Contribution of surface roughness and oxygen-containing groups to the interfacial shear strength of carbon fiber/epoxy resin composites
2023-12-20LIANGYicaiZHANGXinghuaWEIXinghaiJINGDeqiSUWeiguoZHANGShouchun
LIANG Yi-cai,ZHANG Xing-hua,WEI Xing-hai,JING De-qi,SU Wei-guo,ZHANG Shou-chun,*
(1.Research Center of Advanced Thermoplastic Composites Engineering, Institute of Coal Chemistry, Chinese Academy Sciences, Taiyuan 030001, China;2.Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China;3.Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy Sciences, Taiyuan 030001, China;4.National Key Laboratory of Science and Technology on Vessel Integrated Power System, Naval University of Engineering, Wuhan 430033, China)
Abstract: The interfacial shear strength (IFSS) between carbon fibers (CFs) and the matrix is crucial to the performance of CFreinforced polymer composites.To evaluate the contribution of mechanical interlocking and chemical anchoring at the interfaces of a polyacrylonitrile-based CF (TORAYCA T800SC-12000-10E)-reinforced epoxy resin (EP: bisphenol A type epoxy resin and tetrafunctional epoxy resin) composites,the surface roughness and content of oxygen-containing functional groups of the CFs were respectively altered by ammonia treatment and electrochemical oxidation.The results showed that ammonia treatment increased the surface roughness without much change to the surface elemental composition,while electrochemical oxidation increased the number of surface oxygen groups without changing the surface roughness.The IFSS of CF/EP composites was tested by the micro-droplet method.The relationships between IFSS,and surface roughness and oxygen content were obtained by linear fitting.The results showed that in the interfacial bonding of CF to epoxy resin,the contribution of chemical anchoring to the IFSS is larger than that of mechanical interlocking.
Key words: Carbon fiber;Epoxy resin;Mechanical interlocking;Chemical anchoring;Interfacial adhesion
1 Introduction
Carbon fiber reinforced polymer composites(CFRPs) are promising materials which are used in a wide field such as military,aerospace,marine and sporting goods[1–5].However,the overall performance of the composites is largely influenced by the interfacial interactions.The interface is crucial in the load transfer process from the relatively weak matrix to the strong reinforcing fibers,which ultimately determines the practical performance of resulting composites[6–8].Good interfacial properties depend on good interfacial bonding.Fiber pull-out is a major cause of composite failure,and the outcome is largely determined by the interfacial adhesion between fibers and matrix[9–13].
At present,it is more widely accepted that the main factors affecting the interfacial adhesion of CFRPs are mechanical interlocking and chemical anchoring[14].However,evaluating their contributions to interfacial adhesion is difficult,and there is still much debate as to whether the dominant factor in interfacial bonding is mechanical interlocking or chemical anchoring.Lu et al.[15]found that the surface roughness of CFs was the main factor affecting the interfacial adhesion between CFs and epoxy resins for plasmatreated CFs.Li et al.[16]found that neither the surface roughness of CFs nor the oxygen-containing functional groups directly influenced the interfacial adhesion between CFs and epoxy resin.Drzal et al.[17]used a pre-reaction method to find that the chemical anchoring between epoxy groups and CF surface dominated the interfacial adhesion.Therefore,to better improve the performance of composites,it is crucial to distinguish the contributions of mechanical interlocking and chemical anchoring to the interfacial adhesion.It is useful to analyze the interfacial adhesion of composites from a microscopic perspective,but most current studies inevitably change both the physical and chemical properties of CFs surface when CFs are treated[15,18–19].By decoupling the surface roughness and oxygen-containing functional groups of CFs,the contribution of mechanical interlocking and chemical anchoring to the interfacial adhesion can be more accurately distinguished.
In this study,the CFs with different surface roughnesses and oxygen contents were obtained,and the interfacial shear strength (IFSS) of CF/epoxy resin was determined by the micro-droplet method.To determine the contribution of mechanical interlocking and chemical anchoring to the interfacial adhesion,the effects of the surface roughness and oxygen-containing functional groups on the IFSS of composites were decoupled,and empirical equations were fitted by investigating their respective contribution to the interfacial adhesion.It was found that chemical anchoring was the dominant factor in the interfacial adhesion between CFs and epoxy,and the contribution coefficient of chemical anchoring to the interfacial adhesion is greater than mechanical interlocking.
2 Experimental
2.1 Materials
Polyacrylonitrile (PAN)-based CFs (Toray Industries’ TORAYCA T800SC-12000-10E,with a diameter of 5 μm,a tensile strength of 5 880 MPa,and a density of 1.80 g/cm3) were used in this study.All fibers were desized by heating at 500 °C for 30 min in nitrogen atmosphere.These desized fibers were named CF0.
The bisphenol A type epoxy (EP) resin (E51)was purchased from Kunshan Lvxun Chemical in China with an epoxy value of 0.52 mol/100g.The curing agent is diethyl toluene diamine (E100) obtained from Albemarle Corporation (NYSE: ALB).And the ratio of epoxy resin to the curing agent is 1∶0.25 by weight.
The tetrafunctional epoxy resins (MF-4101H)was obtained from Hubei Gemvon New Material Co.,Ltd with an epoxy value of 0.90 mol/100 g.The curing agent is E100 and the ratio of epoxy resin to the curing agent is 1∶0.33 by weight.
