A 2D montmorillonite-carbon nanotube interconnected porous network that prevents polysulfide shuttling
2023-12-20ZHOUMingxiaZHOUWenhuaLONGXiangZHUShaokuanXuPengOUYANGQuanshengSHIBinSHAOJiaojing
ZHOU Ming-xia,ZHOU Wen-hua,LONG Xiang,ZHU Shao-kuan,Xu Peng,OUYANG Quan-sheng,SHI Bin,SHAO Jiao-jing,*
(1.School of Materials and Metallurgy, Guizhou University, Guiyang 550025, China;2.Advanced Batteries and Materials Engineering Research Center, Guizhou Light Industry Technical College, Guiyang 550025, China;3.State Key Laboratory of Advanced Chemical Power Sources, Zunyi 563003, China)
Abstract: A commercial polypropylene (PP) separator was modified by a one-dimensional carbon nanotube (CNT) and two-dimensional montmorillonite (MMT) hybrid material (CNT-MMT).Because of the high electron conductivity of the CNTs,and the strong polysulfide (LiPS) adsorption ability and easy lithium ion transport through MMT,the interconnected porous CNT-MMT interlayer with excellent structural integrity strongly suppresses LiPS shuttling while maintaining high lithium-ion transport,producing a high utilization of the active sulfur.Lithium-sulfur batteries assembled with this interlayer have a high lithium-ion diffusion coefficient,a high discharge capacity and stable cycling performance.They had an initial specific capacity of 1373 mAh g-1 at 0.1 C,and a stable cycling performance with a low decay rate of 0.062% per cycle at 1 C after 500 cycles.
Key words: Lithium-sulfur batteries;Polysulfide shuttling;Two-dimensional montmorillonite;Carbon nanotube;Interlayer introduction
1 Introduction
Currently,the demand for the utilization of renewable energy continue to increase.Large-scale energy storage is a key link in achieving the high utilization of renewable energy.Although commercial lithium ion batteries have already penetrated almost every corner of human’s life,their energy density hardly meet the highly-developed economic society[1–2].Consequently,considerable efforts have been making to exploit advanced electrochemical energy storage devices.As a representative of electrochemical energy storge based on conversion-reaction chemistries,the conceptual prototype of lithium-sulfur batteries Li-S batteries was proposed more than half a century ago[3].Li-S batteries possess high theoretical capacity(1 675 mAh g-1) and high energy density (2 600 Wh kg-1),making them attractive in both industry and academia[4].However,due to the uncontrollable growth of lithium dendrites,low conductivity and large volume change of sulfur cathode,as well as the“shuttle effect” of lithium polysulfides (LiPS),the commercialization of lithium-sulfur batteries has been limited[5–6].The major reason is the dissolution of lithium polysulfides in the organic electrolyte solvents,which results in the irreversible loss of active materials,fast capacity decay and poor cycling performance of the battery.To address this issue,many strategies have been explored,such as adding additives in the electrolyte[7],encapsulating sulfur into the highly-conductive matrix[4,8–10],and inserting an interlayer between separator and active electrodes.The interlayer insertion has been recognized as an effective and simple way to suppress the shuttle effect by forming the physical barriers for polysulfide diffusion and/or establishing stronger chemical absorption[11–14].
Many carbon materials have been used as the interlayer materials because of their remarkable structure features and properties[15].For example,the high electronic conductivity of crystalline carbons is conducive to facilitating fast electrochemical reaction kinetics.The large specific area of low-dimensional carbon materials is beneficial for providing sufficient surface area for interacting with polysulfides[16–17].As we all know,the low polarity of carbon materials makes them to trap polysulfides mainly depending on physical adsorption[15,18],which is much lower than the strong chemisorption between polar materials (metal oxides/sulfides[19–20],conductive polymers[21]) and polysulfides.
Natural clay minerals,such as vermiculite and montmorillonite (MMT),are characterized by not only strong polarity,but also high chemical/thermal stability,and favorable ion exchange capability[22–24].Furthermore,it has been reported that lithium ions have a rather low diffusion barrier on MMT surface[25–28],which would be beneficial for fast lithium ion transport and enhances polysulfide conversion kinetics[27,29].The two-dimensional MMT nanosheets would provide more accessible surface for rapid transport of lithium ions and thus facilitate electrochemical reaction kinetics[30].
