Mott-Schottky heterojunction formation between Co and MoSe2 on carbon nanotubes for superior hydrogen evolution
2023-12-20RENXianpeiHUQiweiLINGFangWUFeiLIQiangPANGLiuqing
REN Xian-pei,HU Qi-wei,LING Fang,WU Fei,LI Qiang,PANG Liu-qing
(1.School of Physics and Electronic Engineering, Sichuan University of Science and Engineering, Zigong 643000, China;2.The First Monitoring and Application Center, CEA, Tianjin 300180, China;3.Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai University, Tianjin 300071, China)
Abstract: Molybdenum selenide (MoSe2) has been regarded as an advanced electrocatalyst for the hydrogen evolution reaction(HER).However,its electrocatalytic performance is far inferior to platinum (Pt).Combining semiconductors with metals to construct Mott-Schottky heterojunctions has been considered as an effective method to enhance HER activity.In this work,we report a typical Mott-Schottky heterojunction composed of metal Co and semiconductor MoSe2 on carbon nanotubes (Co/MoSe2@CNT),prepared by a sol-gel process followed by thermal reduction.The characterization and theoretical calculations show that a Co/MoSe2 Mott-Schottky heterojunction can cause electron redistribution at the interface and form a built-in electric field,which not only optimizes the free energy of hydrogen atom adsorption,but also improves the charge transfer efficiency during hydrogen evolution.Thus,the Co/MoSe2@CNT has excellent catalytic activity with a low overpotential of 185 mV at 10 mA cm-2 and a small Tafel slope of 69 mV dec-1.This work provides a new strategy for constructing Co/MoSe2 Mott-Schottky heterojunctions and highlights the Mott-Schottky effect,which may inspire the future development of more attractive Mott-Schottky electrocatalysts for H2 production.
Key words: MoSe2;Co nanoparticles;Mott-Schottky heterojunction;Hydrogen evolution reaction
1 Introduction
Hydrogen,as a renewable and clean energy source,has great potential to replace traditional fossil fuels,thanks to advantages like high energy density and zero effect of combustion product on environment[1–2].Electrocatalytic water splitting is a very promising approach to produce molecular hydrogen[3–5].However,during the actual hydrogen evolution process,it is necessary to overcome a high overpotential,which will reduce the energy conversion rate.Among numerous hydrogen evolution reaction (HER) electrocatalysts,Pt-based materials still remain the most efficient electrocatalysts for HER,which have significantly hindered their practical applications on a large scale because of their high-cost and scarcity[6–7].Therefore,developing efficient and inexpensive non-Pt electrocatalysts has important practical significance for HER.
Till date,layered transition metal dichalcogenides,such as MoS2[8,9],MoSe2[10–11],VS2[12–13],VSe2[14],WS2[15],WSe2[16],MoTe2[17]and WTe2[18]have aroused great interest as promising alternatives to noble metal catalysts because of low costs,natural abundance and adjustable intrinsic electrocatalytic activity.Among these studied alternatives,molybdenum diselenide(MoSe2),which is composed of covalently bonded Mo and Se atoms in 3 parallel planes,has been recently considered as a potential effective catalyst due to its adjustable active sites and excellent stability[19].However,for pure MoSe2,limited by its own electronic structure,the HER performance is not very competitive[20].Therefore,optimizing the electronic structure of MoSe2is the most fundamental method to improve its catalytic performance.For instance,Qian et al.reported that doping of Zn atoms in MoSe2can effectively activate the HER activity of Se atoms in the basal plane and improve the conductivity,which in turn leads to excellent electrocatalytic performance[21].Li and co-workers designed a MoSe2/MoS2heterostructure as an excellent HER electrocatalyst.They found that the rich interfaces in MoSe2/MoS2heterostructure not only provide more active sites for HER,but also promotes charge transfer and therefore the enhanced electrochemical performance[22].Similarly,to optimize the electronic structure of MoSe2,excellent electrocatalysts such as N-MoSe2[23],S-MoSe2[24],Co-MoSe2[25],MoSe2/ZnO and ZnSe/MoSe2have been developed[26–27].