2.2 Surface treatment of the CFs
The surface roughness of CFs was changed by ammonia treatment.The CFs were placed in 28%(mass fraction) ammonia and soaked at room temperature for 48,72,96,120 and 144 h.After treatment,the CFs were repeatedly washed with deionized water to remove the residues on the surface and dried at 120 °C for 3 h.These fibers were named as A-CF1,ACF2,A-CF3,A-CF4and A-CF5.
The CFs were electrochemically oxidized using 5% (mass fraction) NH4HCO3,with the graphite plate as the cathode and the CFs as the anode.The treatment was carried out in 1 L beaker with a distance of 15 cm between the CFs and the graphite plate.The current for the electrochemical treatment was 8 mA and the treatment time was from 1 to 3 min.After treatment,the CFs were repeatedly cleaned with deionized water to remove the residual electrolyte on the surface and dried at 120 °C for 3 h.These fibers were named as E-CF1,E-CF2,E-CF3and E-CF4.
2.3 Preparation of monofilament composites
Single CF was fixed on a metal frame and tensed,then droplets of liquid uncured epoxy resin were applied uniformly on the CF with the tip of a glass rod.The samples were cured at 150 °C for 2 h,then at 180 °C for 2 h.After the samples were cured and cooled,the resin droplet morphology was observed under a microscope and droplets with a diameter of 30-60 μm for testing.The prepared monofilaments were used to test the IFSS between CF and epoxy resin.
2.4 Characterization
2.4.1 Scanning electron microscopy
A field emission scanning electron microscope(JEOL,JSM-7900F,Japan) was used to analyze the surface topography of CFs with an accelerating voltage of 2 kV.
2.4.2 Atomic force microscopy
The surface topography and surface roughness of CFs were characterized by the atomic force microscopy (AFM,Bruker,Germany).The scanning mode of the AFM was taping mode and the scanning area was 2 μm × 2 μm.
2.4.3 X-ray photoelectron spectroscopy
The surface chemical elemental composition of CFs was determined by X-ray photoelectron spectroscopy (Thermo Scientific,ESCALAB 250Xi,US).The operating voltage was 12.5 kV with Al Kα(1 486.6 eV) X-ray source under a vacuum of 8×10-10Pa.In this study,the test flux energy was 50 eV for the full spectrum and 20 eV for the narrow spectrum,and the binding energy of C1s was used as the energy standard for charge correction.The elemental content of CF surface was determined from the corresponding peak areas in full spectrum and the sensitivity factor of the instrument used.
2.4.4 Dynamic contact analysis
The surface free energy of CFs was determined by the force tensiometer (KRUSS,K100SF,Germany).Deionized water (γd=21.8 mN/m,γp=51 mN/m)with strong polarity was chosen as the test fluid,and the test speed was 3 mm/min and the immersion depth was 4 mm.
According to the Wilhelmy method,the advancing contact angle can be calculated from the change in mass by Eq.(1) and the surface free energy of CFs can be calculated by the Neumann equation in Eq.(2)[20–21].
where θ is the contact angle between CFs and test liquid,∆mis the change in mass of the CFs,g is the gravitational constant,dis the fiber diameter,γlis the surface free energy of test liquid,γsis the surface free energy of CFs andβis the empirical constant with a value of 0.000 124 7.
2.4.5 Tensile strength of CF
The tensile strength of CF after ammonia treatment was measured by the electronic strength tester for single fiber (YG001E,China) according to GB9997.The gauge length was 20 mm,and the draw speed was 2 mm/min.At least 20 valid data were selected for analysis.
2.4.6 IFSS of monofilament composites
The micro-droplet method was used to measure the IFSS between CFs and resin matrix from the microscopic perspective.The micro-droplet method allows a more accurate measurement of the instantaneous force during micro-droplet debonding and the results are more intuitive.The IFSS of CFs was measured for evaluating the interfacial property of composites (TOHEL SANGYO CO.,LTD,HM410,Japan)and the test speed was selected to be low.The IFSS can be calculated by Eq.(3)[22].To minimize errors,more than 30 tests were carried out on each sample and the average value was taken as the IFSS of the CF/EP composites.
where τ is the interfacial shear strength,Fis the maximum load at debonding,dis the diameter of CF andLis the length of the CF embedded in the EP micro drop.
3 Results and discussion
3.1 Surface morphology of CFs
The surface topographies of CFs are shown in Fig.1.It can be seen that the surface topographies of CFs after ammonia treatment changed significantly(Fig.1b-f),with radially distributed grooves on the surface.Especially when the treatment time was more than 96 h,the surface topographies of CFs changed most obviously.However,it was observed that the changes in the surface topographies of CFs after electrochemical oxidation changed very little (Fig.1g-j).Even when the treatment time was increased to 3 min,no significant etching was produced (Fig.1j).Qian et al.[23]explained this phenomenon as the chemical etching on the convex part of CF surface was more intense than that on the concave part,the surface of CFs did not change significantly after electrochemical oxidation due to the different degrees of oxidation.Moosburger et al.[24]explained this phenomenon as the result of different anodizing parameters or different etching behavior of CFs.Thus,we obtain etched or un-etched CFs after treatment to investigate the effect of surface roughness on the interfacial adhesion between CFs and epoxy resin.
