3D porous NiCo2(CO3)3/reduced graphene oxide aerogel with heterogeneous interfaces for high-efficiency microwave absorption
2023-12-20WUDandanZHANGHanxiaoWANGZhengyanZHANGYanlanWANGYongzhen
WU Dan-dan,ZHANG Han-xiao,WANG Zheng-yan,ZHANG Yan-lan,*,WANG Yong-zhen,*
(1.College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China;2.Shanxi Joint Laboratory of Coal based Solid Waste Resource Utilization and Green Ecological Development, Taiyuan 030024, China)
Abstract: Advanced electromagnetic absorbing materials (EAMs) with strong absorption and a wide effective absorption bandwidth (EAB),using innovative microstructural design and suitable multicomponents remain a persistent challenge.Here,we report the production of a material by the hydrothermal reduction of a mixture of graphene oxide (GO),Ni(NO3)2·6H2O,and Co(NO3)2·6H2O,resulting in reduced GO (RGO) with a self-assembled 3D mesh structure filled with NiCo2(CO3)3 .The unique microstructure of this assembly not only solves the problem of NiCo2(CO3)3 particles agglomerating but also changes the electromagnetic parameters,thereby improving the impedance matching and attenuation ability.High electromagnetic wave absorption (EMA) was achieved by combining the 3D interconnected mesh structure and the various interfaces between NiCo2(CO3)3 and RGO.The minimal reflection loss (RLmin) was -58.5 dB at 2.3 mm,and the EAB was 6.5 GHz.The excellent EMA performance of the aerogel can be attributed to the multiple reflection,scattering,and relaxation process of the porous 3D structure as well as the strong polarization of the interfacial matrix.n of the interfacial matrix.
Key words: NiCo2(CO3)3/RGO aerogel;Structural design;Interface polarization;Microwave absorption
1 Introduction
Information science and artificial intelligence have developed rapidly in the 5G era,and the popularity of electronic devices has significantly improved along with people’s quality of life.The associated electronic devices have become more intelligent,versatile,and wearable.However,electromagnetic interference and radiation caused by some electronic devices threaten the ecological environment and military security.Therefore,the development of advanced electromagnetic absorbing materials (EAMs),which dissipate electromagnetic wave energy into heat through their inherent magnetic and dielectric losses,has garnered significant attention from an increasing number of theoretical researchers,both at home and abroad[1–2].Over past decades,different materials (including carbon material,ferrite,conductive polymer,etc.) and structures (such as core-shell structure,hollow structure,and porous structure) have been proposed to improve EAMs performance[3–8].Recently,CoCO3and NiCO3have come to the limelight in academia because of their applications as catalysts[9–10],active materials in energy storage[11–13]and EAMs.For instance,the uniform rod-shaped FeCO3/CoCO3microparticles showed a minimum reflection loss (RLmin)value of -31.86 dB at 12.88 GHz[14].Wei et al.developed a butterfly-shaped Ce(OH)CO3hierarchical structure,and theRLminvalue reached -12 dB at 9.76 GHz[15].The above results indicate that carbonate has a wide effective absorption bandwidth (EAB)compared with other absorbing materials.However,due to the lower impedance matching and attenuation capability,carbonates have poor absorbing properties.
Excellent EAMs not only need to demonstrate strong electromagnetic wave absorption (EMA) performance but should also be thin and lightweight to find practical application[16–17].However,it is difficult for traditional multi-component EAMs without structural design to obtain strong attenuation capability and high impedance matching simultaneously.For example,the porous carbon matrix with inlaid Fe3C/Fe3O4micro-particles not only obtainedRLminvalue -26.72 dB at 10.52 GHz with a thickness of 3.15 mm but also possessed a wide bandwidth of 12.93 GHz covering from 5.07 to 18 GHz[18].Wang et al.reported an efficient design strategy to fabricate hierarchical Ti3C2TxMXene/Ni/ZnO array hybrid nanostructures on cotton fabric by using simple hydrothermal methods[19].However,powdered absorbents are usually filled relatively high,resulting in physical agglomeration and poor attenuation.Therefore,multi-component materials possessing unique structures,such as core-shell structures,hollow structures,porous structures,etc.coupled with electromagnetic characteristics could be capable of achieving high EMA properties,and are therefore attractive.The synthesis of Fe/Fe3O4@TCNFs@TiO2magnetic hybrid nanofibers (MHNFs) with three-layer core-shell structure showed anRLminvalue of -44.8 dB at 13.9 GHz with a matching thickness of 1.6 mm[20].Zhang et al.designed epoxy composites filled with hollow nickel nanoparticles to modify graphene by chemical etching method for microwave absorption[21].Zhang et al.constructed a novel ultra-light egg-derived porous carbon foam (EDCF) structure and studied the effects of pore volume and specific surface area on EDCF EMA characteristics[22].Compared with the traditional multi-component materials,the strategy of structurally designing multicomponent materials to reduce electromagnetic wave (EMW) pollution has been regarded as the most efficient method,which not only increases the types of absorbers but also easily obtains new EMA performance.