According to the Mott-Schottky effect[28],when an appropriate metal contacts with a semiconductor,electrons can flow spontaneously at the metal/semiconductor heterostructure interface until the Fermi energy levels on both sides are equal.This flow of electrons can effectively optimize interfacial electron distribution,which may enhance electrocatalytic activity[29].For example,Xue and his co-workers used successive pyrolysis and phosphidation steps to prepare Co/CoP Janus nanoparticles,and their research results indicate that the redistribution of electrons at the Co/CoP heterojunction interface can significantly enhance the HER and OER activity in acid,base and neutral media[30].Chen et al.reported the construction of a free standing vertically arranged hollow porous heterostructure with an enormous number of Co/a-WOxMott-Schottky micro-interfaces on carbon cloth and achieved excellent hydrogen production performance[31].Sun et al.obtained a Mo-MoS2Mott-Schottky heterojunction via in-situ lithiation technology which can significantly optimize the interfacial electron structure of S sites and improve the proton adsorption ability under all pH environments[29].To date,many Mott-Schottky heterojunctions have been constructed to enhance hydrogen evolution performance,such as Ni/CeO2heterojunction[32],Co-NC@W2N Schottky heterojunction[33],and so on.Based on this charge redistribution,the Mott-Schottky effect can be reasonably applied to the design and preparation of MoSe2-based catalysts,which has been rarely reported so far.
In this work,we design a Mott-Schottky electrocatalyst consisting of metal Co and semiconductor MoSe2grownin-situon carbon nanotube (CNT) surfaces (denoted as Co/MoSe2@CNT) by a combined sol-gel and thermal reduction method.Experimental and density functional theory (DFT) calculation results indicate that the Co/MoSe2Mott-Schottky heterojunction induces electron transfer from the MoSe2side to Co side,leading the local electronic reconfiguration at the interface,optimizing the chemisorption free energies of reaction intermediates,and thus improving the intrinsic electrocatalytic activity.Accordingly,the as-synthesized Co/MoSe2@CNT exhibits outstanding electrocatalytic activity towards the HER in 0.5 mol L-1H2SO4medium,as reflected by the overpotential of 185 mV at 10 mA cm-2and Tafel slope of 69 mV dec-1.
2 Experimental section
2.1 Preparation of MoSe2@CNT electrocatalysts
MoSe2@CNT was synthesized by the typical solgel method.In brief,273 mg molybdenum chloride(MoCl5) and 312 mg diphenyl diselenide (C12H10Se2)was dissolved into 30 mL ethanol at room temperature and stirred for 10 min to form a uniform solution.Then,96 mg CNT was added into the prepared solution and sonicated for 30 min to obtain a homogeneous suspension.After drying,a gel-like precursor is obtained,and then transferred into a ceramic boat and heated to 500 °C for 2 h in an argon atmosphere.Afterwards,the product was cooled to room temperature under the protection of argon gas and collected.
2.2 Preparation of Co@CNT and Co/MoSe2@CNT
Co@CNT was synthesised by a thermal reduction method as follow: 5 mg Cobalt Oxalate(CoC2O4·2H2O) was dissolved in 30 mL hot ethanol and for 30 min to achieve a uniform solution.Then,14 mg CNT was added and stirred for 0.5 h to form a well-dispersed suspension.Subsequently,the precursor was formed after drying and transferred into a ceramic boat and heated to 500 ℃ for 2 h in an Ar atmosphere.After that,the product was cooled to room temperature under the protection of Ar gas and collected.
Meanwhile,Co/MoSe2@CNT was also synthesized by adding 50 mg obtained MoSe2@CNT instead of 14 mg CNT while following the synthesis steps.
