,., 2015. MnO2-nanosheet-modified up conversion nanosystem for sensitive turn-on fluorescence detection of H2O2and glucose in blood., 7 (19): 10548-10555, DOI: 10.1021/acsami.5b02188.
Zhang, Y., He, Y. H., Cui, P. P., Feng, X. T., Chen, L., Yang, Y. Z.,., 2015. Water-soluble, nitrogen-doped fluorescent carbon dots for highly sensitive and selective detection of Hg2+in aqueous solution.,5 (50): 40393-40401, DOI: 10.1039/c5ra04653j.
Zhuang, Q. F., Guo, P., Zheng, S., Lin, Q., Lin, Y. Y., Wang, Y.,.,2018. Green synthesis of luminescent graphitic carbon nitride quantum dots from human urine and its bioimaging application., 188: 35-40, DOI: 10.1016/j.talanta.2018. 05.060.
(June 2, 2022;
March 9, 2023;
April 7, 2023)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
#Thetwo authors contributed equally to this work.
. E-mail: lishawang@ouc.edu.cn
(Edited by Ji Dechun)
猜你喜欢
杂志排行
Journal of Ocean University of China的其它文章
- Effects of 5-Azacytidine (AZA) on the Growth, Antioxidant Activities and Germination of Pellicle Cystsof Scrippsiella acuminata (Diophyceae)
- Using Rn-222 to Study Human-Regulated River Water-Sediment Input Event in the Estuary
- Parameterization Method of Wind Drift Factor Based on Deep Learning in the Oil Spill Model
- YOLOv5-Based Seabed Sediment Recognition Method for Side-Scan Sonar Imagery
- A Metadata Reconstruction Algorithm Based on Heterogeneous Sensor Data for Marine Observations
- Large Active Faults and the Wharton Basin Intraplate Earthquakes in the Eastern Indian Ocean