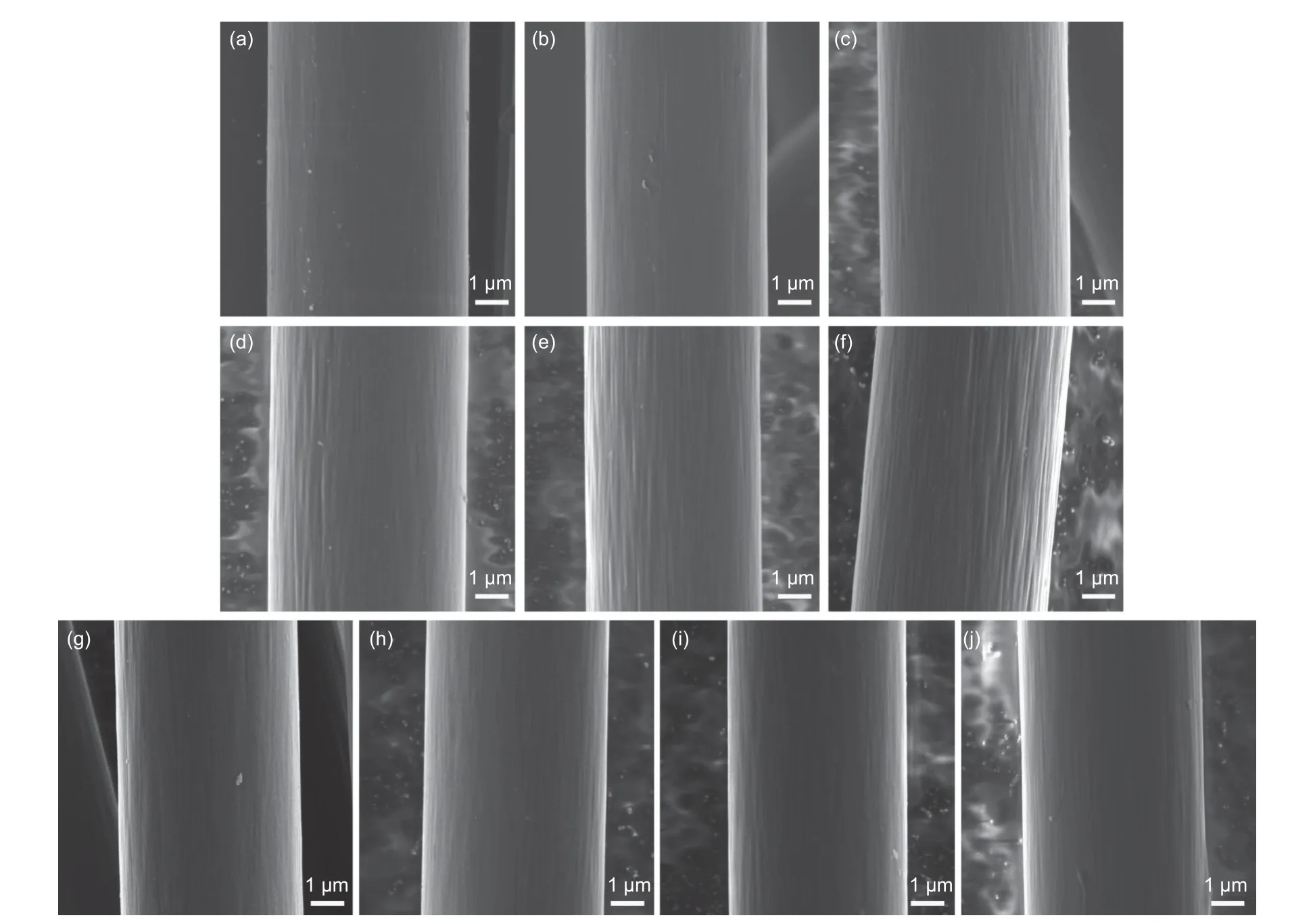
Fig.1 SEM images of CFs: (a) CF0,(b) A-CF1,(c) A-CF2,(d) A-CF3,(e) A-CF4,(f) A-CF5,(g) E-CF1,(h) E-CF2,(i) E-CF3 and (j) E-CF4
AFM imaging was used to obtain further information on the surface roughness of CFs.Since there was no obvious etching on the surface of CFs after electrochemical oxidation,only the CFs with the ammonia treatment were scanned.The 3D surface topographies of CFs with different degrees of etching are shown in Fig.2.The surface roughness of CFs was calculated by the software NanoScope Analysis.To reduce the effect of CF curvature on the roughness calculation,we calculated the surface roughness of CFs in a 1 μm × 1 μm area[25],and the results are listed in Table 1.

Table 1 Average roughness (Ra) values of the CFs obtained from the AFM

Fig.2 AFM images of CFs: (a) CF0,(b) A-CF1,(c) A-CF2,(d) A-CF3,(e) A-CF4 and (f) A-CF5
Similar to the SEM results,the surface of the untreated CFs was very smooth,and deep grooves with radial distribution appeared on the surface of CFs became more and more obvious when the ammonia time was increased.The roughness (Ra) of CF surface increased from 10.2 to 22.4 nm and increased more dramatically when the treatment time was longer than 96 h.The increase of surface roughness and grooves on the surface of CFs are beneficial for the mechanical interlocking between CFs and resin matrix,which is helpful to improve the interface properties.Therefore,we distinguished the effects of ammonia treatment and electrochemical treatment on surface physical properties to investigate their respective effects on IFSS.The coarsening of CFs by ammonia treatment is attributed to the fact that the weak interface layers and defects on the surface of CFs could be peeled off and removed by ammonia[26–27].