2.3 Materials characterization
The morphology of the samples was analyzed using a JSM-7610F scanning electron microscopy(SEM).Morphological and microstructural observations were performed using a transmission electron microscopy (TEM,FEI,Tecnai G2).The phase and crystalline structures of the samples were obtained by a Bruker D8-Advance.Chemical states of the samples were analyzed via X-ray photoelectron spectroscopy(XPS,Thermo-VG Scientific,ESCALAB 250).Raman spectra were carried out on the Renishawin Via micro-Raman spectrometer with 532 nm laser excitation.
2.4 Electrochemical measurement
All electrochemical measurements were conducted using a traditional 3 electrode system on a CHI 604E electrochemical workstation.In a three-electrode system,electrocatalyst modified glassy carbon electrode (GCE) was used as the working electrode,and an Ag/AgCl (in 3.5 mol L-1KCl solution) electrode and a Pt wire electrode were used as the reference electrode and the counter electrode,respectively.The preparation of the working electrode is described as follows: 5 μL mixed liguor of 2.0 mg catalyst,100 μL ethanol,400 μL deionized water and 40 μL Nafion solution (5 wt% Sigma-Aldrich) was dropped onto the polished GCE surface and dried.Linear sweep voltammetry (LSV) was conducted in 0.5 mol L-1H2SO4saturated with N2at a scan rate of 10 mV s-1and the overpotentials were calibrated by the use of equationERHE=EAg/AgCl+0.205+0.0591×pH.All the overpotentials were converted into a reversible hydrogen electrode (RHE).The electrochemical impedance spectroscopy (EIS) was recorded in the same configuration with an AC voltage amplitude of 5 mV atη=0.4 V from 106to 10-2Hz.The electrochemical surface area (ECSA) of the samples was estimated by cyclic voltammograms with different scan rates (20-100 mV s-1) in the 0.1-0.2 Vvs.RHE region.
2.5 Computing methods
The detailed calculation information has been provided in the electronic supplementary information.
3 Results and discussion
The morphology and microstructure of the prepared catalysts was studied using SEM and TEM.From Fig.1a-c,it can be clearly seen that the 3 samples display a carbon nanotube network morphology.This structure is expected to accelerate the rapid diffusion of electrolyte ions and the release of the H2gas bubbles by water splitting.There are no significant changes in morphology for MoSe2@CNT and the CNT network structure is well preserved in Co/MoSe2@CNT after the growth of Co nanoparticles.The bright field TEM image of Co/MoSe2@CNT shown in Fig.1d indicates that there are some dark areas within and on the surface of CNTs.The high resolution TEM (HRTEM) image shown in Fig.1e clearly illustrate that the dark areas are Co/MoSe2heterojunction,indicating that the nanosheets were composed of 2-4 atomic layers stacking together.In addition,it can be seen that the length of lattice stripes does not exceed 10 nm.Due to the confinement effect of CNT,MoSe2nanosheet has ultra-thin thickness and ultra-short lattice length,which can provide a large number of exposed active edges and potentially lead to enhanced electrocatalytic activity.This confinement effect of CNT has also been reported in previous literatures[13,34].Careful examination of the flake's inner plane under HRTEM (Fig.1f)revealed the prevalent presence of Co/MoSe2heterointerfaces.The well-resolved interplanar spacings of 0.21 and 0.67 nm are attributed to the Co (111) and MoSe2(002),respectively.This indicates the successful formation of Co/MoSe2Mott-Schottky heterojunction.In addition,the inter-planar spacings of 0.35 nm is distributes to the (002) lattice planes of CNTs,indicating a close contact was formed between Co/MoSe2and CNTs during the material synthesis process.Moreover,the high-angle annular dark-field scanning transmission electron microscopy (HAADFSTEM) and the elemental mapping analysis (Fig.1g)were also used to obtain the spatial distribution of the elements,proving the presence of C,Co,Mo and Se elements on the surface of Co/MoSe2-CNT.These results demonstrate the successful construction of the Co/MoSe2Mott-Schottky heterojunction on the CNTs.