The aerogels with macroscopic 3D interconnected porous structures can solve the issues related to agglomeration and disordered arrangements.In addition,ultra-low bulk density[23],large specific surface area[24],and rich functional groups[25]of graphene aerogel (GA) provide multiple interfaces,tortuous spaces,and rich polarization positions for the effective attenuation of microwaves,which is ideal for EAMs[26].In this work,low-cost NiCo2(CO3)3(NC)and GA were selected as the matrix materials and the support material,respectively.
Here,we synthesized NiCo2(CO3)3/RGO (NCR)hydrogel with a 3D porous structure and multi-interfacial hybrid matrix by hydrothermal method.The reduction of graphene oxide (GO) and the hybridization of carbonates were completed in only one step.The high EMA (RLmin=-58.5 dB) and EAB (maximum 6.5 GHz) can be achieved by combining the interfacial matrix and macroscopic 3D interconnected mesh structures.In addition,the effective EMA performance (RL≤ -10 dB) can cover the 4.5-18 GHz of the frequency band by adjusting the sample thickness.Overall,the strategy of the structural design of multicomponent materials to form aerogel plays an important role in making ultralight and high-performance EAMs.
2 Experimental section
2.1 Materials
Graphite flakes (>98%) with the size of 325 mesh were purchased from Alfar Aesarc’s reagent company and used to synthesize GO.Ni(NO3)2·6H2O and Co(NO3)2·6H2O were purchased from Tianjin Chemical Co.,Ltd.(Tianjin,China).Deionized water used for all experiments was produced by our laboratory.
2.2 Synthesis of NiCo2(CO3)3/RGO (NCR)
The GO was synthesized by the improved Hummer's method[27].First,33 mL of deionized water was poured into a beaker containing 0.167 g GO,followed by ultrasonic treatment for 4 h,after which uniform GO dispersion in water was obtained.Subsequently,1.22 g of Ni(NO3)2·6H2O and 2.44 g of Co(NO3)2·6H2O were added to the GO water dispersion,and then 1.27 g of CO(NH2)2was added stirred magnetically for 20 min.Then,the solution was moved to the PTFE-lined autoclave and maintained at 180 °C for 12 h to form the NiCo2(CO3)3/RGO hydrogel.Finally,the hydrogel was freeze-dried to get the 3D NiCo2(CO3)3/RGO (NCR-1) aerogel.The process is shown in Fig.1.For comparison,under the same conditions as above,NiCo2(CO3)3/RGO (NCR-2) was synthesized by adding 0.54 g of Ni(NO3)2·6H2O,1.08 g of Co(NO3)2·6H2O and 0.56 g of CO(NH2)2,and NiCo2(CO3)3(NC) synthesized without the addition of graphene.

Fig.1 Synthetic procedure of NCR aerogel
2.3 Characterization
The crystallography and phase structure of samples was analyzed by X-ray diffraction (XRD) in the range of 5°-80°.Diffraction used Cu targetKα rays and Ni metal for filtering.The surface microstructure and composition of our samples were studied by scanning electron microscopy (SEM,TESCAN MIRA LMS,America) equipped with energydispersive X-ray spectroscopy (EDS) analysis facility.X-ray photoelectron spectroscopy (XPS,Thermo Scientific K-Alpha) was recorded for elemental analysis.The characteristic peak intensity and position of free carbon in the sample were determined by Raman spectrometer (HORIBA Scientific LabRAM HR Evolution under 532 nm laser).The ring specimens with an outer diameter of 7.00 mm and an inner diameter of 3.00 mm were used to measure EMA.The vector network analyzer (Anritsu,MS46322B,Japan) calibrated with a co-axial transmission line was used to measure the complex permittivity and permeability in 2-18 GHz.