Herein,multi-walled carbon nanotubes (CNT)and 2D MMT nanosheets were hybridized to develop a 1D/2D porous interconnected network as an interlayer between the commercial Celgard 2500 separator(PP) and cathode.The highly conductive CNT endowed the fast electron transport ability,while the strong LiPS-adsorption ability and low lithium ion diffusion barrier of 2D MMT ensured the instant polysulfide conversion reaction.Both electronic/ionic conduction pathways were achieved in the porous CNTMMT network,forming an effective barrier for inhibiting the polysulfide shuttle.Finally,the Li-S batteries with the porous network interlayer delivered a high discharge specific capacity of 785.4 mA h·g-1at 1 C and a low capacity decay rate of 0.062% per cycle over 500 cycles.
2 Experimental
2.1 Fabrication of 2D MMT nanosheets
The 2D MMT nanosheets were obtained by exfoliating MMT powders by ion-exchange[31].Specifically,6 g MMT powder was added into 450 mL 1 mol L-1LiCl aqueous solution and stirred at 80 °C for 24 h for ion exchange.The suspension was then cooled down to room temperature and then was cleaned by dialysis with deionized water until the chloride ions could not be detected.The as-obtained suspension was centrifuged at 3 500 r min-1for 2 h to obtain supernatant.Subsequently,the centrifuged supernatant was freeze-dried to obtain 2D MMT nanosheets.
2.2 Preparation of the sulfur cathode
Kichen Black (KB) (Colruder) and sulfur nanopowder were evenly mixed (weight ratio of KB∶S=3∶7) and then in Ar atmosphere heat preservation for 10 h under 155 °C.The multiwall carbon nanotubes (CNT) and poly-(vinylidene fluoride)(PVDF) were mixed at a weight ratio of 8∶1∶1,and then N-methyl-2-pyrrolidinone (NMP) was added and ground to prepare a slurry,which was then coated on carbon-coated aluminum by using a scraper.After preliminary drying,the residual solvent was removed by vacuum drying at 60 °C for 12 h,and then was perforated onto a disk with a diameter of 12 mm as the cathode.The quality of sulfur load of was approximately 1.0 mg cm–2.
2.3 Li2S deposition and dissolution test
The CNT/MMT (weight ratio of CNT∶MMT=4∶1) was mixed with PVDF with a weight ratio of 9∶1.And then,the NMP was added to prepare the slurry.The fully mixed slurry was coated on the aluminum foil to fabricate the cathode (CNT-MMT electrode).Lithium metal and PP were used as anode and separator,respectively.Li2S8electrolyte in amount of 20 μL (0.2 mol L-1Li2S8in TETRAGLYME=100%,volume fraction) was dripped on the cathode,followed by adding 20 μL normal electrolyte(1 mol L-1LiTFSI in DOL/DME (v/v)=1∶1,2%LiNO3) on the lithium anode.For the control sample,MMT or CNT was mixed with PVDF in a weight ratio of 9∶1 to prepare MMT or CNT electrode and battery.The batteries were tested for dissolution and deposition on the Neware CT 4008 battery test system.For the deposition test,the battery was first discharged at a constant current of 0.113 mA until the voltage reaches 2.06 V,and then kept at a constant voltage of 2.05 V until the current was nearly close to zero.And for the dissolution test,the battery was first discharged to 1.7 V at a constant current of 0.1 mA,and then discharged to 1.8 V at 0.01 mA,and then charged at a constant voltage of 2.4 V to ensure that the sulfur species in the battery could be adequately converted to Li2S.
2.4 Preparation of the interlayer
The powder of MMT and CNT (weight ratio of CNT∶MMT=4∶1) was mixed with PVDF in a weight ratio of 9∶1 and then NMP was added to prepare the slurry,and next absolute ethanol was added for sonicated dispersion.The dispersion was vacuum filtered on a Celgard 2500 separator (PP).Afterwards,the sample was dried in vacuum at 60 °C for 12 h to remove the residual solvent to obtain CNT-MMTmodified separator (denoted by PP-CNT-MMT).In a control experiment,the mixture slurry of CNT or MMT and PVDF (mass ratio of 9∶1) coated on the PP,leading to CNT or MMT-modified separators (denoted by PP-CNT,PP-MMT).All samples were prepared into 16 mm diameter of the disc,and the mass loading of material was about 0.28 mg cm-2.