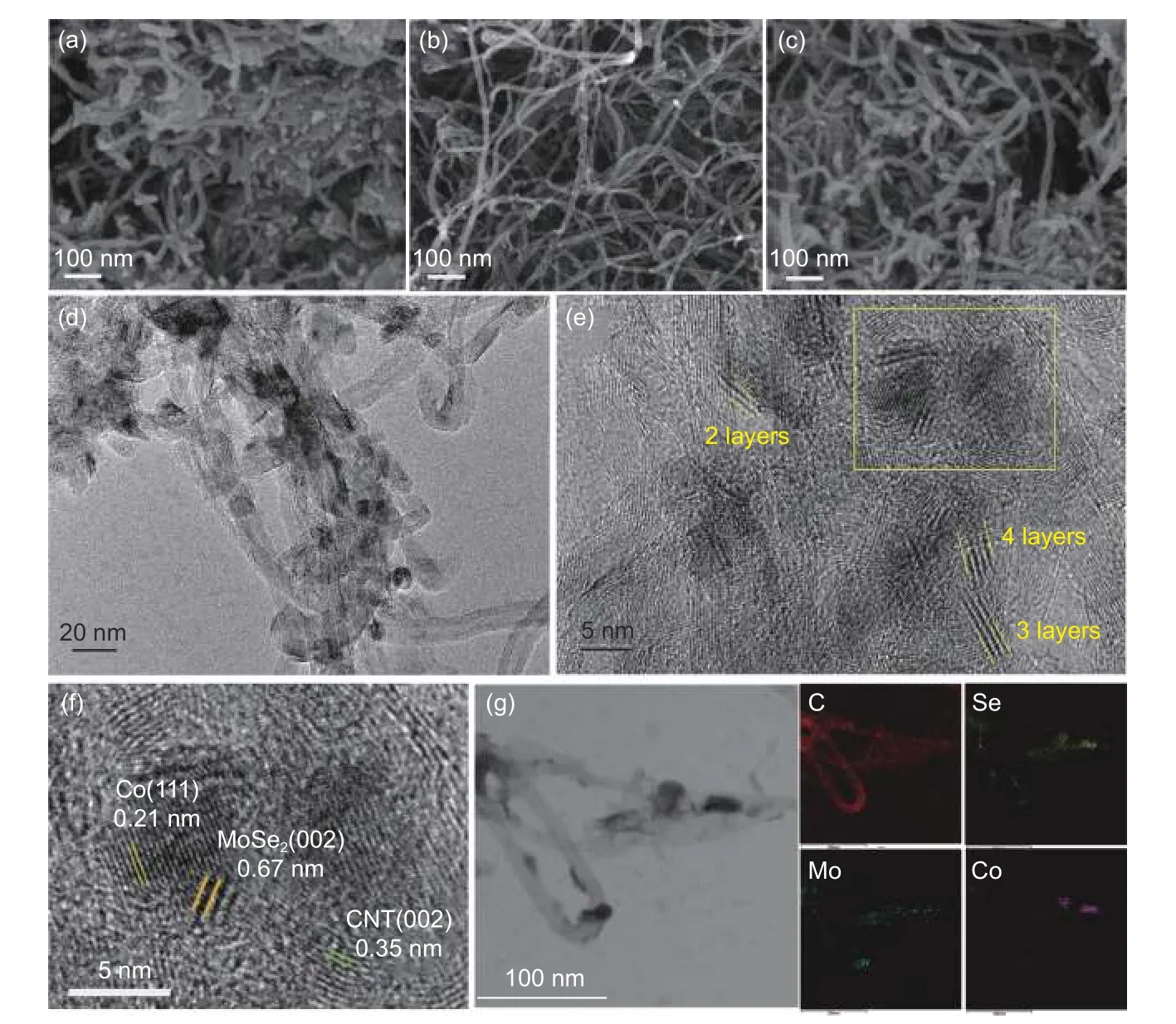
Fig.1 (a-c) SEM of MoSe2@CNT,Co@CNT and Co/MoSe2@CNT.(d) TEM image and (e) HRTEM image of Co/MoSe2@CNT.(f) An enlarged region indicated by a box in (e).(g) HAADF-STEM image and corresponding element mappings of the Co/MoSe2@CNT sample
To validate the mixed phase and chemical structure,XRD patterns of the Co@CNT,MoSe2@CNT and Co/MoSe2@CNT samples were collected as shown in Fig.2a.The sharp peak at 2θ=26.1° in all the curves can be readily indexed to the (002) crystal plane of CNTs.For MoSe2@CNT and Co/MoSe2@CNT,the diffraction peaks at about 13.6°,31.9°,37.2° and 53.6° corresponded to the (002),(100),(103) and (110) crystal planes of MoSe2,and the diffraction peaks at 44.3° and 51.5° corresponded to the (111) and (200) crystal planes of Co (PDF#97-067-1069),respectively.Besides these peaks,three small weak diffraction peaks were identified at 36.5°,42.3° and 61.5°,which correspond to the (111),(200)and (220) phases of CoO (PDF#97-007-6638),respectively,proving the small presence CoO phase in the Co@CNT and Co/MoSe2@CNT samples.Fig.2b suggested the Raman spectra of MoSe2@CNT and Co/MoSe2@CNT,validated the coexistence of the 1T and 2 H MoSe2phases.The peaks at 234.1 and 276.7 cm-1represent the out-of-planeA1gand in-planeE12gvibration modes of 2H MoSe2[35],respectively,while the unique peaks at 118.5,144.1,365.1 and 455.4 cm-1belonged to theJ1,J2,J4andJ3phonon modes of 1TMoSe2,respectively[36].In addition,the B12gmode,which is inactive for MoSe2bulk,is present at 332.9 cm-1for MoSe2@CNT and Co/MoSe2@CNT[37],confirming their few-layered composition.

Fig.2 (a) XRD patterns of Co@CNT,MoSe2@CNT and Co/MoSe2@CNT.(b) Raman spectra of Co/MoSe2@CNT and Co/MoSe2