3 Results and discussion
The NCR aerogel was synthesized by a simple in situ hydrothermal method and freeze-drying process.A certain amount of NiCo2(CO3)3nanoparticles were uniformly added to the GO solution,as shown in Fig.1.The NiCo2(CO3)3could be adsorbed by the GO sheet thanks to the presence of polar groups (such as ―OH,―COOH,etc.).Through a simple hydrothermal process,graphene nanosheets with carbonate were assembled into hydrogels.During the freeze-drying process,the NCR aerogel with 3D mesh structures composed of graphene and carbonate can be collected from the solvent.
The crystallography and phase structure of our samples were analyzed by XRD.The characteristic diffraction peaks of NC and NCR matched well with CoCO3(PDF Card No.78-0209) as observed in Fig.2a.This result suggests that nickel is likely embedded in the lattice of the cobalt base phase and cannot be detected[28–30].It also shows that NC and NCR have good crystallinity,and the dynamic transformation of nanostructure during preparation do not cause phase transformation.The peaks intensity of NC carbonate without graphene are the largest.The intensity of carbonate diffraction peaks of NCR-1 and NCR-2 decreased with graphene content.The carbonate diffraction peak intensity of NCR-2 is weaker because of the graphene content.RGO was observed at a wide peak at about 25°,consistent with the diffraction peak position in other papers[31–32],indicating that GO has been efficiently reduced to graphene.The intensity of the peak at 25° increased with the added graphene content.
Fig.2b displays the digital photograph of the ultralight NCR aerogel.The microscopic morphology of NC,NCR-1 and NCR-2 composites are exhibited in Fig.2(c-i).The freeze-dried graphene aerogel shows uniform interconnected pores.NC without graphene is spherical particles (Fig.2c).A large number of spherical NiCo2(CO3)3particles in the pore structure surrounded by graphene sheets are observed in the fracture surface of the NCR-1 composite.The synthesized NiCo2(CO3)3fills the mesh space of graphene and forms a tight 3D mesh structure to increase the interface (Fig.2d-f).This is beneficial for facilitating more interface polarization upon encountering electromagnetic waves[33].And the 3D mesh structure also extends the reflection path of electromagnetic waves and finally dissipates in the form of heat.The 3D structure formed by NCR-2 composites is looser,with few villous pores and carbonate particles attached to the surface with the decrease of the ratio of carbonate to graphene (Fig.2g-l).The number of interfaces between 3D mesh graphene and carbonate particles is also less than NCR-1 composites,which is not beneficial to the absorption and dissipation of electromagnetic waves.The corresponding element mapping for NCR-1 (Fig.2e) is shown in Fig.2j–m.From the element mapping shown in Fig.2j–m,we can conclude that the Co,Ni,O and C elements are uniformly distributed on the NCR-1 surface.
The chemical bonds and electronic states of NC,NCR-1 and NCR-2 were characterized by XPS(Fig.3a-e).Fig.3a displays the XPS survey spectrum of all the elements.Fig.3b shows the C 1s XPS spectrum of NCR-1,which reveals 3 peaks at 284.8,286.1 and 289.0 eV,corresponding to C―C/C―O and C=C,respectively,indicating a complete reduction of GO[34–35].It can be found that the corresponding integrated area of C―C bonds in the sample increases with the graphene content,while the opposite is true for C―O and C=C bonds.Fig.3c exhibits the O 1s XPS spectrum at 531.6 and 532.7 eV.The peak at 531 eV is attributed to the carbonate[36].In particular,various oxygen-containing functional groups of GO introduce a large number of internal defects that favor dipolar polarization losses.The peak at 531 eV corresponds to C=O.The C=O bond is beneficial to improve the conductivity of materials to gain excellent conduction losses and enhance the EMA performance of the sample.In addition,the oxygen-containing group bonds prove that GO is effectively reduced.As shown in Fig.4d,for the spin-orbit splitting of the high-resolution Ni 2p spectrum,two components of Ni 2p3/2and Ni 2p1/2can be seen (Fig.3d)[37–38].Each component is fitted by Ni (II) and satellite peak.Ni 2p3/2and Ni 2p1/2peaks can be further fitted into 2 peaks at 862.5 and 879.6 eV,respectively.In addition,two satellite signals marked "Sat" at 856.9 and 874.4 eV are observed,respectively.The Ni 2p3/2-Ni 2p1/2energy separation is 17.1 eV,which is a signature of the Ni 2p oxidation state[39].The spectra and fitting results of Co 2p are similar to Ni 2p (Fig.3e).The two main spin-orbit double peaks for Co 2p3/2and Co 2p1/2occur at 786.8 and 802.6 eV.The "Sat" peaks of Co 2p are located at 787.1 and 798.0 eV,respectively,indicating the presence of NiCo2(CO3)3[10–11].The presence of graphene in NCR may lead to a change of electronic states,which may cause a shift in the peak positions of Ni and Co.