2.5 Material characterizations
Atomic force microscope,(AFM,Bruker,Dimension ICON),field emission scanning electron microscopy (SEM,ZEISS SUPRA 40) and transmission electron microscopy (TEM,FEI Tecnai G2 F20) were used to observe the micro-morphology of the 2D MMT.X-ray diffraction (XRD,Empyrean) using CuKα radiation (45 kV,40 mA,λ=0.154 nm,2θ=10°-60°) was used to characterize the crystal structure of the samples.The Fourier transform infrared spectrometer (FTIR,Termo Fisher Niolet iS10) was used to determine the functional groups and chemical bonds of materials.X-ray photoelectron spectroscopy (XPS,Perkin Elemer PHI) was used to characterize the interaction between 2D MMT and polysulfides.
2.6 Electrochemical measurements
The Li-S batteries were assembled with polypropylene (PP),PP-CNT,PP-MMT and PP-CNT-MMT,respectively,lithium metal as the anode,and sulfur as the cathode.20 μL lithium sulfur electrolyte (1 mol L-1LiTFSI in DOL/DME (v/v)=1∶1,2% LiNO3) was drip-added to both sides of the separator.Discharged and charged galvanostatic of the batteries was performed on the LAND 2001A instrument.Cyclic voltammetry (CV) and electrochemical impedance tests (10 mHz to 1 000 kHz) were conducted on a CHI604e electrochemical workstation.
3 Results and discussion
Morphology of the 2D MMT nanosheets was characterized by AFM.As shown in Fig.1a,the 2D MMT nanosheets had a thickness of about 1.4 nm,which corresponded to the monolayer MMT exposing larger surface area for lithium ions and polysulfides.Scanning electron microscopy (SEM) further confirmed their sheet-like morphology (Fig.1b).The TEM-SAED showed the crystalline structure of the 2D MMT (Fig.1c),which indicated the atomic arrangement of the MMT.The crystalline planes (100)and (110) could be identified on the SAED.The XRD results of the 2D MMT and MMT lamellar crystals have been compared in Fig.1d.As we can see,the two samples have distinct diffraction patterns.The(001) peak assigning to the interlayer spacing of MMT nanosheets locate at 6.2° and 5.9° for the 2D MMT and MMT crystals,respectively,indicating that the decreased interlayer spacing after the exfoliation,which is probably due to the smaller size of lithium ions than the original interlayer cations (such as Na+and Mg2+) in the MMT crystals.Notably,the peak of the MMT lamellar crystal at 26.6° is due to quartz impurity existing in the MMT crystals.The basically consistent Fourier transform infrared spectra (FTIR)of the 2D MMT and MMT suggest the consistency of the functional groups and chemical bonds of MMT before and after the ion exchange exfoliation (Fig.1e).The FTIR results were consistent with the above SAED and XRD results.The absorption peak at 3 610 cm-1is attributed to tensile vibration of the―OH groups.The absorption peaks at 3 440 and 1 640 cm-1belong to the stretching and binding modes of water molecule,respectively.The peaks at 1 040 and 472 cm-1are attributed to the stretching vibration of Si―O―Si groups and Si―O―Al bonds,respectively.It has been reported that the Si―O―Si group on the clay surface is able to attract lithium ions[32].These polar bonds endow the MMT with good chemical adsorption ability for polysulfides.The Zeta potential of -19.3 mV for (Fig.1f) the as-obtained 2D MMT aqueous suspension revealed the negative charges from the ―OH groups and the silicon atoms[33].The negatively charged MMT could provide additional pathways for the lithium ion transportion.