An in-depth analysis was conducted to resolve the surface chemical composition and electronic state using XPS.The investigation spectrum in Fig.3a shows the presence of Co,Mo,Se,O and C elements in Co/MoSe2@CNT,which is very consistent with the EDS results (Fig.1g).Fig.3b shows Mo 3d core-level XPS spectra of the 2 samples.The two main peaks at 232.23 and 229.15 eV,corresponding to Mo 3d3/2and Mo 3d5/2,respectively,ascribed to 1T-MoSe2,while the 2 peaks located at 232.83 and 230.39 eV belong to 2H-MoSe2[38–39],suggesting that our sample contains a mixture of the 2 phases,which is consistent with the Raman spectra in Fig.2b.The peak at 235.67 eV belongs to Mo6+3d3/2and is due to the partial oxidation of Mo4+[40]in air.Likewise,the peaks of Se 3d can also be deconvoluted into 2 pairs of peaks (Fig.3c).For Co/MoSe2@CNT,a pair with lower energies (56.33 eV for Se 3d3/2and 54.81 eV for Se 3d5/2) is attributed to the 1T phase,and a pair with higher energies (57.07 eV for Se 3d3/2and 55.66 eV for Se 3d5/2) to the 2H phase[41].Furthermore,the electronic interaction between Co and MoSe2induces a positive shift of about 0.1 eV in the Se peaks compared with the MoSe2@CNT,which indicates the decreasing surface electron density of MoSe2because of Co load.Fig.3d presents the high-resolution spectra of Co 2p for Co/MoSe2@CNT and Co@CNT.The Co 2p spectra of Co/MoSe2@CNT exhibit 6 peaks,including Co02p at 778.97 and 793.89 eV,Co2+2p at 781.21 and 797.55 eV,and 2 shakeup satellites (Sat.) peak at 802.85 and 785.56 eV,respectively[42–43].Notably,the binding energy of transition metal Co of Co/MoSe2@CNT negatively shifts by~1.4 eV as compared with that in Co@CNT,indicating the Co/MoSe2heterojunction could lead to an increase in the electron density of Co.