3.2 Surface chemical composition of CFs
The XPS wide spectra results of CF surface after ammonia treatment and electrochemical oxidation are shown in Fig.3 and Table 2.It can be seen that the main elements on the surface of CFs are C,N and O.The carbon content on the surface of the untreated CFs was about 88.89%,and the oxygen and nitrogen contents were about 8.66% and 2.45%,respectively.Table 2 illustrated that the elemental content of CFs surface was not significantly changed when the ammonia treatment time increasing.However,the elemental content of CFs surface changed significantly after anodic oxidation,the oxygen element content increased from 8.66% to 15.43%,the nitrogen element content remained essentially unchanged,and the O/C ratio increased from 9.74% to 18.89%.The electrochemical oxidation mechanism of CFs has been widely studied,and the simplified step-wise progression mechanism for the oxidation can be shown in Eq.(4)[28–29].
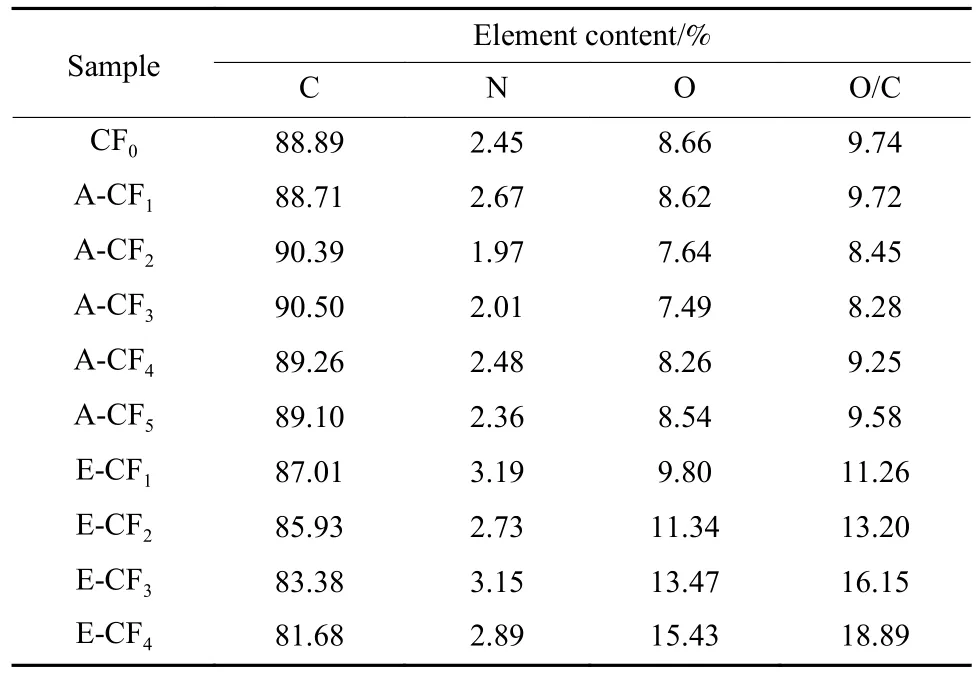
Table 2 Elemental content of the CFs surface
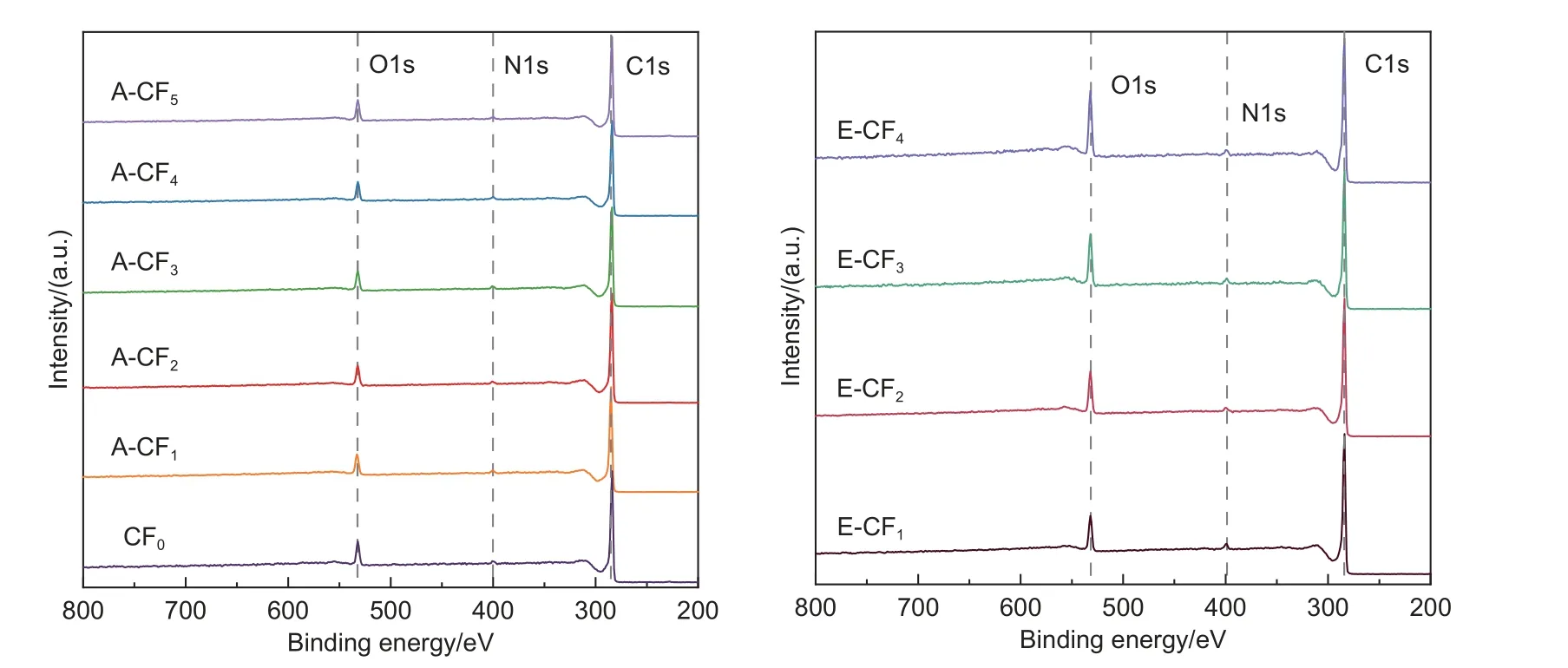
Fig.3 XPS wide spectra of the CFs
The increase in oxygen content indicated that the chemical properties of CF surface become more active after electrochemical oxidation[23].And the increasement of surface activity provides more active sites for the interfacial adhesion between CFs and epoxy resin,contributing to the chemical anchoring between fibers and resin matrix[30].
To obtain further information on the chemical properties of CF surface,the XPS C1s spectra of CFs surface were split and the results are shown in Fig.4 and Table 3.It can be seen that the functional groups on the surface of CFs were aromatic and aliphatic carbons (C―C,284.3-284.7 eV and 285-285.3 eV), alcohol hydroxyl or ether groups (C―O,285.7-286.6 eV),carbonyl groups (C=O,287.5-288.1 eV)and carboxyl or ester groups (―COO,288.6-289.1 eV)[31–34].The C―O and ―COO groups were the main functional groups on the surface of CFs.The type and amount of oxygen-containing functional groups on the CFs surface were not significantly changed after ammonia treatment,which indicated that the ammonia treatment did not change the number of polar groups on the CF surface.But the oxygen-containing functional groups on the CF surface were significantly changed after electrochemical oxidation,especially the C―O and ―COO groups,which can form covalent bonds with epoxy group and is beneficial to the interfacial adhesion between CFs and epoxy resin[35].Since the main groups that react with epoxy group are hydroxyl and carboxyl groups,the chemical properties in this study refer to the ability of the CFs surface to react with the epoxy resin.Combined with the results of the CF surface morphology analysis,it can be concluded that the ammonia treatment could change the morphological structure of CF surface without changing the chemical properties of CF surface.While the electrochemical treatment changed only the chemical properties of CF surface.
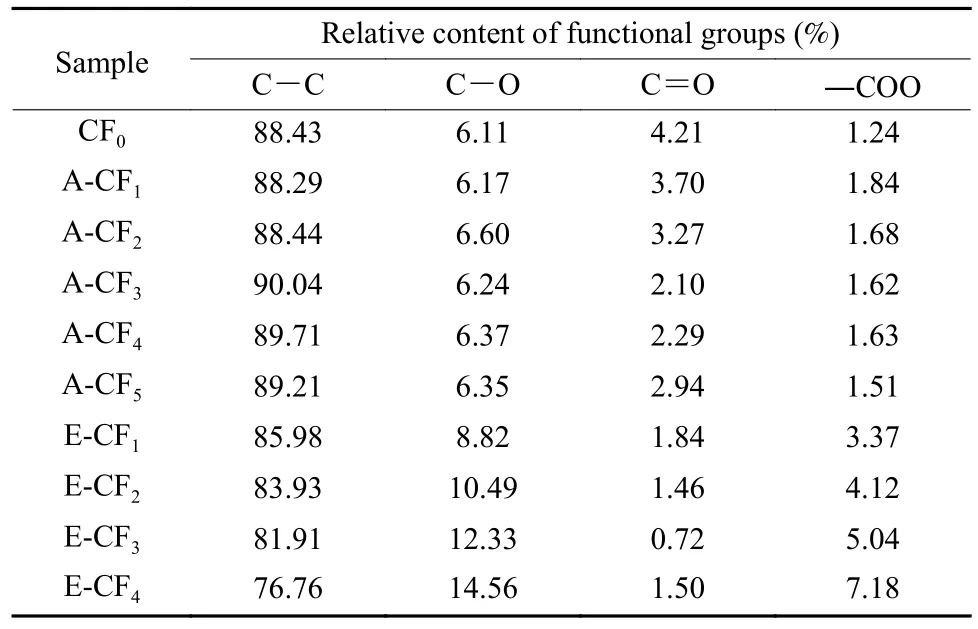
Table 3 Relative contents of functional groups on the surface of CFs

Fig.4 C1s XPS spectra of the CFs
Thus,two series of CFs displayed different surface properties.The A-CF specimens were characterized by various surface roughness with unchanged chemical properties,and the E-CF specimens were marked by various surface chemistries with unchanged physical properties.By comparing the IFSS between these CFs and epoxy resin,the contribution factor to the CF/EP interface adhesion from mechanical interlocking and chemical anchoring can be determined.