Fig.3 (a) XPS spectra of NC,NCR-1 and NCR-2.XPS spectra of (b) C 1s,(c) O 1s,(d) Ni 2p,and (e) Co 2p of NC,NCR-1 and NCR-2.(f) Raman spectra of NC,NCR-1 and NCR-2

Fig.4 (a,b) The real part (ε') and the imaginary part (ε") of complex permittivity,(c,d) the real part (μ') and the imaginary part (μ") of complex permeability of NC,NCR-1,NCR-2
The Raman spectra of NC,NCR-1 and NCR-2 are shown in Fig.3f.For NC,it can be observed that the symmetrical telescopic vibration of the ―CO3group is 1 085 cm-1and the in-plane bending vibration is 704 cm-1.The location of the peak is consistent with previous reports[40],definitevely proving the existence of carbonate.The two spectral peaks of the―CO3group are changed significantly when graphene oxide is added.The differences in Raman spectra are likely to be related to crystallographic orientations.The spectral peak intensity generated by the symmetrical telescopic vibration of C―O of NCR-1 is very weak and difficult to confirm.And the spectral peak generated by the bending vibration in the C―O plane is slightly enhanced.However,only the spectral peaks generated by the C―O symmetric telescopic vibration of NCR-2 are distinguishable after adding graphene oxide.Similar results have been observed in other carbonate structures,which are caused by the incorporation of cations[40–41].The characteristic peaks ofDandGbands of graphene were observed at 1 323 and 1 578 cm-1related to disordered carbon and sp2hybrid carbon,respectively.The higher intensity ratio (ID/IG) in Raman spectroscopy could be attributed to the increase in the number of smaller sp2carbon domains in graphene after chemical reduction and graphene content.This indicates that NCR-1 has better electromagnetic absorbing performance because of its stronger dielectric property than NCR-2.The peaks of both theDandGbands shifted,which indicates a significant charge transfer between the graphene nanosheets and the NiCo2(CO3)3particles.Most of the oxygen-containing groups of the NCR-1 are removed by reduction.The residual functional groups improve proper impedance matching and also lead to additional dipole polarization and defective polarization relaxation.In addition,due to the existence of multiple dissipation modes,the residual functional groups are more conducive to dissipation in the form of thermal energy by interacting with electromagnetic waves.The interaction between graphene sheets and NiCo2(CO3)3granules is contributed to improve their EMA performance.
The complex permittivity (εr=ε'-iε") and permeability (μr=μ'-iμ") can be obtained to study the possible EMA mechanism of NC,NCR-1,NCR-2 at 2-18 GHz (Fig.4).Among them,the real part (ε' andμ') of complex permittivity and permeability represent the electromagnetic storage capacity of NC,NCR-1,NCR-2 and the imaginary part (ε" andμ") of them represents the dielectric and magnetic energy dispersion capacity of NC,NCR-1,NCR-2,respectively.Fig.4a-d show electromagnetic parameters of NC,NCR-1 and NCR-2.Theε' values of NC,NCR-1 and NCR-2 decrease with frequency,from 6.19 to 4.96,10.25 to 5.22,9.87 to 5.05,respectively.With the addition of conductive graphene,theε' value of NCR is much higher than that of NC (Fig.4a).And theε' of NCR-1 is significantly higher than that of NCR-2 in 8-18 GHz.Theε" value of NCR of the 3D mesh structure is the largest,which is much higher than that of the granulated NC absorber,indicating that NCR has a stronger dielectric energy dissipation capacity(Fig.4b).Theε" of NCR-1 is higher than that of NCR-2.
As far as we know,EMA contributes to 2 types of losses: dielectric and magnetic loss.For all samples,theμ' value at 2-18 GHz fluctuates between 1.13 and 0.97,while theμ" value fluctuates in the range from -0.03 to 0.21 (Fig.4(c,d)).The negativeμ" value shows that the magnetic energy generated by the material's induced magnetic field is converted into electrical energy.Similar results have been observed in some magnetic absorbers[42].It is worth mentioning that 2 resonant peaks are displayed inμ",which is contributed to the natural resonance at about 8.2 GHz and the exchange resonance at about 14.2 GHz,respectively[22,43].