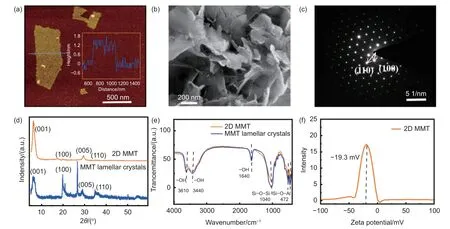
Fig.1 Characterization of 2D MMT.(a) AFM image and the corresponding height profile (inset),(b) SEM image and (c) SAED pattern of the MMT nanosheets.(d) XRD patterns and (e) FTIR spectra of the 2D MMT nanosheets and MMT lamellar crystals.(f) Zeta potential plot of the aqueous suspensions containing 2D MMT
Strong adsorption ability between the interlayer material and polysulfides is an important prerequisite for ensuring efficient polysulfide conversion.Therefore,a visual adsorption test was conducted (Fig.2a).Specifically,MMT (20 mg) and CNT (20 mg) were added into 10 mL Li2S6solution (2 mmol L-1Li2S6in DOL/DME=1∶1 vol%),respectively.After 48 h,the color of the solution containing MMT changed a lot and became almost colorless,while that the one containing CNT interlayer was light yellow.In contrast to the dark yellow of Li2S6solution,the MMT showed higher suppression ability toward LiPS than that of the CNT,which is attributed to the high polarity of MMT and excellent ion accommodation ability between their nanosheets[15,34–35].X-ray photoelectron spectroscopy (XPS) was utilized to characterize the adsorption mechanism of 2D MMT on polysulfides.Fig.2b shows the O 1s spectra of 2D MMT before and after adsorbing Li2S6.The O 1s spectrum of 2D MMT before the adsorption contained 3 characteristic peaks of Al/Mg―O (531.94 eV),Si―O (532.61 eV),and Si―OH (533.87 eV).However,the Si―OH peak at 533.87 eV disappeared after the adsorption while the O―S (531.27 eV) and Li―O (530.67 eV) signals appearred.In Fig.2c the Li 1s spectrum showed the formation of Li―O bond (57.29 eV) after adsorption,further indicating the chemical interaction between the oxygen groups on MMT and polysulfides.To study the polysulfide conversion reaction kinetics,Li2S nucleation test was conducted on the MMT,CNT and CNT-MMT based electrodes,respectively,by using Li2S8as active material (Fig.2d-f,see details in experimental section).Notably,the electrostatic potential discharge curves of the MMT-electrode (Fig.2d)failed to detect nucleation peaks of Li2S,which might be due to poor electrical conductivity.The CNTMMT-electrode (Fig.2f) and the CNT-electrode(Fig.2e) showed the Li2S nucleation capacities of 229.7 and 196.2 mAh g-1,respectively.The 1DCNT/2D-MMT hybrid was more favorable for the conversion of LiPS to Li2S.Furthermore,the CNTMMT-electrode had an earlier responsivity peak time(2 575 s) than the CNT-electrode (6 203 s),hinting its faster conversion kinetics.The fast conversion kinetics is attributed to the stronge adsorption capacity and low lithium ion diffusion barrier of MMT.In addition,electrostatic potential charge experiments were performed to investigate the dissolution kinetics of Li2S.As shown in Fig.2g,the CNT-MMT-based electrode showed higher oxidation current density (1.903 mA)and earlier rinsing time (250 s) than CNT (1.119 mA and 500 s),while the oxidation current of the MMTbased electrode was almost 0,indicating the CNTMMT had the lowest overpotential and fast conversion from Li2S to LiPS.
The 1D-2D interconnected network was investigated as the interlayer for suppressing LSBs.Commercial Celgard separators blade-coating with CNT-MMT and CNT based interlayers were fabricated and denoted as PP-CNT-MMT and PP-CNT,respectively.The cross-sectional SEM and energy dispersive spectroscopy (EDS) (Fig.2h-i) showed that the CNT and MMT were well integrated and uniformly distributed in the whole interlayer,in which the MMT nanosheets adsorb polysulfides through abundant —OH.Meanwhile,the CNT provides a high-conductive network,which is beneficial for promoting the electrochemical conversion of polysulfides.The low mass loading(about 0.28 mg cm-2) the thin thickness of the interlayer avoided the reduction of the gravimetric/volumetric energy density of the battery.
SEM images showed completely different surface topography of the separators.In Fig.3a the pristine PP showed obvious porous structure,which easily allows the polysulfides to pass through the separator.In sharp contrast,the PP-MMT (Fig.3b),PP-CNT (Fig.3c) and PP-CNT-MMT (Fig.3d) coating presented relatively smooth surface.The interlayer formed a barrier to cover the porous structure of PP,physically blocking the polysulfide shuttling.According to the contact angle measurement results(Fig.3e-h),the CNT-MMT-modified separator showed the best electrolyte wettability.Thus,the PPCNT-MMT could facilitate lithium ion transport.The high magnification SEM image clearly exhibited the interconnected porous network formed by the 1D CNT and 2D MMT Fig.3i.The CNT not only increased the conductivity of the interlayer,but also prevented the stacking of 2D MMT nanosheets,which was beneficial for the explosion of the polar surfaces of 2D MMT nanosheets.The uniform distribution of CNT and MMT enhanced the adsorption for polysulfides due to the larger exposed surface area and promoted the polysulfide transformation owing to the well-interconnected ion/electronic transport network.As shown in Fig.3j,the PP-CNT showed small cracks even after folding,whereas the CNT-MMT modified separator had no cracks or fissures after being folded.The porous CNT-MMT interlayer could maintain good structural stability to inhibit polysulfide shuttling well during the battery cycling.