In order to study the effect of Mott-Schottky heterojunction on the catalytic activity of the Co/MoSe2@CNT,the HER performance was evaluated using a traditional three-electrode system in the N2-saturated 0.5 mol L-1H2SO4solution.A set of reference samples,including Co@CNT,MoSe2@CNT and commercial Pt foil,were also examined and compared under the same test condition.The linear sweep voltammetry (LSV) curves of the catalysts are displayed in Fig.4a.Undoubtedly,Pt catalyst shows impressive HER activity with an overpotential of 31 mV(η10) to drive the current density of 10 mA cm-2,which is well consistent with the values of previous reports[44].The Co/MoSe2@CNT only requires a small overpotential of 185 mV to deliver a current density of 10 mA cm-2,significantly better than those of Co@CNT (439 mV) and MoSe2@CNT (239 mV)(Fig.4b).In addition,in striking contrast to Co@CNT and MoSe2@CNT,the Co/MoSe2@CNT exhibits a higher current density as the applied potential negatively increases,implying better HER catalytic activity.When the cathode current density is greater than 90 mA cm-2,the electrocatalytic performance of Co/MoSe2@CNT is better than that of Pt,highlighting the key contribution of Co/MoSe2Mott-Schottky heterojunction.

Fig.4 (a) LSV polarization curves,(b) η10 values,and (c) the corresponding Tafel plots of Co@CNT,MoSe2@CNT,Co/MoSe2@CNT,and Pt catalyst at a sweep rate of 10 mV s-1 in 0.5 mol L-1 H2SO4,respectively.(d) The Nyquist plots with the equivalent circuit given inset.(e) Electrochemical cyclic voltammogram of Co/MoSe2@CNT at various scan rates (20-100 mV s-1).(f) Cdl values of the different Pt-free catalysts
The corresponding Tafel plots are shown in Fig.4c.As expected,Pt foil shows the optimal Tafel slope of 36 mV dec-1.The Tafel values for Co/MoSe2@CNT,MoSe2@CNT,and Co@CNT are determined to be 69,75 and 120 mV dec-1,respectively.The Tafel slope is generally considered to be closely related to the catalysis reaction kinetics,so Co/MoSe2@CNT exhibits enhanced HER kinetics than MoSe2@CNT and Co@CNT.Notably,the HER performance of the obtained Co/MoSe2@CNT,within the context of overpotential (185 mV) at 10 mA cm-2and Tafel slope (69 mV dec-1),is comparable to the most active MoS2-based and MoSe2-based HER electrocatalysts (Table S1).
Additionally,electrochemical impedance spectroscopy (EIS) measurements were performed to analyze the reaction kinetics in catalytic process.Analysis of the Nyquist plots (Fig.4d) shows that the chargetransfer resistance (Rct) of Co/MoSe2@CNT electrode is much lower than those of MoSe2@CNT and Co@CNT,indicating faster and more efficient electron transfers during the electrocatalytic process at the Co/MoSe2interface.
The electrochemical active surface area (ECSA)of the catalyst can be calculated by the electrochemical double-layer capacitance (Cdl) value.The Cdlof Co/MoSe2@CNT,MoSe2@CNT and Co@CNT are estimated by cyclic voltammetry (CV) tests within the non-Faradaic potential range (0.10-0.20 V vs.RHE) at different scanning rates (20-100 mV s-1,Fig.4e and Fig.S1).As displayed in Fig.4f,theCdlvalue of Co/MoSe2@CNT is 8.09 mF cm-2,which is signifiantly larger than MoSe2@CNT (3.43 mF cm-2) and Co@CNT (6.44 mF cm-2).This result also affirms that the formation of Co/MoSe2heterojunction is conducive to the increase of active sites,which leads to an increase in electrochemical activity.Other than the excellent HER activity,the Co/MoSe2@CNT also shows good stability (Fig.S2).