3.3 Dynamic contact angle analysis
Table 4 illustrates the results of the dynamic contact angle tests for all the CFs.Because of the strong polarity of water and weak polarity of CF0,the contact angle of CF0against deionized water was relatively larger.According to the XPS results,the ammonia treatment did not introduce polar functional groups on the CF surface and did not increase the surface polarity of CFs.Therefore,the contact angle of CFs against deionized water did not drop sharply after ammonia treatment,and the surface energy calculated from the contact angles only changed slightly.However,the oxygen element content and functional groups of CFs surface increased significantly after electrochemical treatment.Therefore,the dynamic contact angles against deionized water dropped markedly,and the surface free energy correspondingly increased significantly,and the surface free energy correspondingly increased significantly.
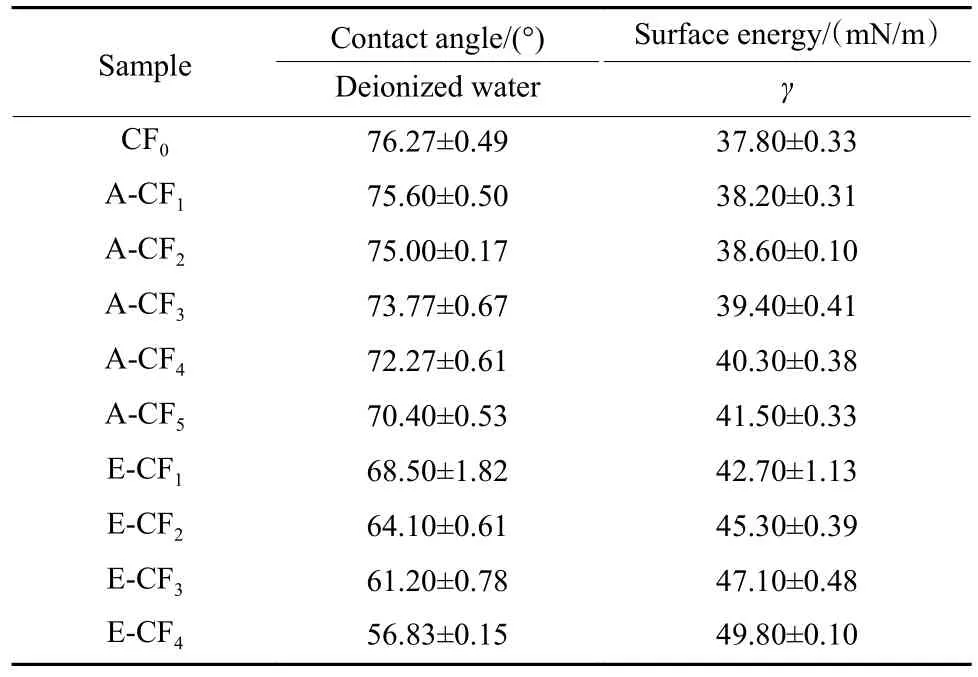
Table 4 Dynamic contact angle and surface free energies of CFs
According to the results of dynamic contact angle test,the CF surface still maintained weak polarity after the ammonia treatment,while the polarity of CFs surface was obviously increased after electrochemical treatment,which is consistent with the XPS results.The increase of surface polarity is conducive to the interface adhesion.
3.4 Tensile strength analysis
The tensile strengths of the CFs after ammonia treatment are shown in Fig.5.It was found that the tensile strength of the CFs did not decrease significantly after ammonia treatment.This is due to the fact that the ammonia treatment only peeled off the weak interfacial layers and defects on the CF surface and did not deeply affect the graphite layer structure of the CFs.The slight decrease in tensile strength did not affect the interfacial shear strength of the CFs and resin.