Fig.3 (a-d) SEM images of PP,PP-MMT,PP-CNT and PP-CNT-MMT.(e-h) The electrolyte contact angles on various separators.(i) High magnification SEM image of PP-CNT-MMT.(j) Digital photos of PP-CNT and PP-CNT-MMT before and after folding
Li-S coin cells were assembled to evaluate the electrochemical performances with the modified separators.Within Li-S batteries,the rapid diffusion of lithium ions can accelerate the uniform deposition of polysulfides.The CV curves of the batteries with different separators were measured at different scan rates as shown in Fig.4(a-d).The lithium ion diffusion coefficient (DLi+) was calculated based on Randles-Sevick equation according to the CV curves.TheDLi+has been compared in Fig.4e.TheDLi+of PP-MMTCNT is much larger than those of PP,PP-MMT and PP-CNT.This could be explained by the following 3 reasons: (1) The interconnected porous CNT-MMT hybrid network,(2) low lithium ion transport barrier on the 2D MMT,(3) the better affinity of the CNTMMT to electrolyte.

Fig.4 (a-d) CV curves of the batteries with different separators at the scan rates of 0.1,0.2,0.3 and 0.4 mV s-1.(e) Lithium ion diffusion coefficient(DLi+) of the cells with PP,PP-MMT,PP-CNT and PP-CNT-MMT
The Li-S batteries using PP-CNT-MMT exhibited superior electrochemical performance.This was due to the enhanced polysulfide conversion kinetics of the MMT-CNT interlayer that could act as a secondary collector for adsorbing polysulfides and reusing the sulfur species dissolved in the electrolyte.In Fig.5a,the Nyquist plots of the batteries displayed semicircles at the high-frequency range,corresponding to the charge transfer resistance (Rct).The batteries using PP-CNT-MMT (78.2 Ω) and PP-CNT (45.5 Ω)had lowerRctthan those using the pristine PP (105.8 Ω)and PP-MMT (96 Ω).The low charge transfer resistances were ascribed to the high electronic conductivity of the CNT,which was conducive to promoting the redox conversion of polysulfides.As shown in the GCD curves at 0.1 C (Fig.5b),the battery with PPCNT-MMT delivered an initial discharge capacity of 1 337 mAh g-1,much higher than those with PP-MMT(1 147 mAh g-1),PP-CNT (1 199 mAh g-1) and pristine PP (1 167 mAh g-1).Additionally,the battery with PP-CNT-MMT exhibited a polarization of 0.185 V,smaller than those with PP-MMT (0.190 V),PP-CNT (0.194 V) and pristine PP (0.203 V).These results indicated that PP-CNT-MMT accelerated the electrochemical redox reaction kinetics,contributing to the increase of the utilization of active substances.The rate performance of the cells was shown in Fig.5c.The cell with PP-CNT-MMT had the highest specific capacity of discharge at all rates from 0.1 C to 3 C,with a specific capacity of 536 mAh g-1at 3 C.When the current density switched back to 0.1 C,the cell with PP-CNT-MMT delivered a specific capacity of 751.8 mAh g-1.The PP-CNT and PP-MMT had comparable discharge specific capacities and both outperform the original PP separator.At high current densities,the cell using PP-CNT separator had a slightly higher specific discharge capacity than those using PP-MMT separator,which was attributed to the intrinsic electronic insulation of MMT.The CV curves (Fig.5d) of the cells with PP-CNT-MMT overlaped well for the first 3 cycles,indicating good chemical stability and reversibility.