In order to clarify the compositional synergy between the metallic Co and the semiconducting MoSe2phases in the Mott-Schottky electrocatalyst,the basic principles that endow the excellent HER performance of Co/MoSe2@CNT is further studied.On the basis of semiconductor physics theory,the work functions of the metal Co and the semiconductor MoSe2play a decisive role in determining the electron transfer behavior at the Co/MoSe2interface.As shown by the energy band diagrams (Fig.5),the work functions of Co and MoSe2are estimated to be 5.0 and 4.57 eV[22,45],respectively,satisfying the requirement for establishing a Schottky barrier.Thus,once Co and MoSe2are in direct contact,electrons spontaneously transfer from MoSe2to Co side across the Co/MoSe2interface until the Fermi levels reach equilibrium on both sides.Consequently,electrons will be enriched on the nucleophilic Co side,while holes will accumulate on the electrophilic MoSe2side,creating a built-in electric field to provide a directional high-speed way for continuous electron flow.
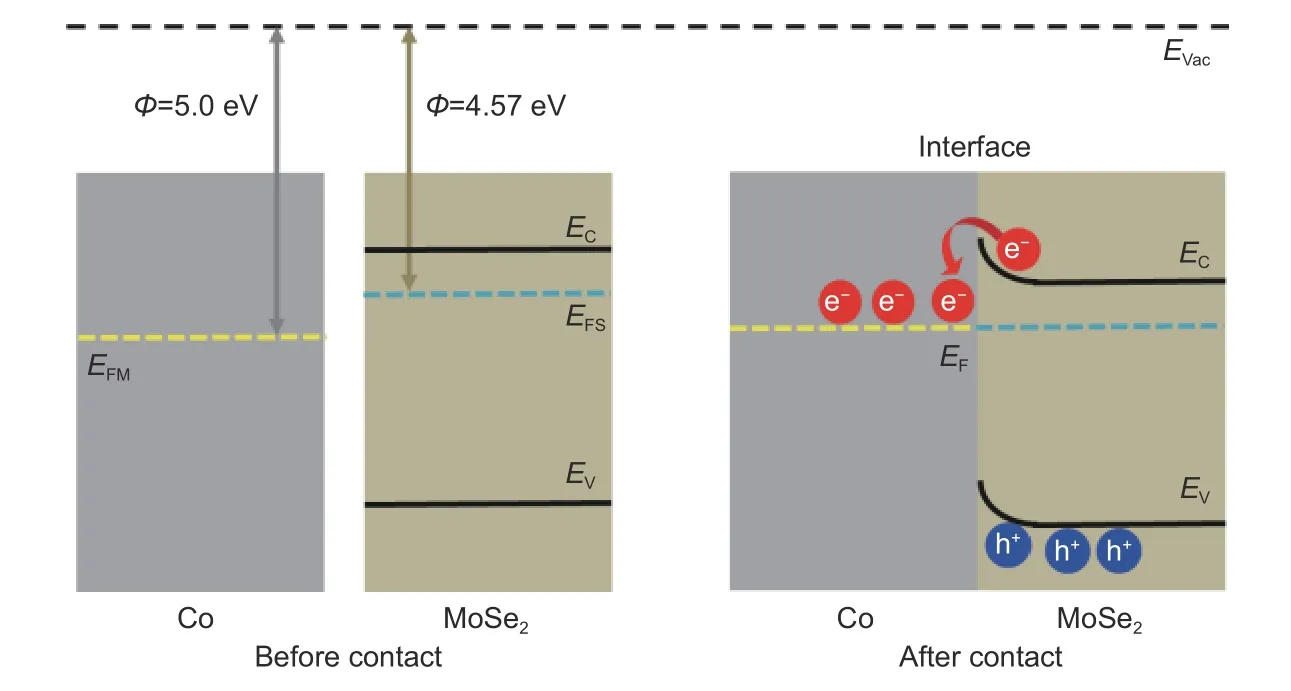
Fig.5 The proposed energy band diagrams of Co and MoSe2 before contact and after contact (Mott-Schottky heterojunction),where EF,Evac,Ev and Ec represents the Fermi energy,vacuum energy,valence band and conduction band potentials,respectively
The enhancement mechanism of Co/MoSe2heterojunction for HER was elucidated by density functional theory (DFT) calculations.The computational models of Co,MoSe2and Co/MoSe2heterostructure are shown in Fig.S3.The density of states (DOS) diagrams (Fig.6a-c) show that compared with a single MoSe2,the energy gap of Co/MoSe2is partially occupied by the Co 3d orbit,which can effectively reduce the energy barrier of the electron transmission and improve the electronic conductivity,thereby accelerating reaction kinetics.The difference in charge density of the Co/MoSe2heterojunction shows the electron accumulation on Co side (yellow region) and electron depletion on the adjacent MoSe2side (cyan region)(Fig.6d),which further affirms the electron transfer from MoSe2to Co and agrees well with the XPS results.It is well known that the electrocatalytic activity is closely related to the free energy adsorbed by hydrogen onto the catalyst surface,and the ΔGH*of an ideal HER catalyst is close to zero[46].The calculated ΔGH*of 3 constructed modes are given in Fig.6e.The ΔGH*of the bare MoSe2is determined to be 1.892 eV,indicating its poor HER characteristics.Moreover,when Co atom is coupled with MoSe2,the ΔGH*value significantly reduces down to -0.278 eV,indicating that the HER activity of MoSe2can be greatly improved by growing Co on its surface.