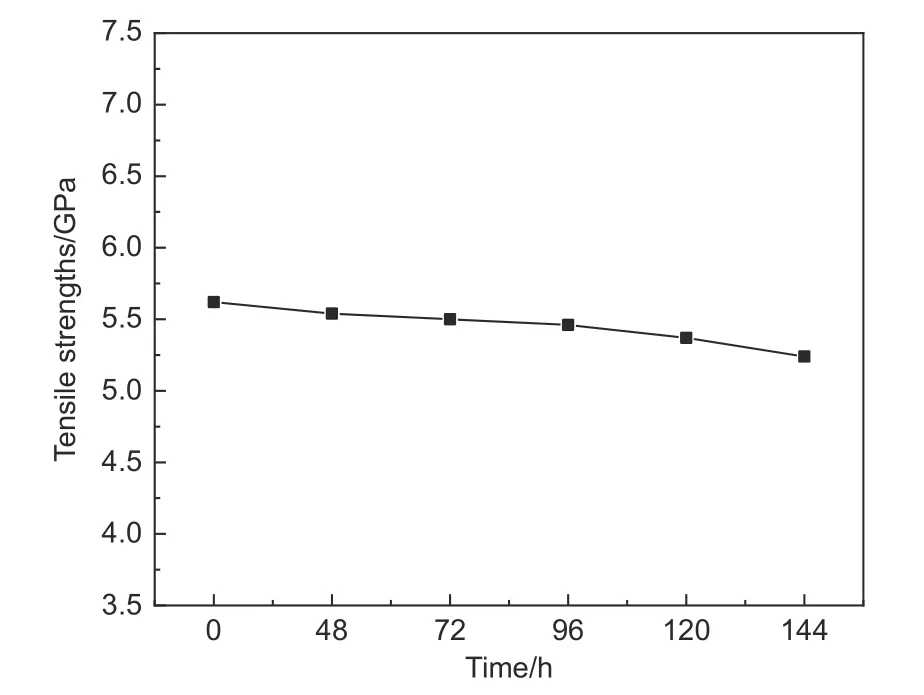
Fig.5 The tensile strength of CFs after ammonia treatment
3.5 Interfacial properties of the CFs
The influence of roughness and oxygen content on IFSS is shown in Fig.6.It was found that the IFSS increased with increasing the roughness and oxygen content of CFs surface,and the relationships basically satisfied a linear relationship.It is well known that the increase in roughness is beneficial to the mechanical interlocking of CFs with resin matrix,and the increase in the oxygen content is conducive to the chemical anchoring.The IFSS was mainly determined jointly by the mechanical interlocking,chemical anchoring and the sum of other factors.
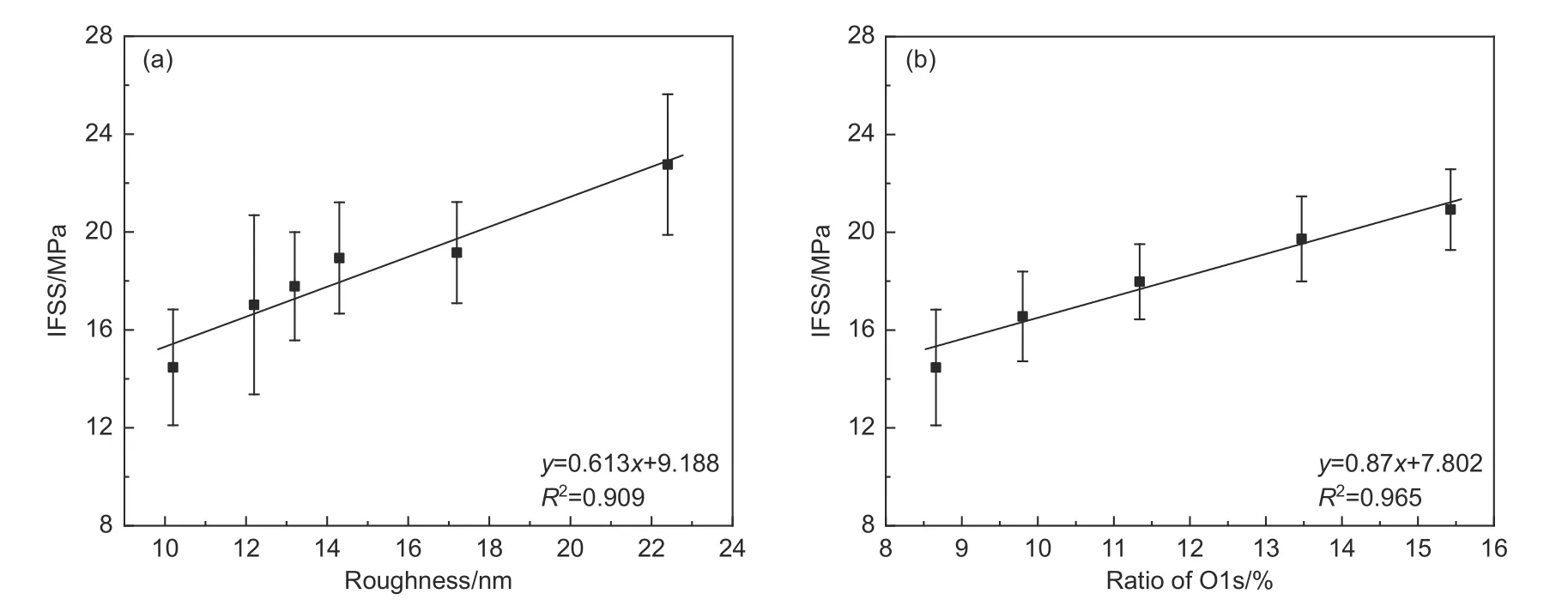
Fig.6 Variation of IFSS in bifunctional epoxy systems (a) with surface roughness and (b) with the O1s content on surface
However,it is not possible to directly compare the contribution of the roughness and oxygen content to the interfacial adhesion,as different levels of treatment can lead to different results.Therefore,we investigated the extent to which roughness and oxygen content increased interfacial adhesion and compared them from the perspective of contribution coefficient.To more intuitively compare the contribution coefficient of the roughness and oxygen-containing functional groups to the interfacial adhesion,we assumed that the roughness and oxygen content were each linearly related to the IFSS and they can be expressed as follows:
wherex1andx2stand for roughness and oxygen content,respectively,a1anda2stand for the factors of the influence of the roughness and oxygen content on the IFSS,b1stands for the effect of other properties except the roughness on the CF surface,b2stands for the effect of other properties except the oxygen content on the CF surface.Thus,only one variable,roughness or oxygen content was included in the obtained relationship.In the A-CF case,the factor is the roughness,and in the E-CF case,the factor is the oxygen content.As shown in Fig.6,by linear fitting the obtained IFSS relationships,we obtained the relationships between IFSS and the roughness or the oxygen content.If the assumption was true,the value ofb1stands for the contribution of chemical anchoring and other factors in the interfacial adhesion between the CF0and epoxy resin,and the value ofb2stands for the contribution of mechanical interlocking and other factors in the interfacial adhesion between the CF0and epoxy resin.Therefore,the contributions of other factors calculated from the values ofb1andb2should be identical.The contributions of the roughness and oxygen content of the CF0at this point were calculated to be 6.25 and 7.53 MPa,respectively.The valueb1after the contribution of oxygen content was deducted was the contribution of other factors of the CF0at this time.The valueb2after the contribution of the roughness was deducted was also the contribution of other factors.The corresponding results obtained were 1.65 and 1.55 MPa,which were largely consistent.Therefore,it can be concluded that the hypothesis was valid and the relationship between IFSS and the roughness and oxygen content conformed to the equation as follows:
whereXstands for the value of the surface roughness of CF,nm.Ystands for the oxygen content of CF surface,%.From Eq.(7),the contribution coefficient of chemical anchoring is higher than that of mechanical interlocking in the interfacial adhesion between CFs and epoxy resin.And with all other factors of CFs surface remaining unchanged,the contribution coefficient of chemical anchoring is 58.7% while the contribution coefficient of mechanical interlocking is 41.3%.Therefore,in order to control the properties of composites,the surface treatment should be designed according to the different contribution coefficients.