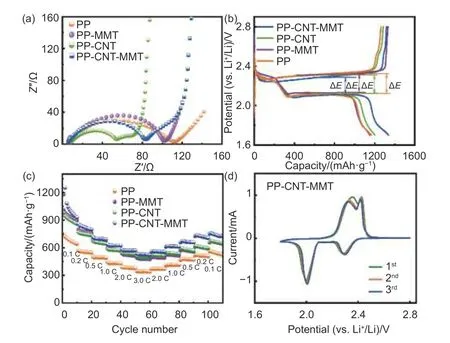
Fig.5 (a) Nyquist plots,(b) galvanostatic charge/discharge profiles at 0.1 C and (c) rate performance of the Li-S battery with PP-CNT-MMT,PPCNT,PP-MMT and PP,respectively.(d) CV curves for the 1st,2nd and 3rd cycles of the cell with PP-CNT-MMT at a sweep rate of 0.1 mV s-1
The cycling stability of the batteries with different separator was also studied in Fig.6a-c.Since the 2D MMT endowed the MMT/CNT hybrid interlayer with excellent structural integrity,good chemisorption for lithium polysulfides,and low lithium ion transport energy barrier,the battery with PP-CNTMMT displayed superior initial discharge capacity of 988.4 mAh g-1at 0.2 C.A high capacity of 817 mAh g-1could be retained over 100 cycles (Fig.6a).In contrast,the batteries with PP-MMT,PP-CNT and pristine PP decayed from 912,826,805 mAh g-1to 723,666,559 mAh g-1,respectively.The highest capacity of the batteries with PP-MMT demonstrated the highest sulfur utilization.As shown in Fig.6b,the discharging specific capacity of the battery using PPCNT-MMT was superior to others at 0.5 C.The longterm cycling performance of these batteries at 1 C were also compared in Fig.6c.For the cell with PPCNT-MMT,the specific capacity was 784 mAh g-1at 1 C with a capacity decay rate of 0.062% per cycle over 500 cycles,much lower than that using PP(0.090%) and PP-CNT (0.065%).

Fig.6 The cycle performance of the batteries with PP-CNT-MMT,PP-MMT,PP-CNT and PP at (a) 0.2 C and (b) 0.5 C with sulfur loading of 1.0 mg cm-2.(c) The long-term cycling performance of these batteries at 1 C
4 Conclusion
In summary,the CNT-MMT hybrid interlayer has been prepared to inhibit the shuttling of LiPS.The high electronically-conductive CNT offered fast electron transport passages and avoided the re-stacking of 2D MMT nanosheets.The 2D MMT nanosheets effectively adsorbed LiPS and accelerated the electrochemical reaction kinetics owing to the low energy barriers for lithium ion transportation.Moreover,the good film formation of 2D MMT provided good structural stability during the battery service.Last but not the least,the formed porous interconnected ion/electronic network ensured the instant conversion of the adsorbed LiPS.Consequently,the batteries with PPCNT-MMT delivered a high initial specific capacity of 1 337 mA h g-1at 0.1 C and a low capacity decay of 0.062% per cycle at 1 C over 500 cycles.This work presents that the suppression ability for the polysulfide shuttling of the PP-CNT-MMT has obvious and its potential application in high-energy-density lithiumsulfur batteries.
Data availability statement
The data that support the findings of this study are openly available in Science Data Bank at https://www.doi.org/10.57760/sciencedb.j00125.00061 or https://resolve.pid21.cn/31253.11.sciencedb.j00125.00061.
Acknowledgements
This work was supported by National Natural Science Foundation of China (51972070,52372185 and 52062004),Guizhou Provincial High Level Innovative Talents Project (QKHPTRC-GCC[2022]013-1),Innovation Team for Advanced Electrochemical Energy Storage Devices and Key Materials of Guizhou Provincial Higher Education Institutions (QianJiaoJi[2023]054),Guizhou Provincial Science and Technology Projects (QKHJC[2020]1Z042,QKHZC[2021]YB317) and Cultivation Project of Guizhou University (GDPY[2019]01) .
杂志排行
新型炭材料的其它文章
- Carbon-based photothermal materials for the simultaneous generation of water vapor and electricity
- 生物质炭材料作为金属空气电池阴极的研究进展
- 3D porous NiCo2(CO3)3/reduced graphene oxide aerogel with heterogeneous interfaces for high-efficiency microwave absorption
- 煤基富氧多孔炭纳米片的制备及其超级电容器性能
- Mott-Schottky heterojunction formation between Co and MoSe2 on carbon nanotubes for superior hydrogen evolution
- A one-pot method to prepare a multi-metal sulfide/carbon composite with a high lithium-ion storage capability