Fig.6 DOS curves for (a) Co,(b) MoSe2 and (c) Co/MoSe2 heterojuctions;(d) The charge density difference in the interface between Co and MoSe2;(e) Gibbs free energy diagrams of Co,MoSe2 and Co/MoSe2
According to the experimental results and theoretical calculations,the enhanced HER performance of Co/MoSe2@CNT can be reasonably attributed to the Mott-Schottky heterojunction effect and structural merits.First,due to the different work functions of Co and MoSe2,the formation of Co/MoSe2heterojunction leads to the electronic redistribution at the Co/MoSe2interface,thereby improving the charge transfer efficiency and reducing the reaction energy barriers,and therefore enhancing the intrinsic HER activity.Second,the complex CNT-intertwined networks not only expands the exposure of electrocatalytic active components and increases the number of active sites,but also shortens the diffusion pathway of hydrogen ion.
4 Conclusions
In summary,a well-formed Co/MoSe2Mott-Schottky heterojunction has been successfully synthesized on the surface of CNTs through an effective method involving sol-gel and thermal reduction approach.The results show that Co/MoSe2@CNT exhibits outstanding HER performance in acidic medium,with an overpotential of 185 mV at a current density of 10 mA cm-2and a small Tafel slope of 69 mV dec-1.The experimental demonstrations and theoretical calculations suggest that such excellent HER performance could be attributed to the Mott-Schottky effect between Co nanoparticles and MoSe2nanosheets.Therefore,the design concept and synthetic approach in this work can be expected to provide guidance for developing efficient Mott-Schottky heterojunction electrocatalysts for hydrogen evolution.
Data availability statement
The data that support the findings of this study are openly available in Science Data Bank at https://www.doi.org/10.57760/sciencedb.j00125.00063 or https://resolve.pid21.cn/31253.11.sciencedb.j00125.00063.
Acknowledgements
This work was supported by the Sichuan Natural Science Foundation Project (22NSFSC0335) and the fund of the State Key Laboratory of Catalysis in DICP(N-22-14).
杂志排行
新型炭材料的其它文章
- Carbon-based photothermal materials for the simultaneous generation of water vapor and electricity
- 生物质炭材料作为金属空气电池阴极的研究进展
- 3D porous NiCo2(CO3)3/reduced graphene oxide aerogel with heterogeneous interfaces for high-efficiency microwave absorption
- 煤基富氧多孔炭纳米片的制备及其超级电容器性能
- A 2D montmorillonite-carbon nanotube interconnected porous network that prevents polysulfide shuttling
- A one-pot method to prepare a multi-metal sulfide/carbon composite with a high lithium-ion storage capability