To verify the contribution coefficient of the roughness and oxygen-containing functional groups to the interfacial adhesion in different epoxy resins,the bifunctional resin (E51) in the system was replaced by the tetrafunctional resin (MF-4101),and the IFSS of the CFs reinforced tetrafunctional epoxy resin is shown in Fig 7.It can be seen that the value of IFSS has been significantly improved on the whole.Compared with the roughness,the value of IFSS increased more obviously with increasing the content of oxygen-containing functional groups.By fitting the 2 relationships linearly,it can be obtained the relationship of IFSS to the roughness and oxygen containing functional groups in the tetrafunctional system as well as their contribution coefficients (kin Fig.7).It can be seen that the chemical anchoring was the dominant factor in the interfacial adhesion,and in the tetrafunctional system,the contribution coefficient of interface adhesion by chemical anchoring is much higher than that by mechanical interlocking.The reason for this result we speculated is that there are more epoxy groups in the tetrafunctional resin than in the bifunctional resin,which improves the chemical anchoring of composites.

Fig.7 Variation of IFSS in tetrafunctional epoxy systems (a) with the surface roughness and (b) with the O1s content on surface
To verify the obtained formula,the T800SC CF desized by acetone (D-CF) was substituted for the CF0.Unlike the two carbon fiber treatments mentioned above,D-CF has a surface roughness (Ra) of 10.5 nm and a surface oxygen content of 10.40%.Using the same method,the IFSS between D-CF and bifunctional epoxy resin was measured to be 16.3 MPa,and that with tetrafunctional epoxy resin was 19.5 MPa,while the calculated values are 17.1 MPa and 20.8 MPa respectively.Comparing the experimental values with the calculated ones,there was little difference between them in different epoxy systems.Therefore,we believed that the surface properties of the T800SC CF basically conformed to the above relationships.
4 Conclusion
In this work,by decoupling the roughness and oxygen containing functional groups of the CF surface,the contributions of mechanical interlocking and chemical anchoring to the interfacial adhesion between CFs and epoxy resin were obtained.The increase in the surface roughness is beneficial to the mechanical interlocking in composites and the increase in the oxygen content is beneficial to the chemical anchoring in composites.Through linear fitting,we obtained the relationship between the IFSS and the roughness as well as the oxygen content.The other factors on CF surface remains constant,chemical anchoring was the dominant factor of the interfacial adhesion between CFs and epoxy,and the contribution coefficient of the interfacial adhesion by chemical anchoring is higher than that by mechanical interlocking.
Data availability statement
The data that support the findings of this study are openly available in Science Data Bank at https://www.doi.org/10.57760/sciencedb.12717 or https://resolve.pid21.cn/31253.11.sciencedb.12717.
Acknowledgements
This work was supported by the Key Research and Development Program of Shanxi Province(202003D111002),Major Science and Technology Project in Shanxi Province (202101040201003);the National Natural Science Foundation of China(51903249) and the Innovation Fund Project of Shanxi Institute of Coal Chemistry,Chinese Academy of Sciences (SCJC-XCL-2022-12).
杂志排行
新型炭材料的其它文章
- Carbon-based photothermal materials for the simultaneous generation of water vapor and electricity
- 生物质炭材料作为金属空气电池阴极的研究进展
- 3D porous NiCo2(CO3)3/reduced graphene oxide aerogel with heterogeneous interfaces for high-efficiency microwave absorption
- 煤基富氧多孔炭纳米片的制备及其超级电容器性能
- Mott-Schottky heterojunction formation between Co and MoSe2 on carbon nanotubes for superior hydrogen evolution
- A 2D montmorillonite-carbon nanotube interconnected porous network that prevents polysulfide shuttling