Based on the electromagnetic parameters,the tangent loss of the dielectric and magnetic can be calculated to reflect the electromagnetic loss capability.As shown in Fig.5(a,b),it is further explained that the NCR aerogel upon adding graphene increases the dielectric tangent loss of NC.Due to the strong interfacial polarization,dipole relaxation,and multiple reflections of 3D mesh structure formed by carbonate and graphene,NCR has a higher dielectric tangent loss (ε"/ε').Theε"/ε' value of NCR of the 3D mesh structure is the largest,which is much higher than that of the granulated NC absorber.In contrast,theε"/ε'value of NCR-1 is also greater than NCR-2,indicating that NCR-1 has a stronger dielectric loss capacity(Fig.5a).There are 3 reasons for the above results.First,the number of polarization centers increases due to abundant defects in the graphite sheet.Secondly,the 3D self-assembled materials have a large number of electric dipoles and heterogeneous interfaces.Finally,the rich pore structure can also promote multiple reflections and scattering after electromagnetic wave entry to aid its dissipation into the thermal energy.Fig.5b shows the magnetic tangent loss (μ"/μ')of NC,NCR-1 and NCR-2,this parameter could reflect the magnetic loss capacity of EMAs.The eddy current loss is calculated by the following Equation[44].

Fig.5 (a,b) Dielectric and magnetic loss tangents (ε″/ε′,μ"/μ'),(c) C0–f curves and (d) attenuation constant of NC,NCR-1,NCR-2
The magnetic loss may be caused by the eddy current loss in 2-18 GHz.As shown in Fig.5c,the value of theC0curve fluctuates little,which again indicates that the magnetic loss capacity is not strong in NC,NCR-1 and NCR-2.Generally,in addition to dielectric and magnetic loss,the effective complementarity between relative permittivity and permeability has a great influence on the EMA performance.Due to the large number of loss factors,an indicator is needed to measure the comprehensive loss capacity.As an important parameter to measure the electromagnetic loss of materials,the attenuation constant (α) can be obtained by the following Equation[45]:
As shown in Fig.5d,compared with other samples,NCR-1 has the largest attenuation constant because of its highest permittivity.In 2-12 and 14-18 GHz,theαof NCR-1 is greater than NCR-2 with a similar structure,which also indicates that the amount of added graphene is not better.The structural density of NCR-2 and heterogeneous interfaces composed of carbonate particles and graphene decrease with increasing graphene content.It deteriorated the loss performance of NCR-2 and affected electromagnetic dissipation.Since the rich pore structure of the compact 3D mesh structure composed by NiCo2(CO3)3/RGO lengthens the reflection path and graphene addition also increases interface polarization,dipole relaxation,and surface eddy current loss,theαof NCR-1 is much greater than granular NC absorber.
Based on the complex permittivity and complex permeability,theRLvalue can be calculated by the Equation[46–50]:
wherecanddare the speed of light and the thickness of the absorber,respectively.Andfis the wave frequency.The reflection loss is directly related to the amount of microwave absorption.The EAB and theRLminare the most important indicators to judge the EMA capacity of materials.The maximumRLshifts significantly to the lower frequencies as the sample thickness increases from 0.5 to 5.0 mm.It can be expressed by the following formula[51–52]:
wheredmandfmare the best matching thickness and matching frequency,respectively.According to some specific applications,the EMA characteristics can be adjusted by changing samples’ thickness.
In Fig.6a,theRLminof NC is -13.1 dB at 14.3 GHz,the thickness is 2.3 mm.The interface polarization,dipole relaxation,and electron polarization of NC are weaker than others because of its singlecomponent NiCo2(CO3)3.And the granular NC surface has fewer instances of reflection and scattering.The above shows that the electromagnetic loss and attenuation capacity of the NC is very low,which affects the final electromagnetic wave dissipation ability.TheRLminof NCR-1 with the 3D mesh structure added a certain graphene can reach -58.5 dB,corresponding to a frequency of 14.08 GHz,a thickness of 2.3 mm,and an EMB of 6.5 GHz (Fig.6b).In addition,the effective EMA ability (RL≤-10 dB) can cover the 4.5-18 GHz of the frequency band by adjusting sample’s thickness.In Fig.6c,it is clearly shown that the absorption performance of NCR-2 decreases with increased graphene content.The thickness is 2.3 mm,theRLminof NCR-2 is -41.9 dB at 11.36 GHz,and it shows an EAB of 6.3 GHz.It can be found that the 3D mesh structure formed by adding graphene helps to improve the EMA of NC.The hydrothermal reduction will remove a large number of oxygen-containing groups.The defects,disordered sites,and residual functional groups not only improve impedance matching but also lead to additional dipole polarization and defect polarization relaxation in alternating electromagnetic fields.
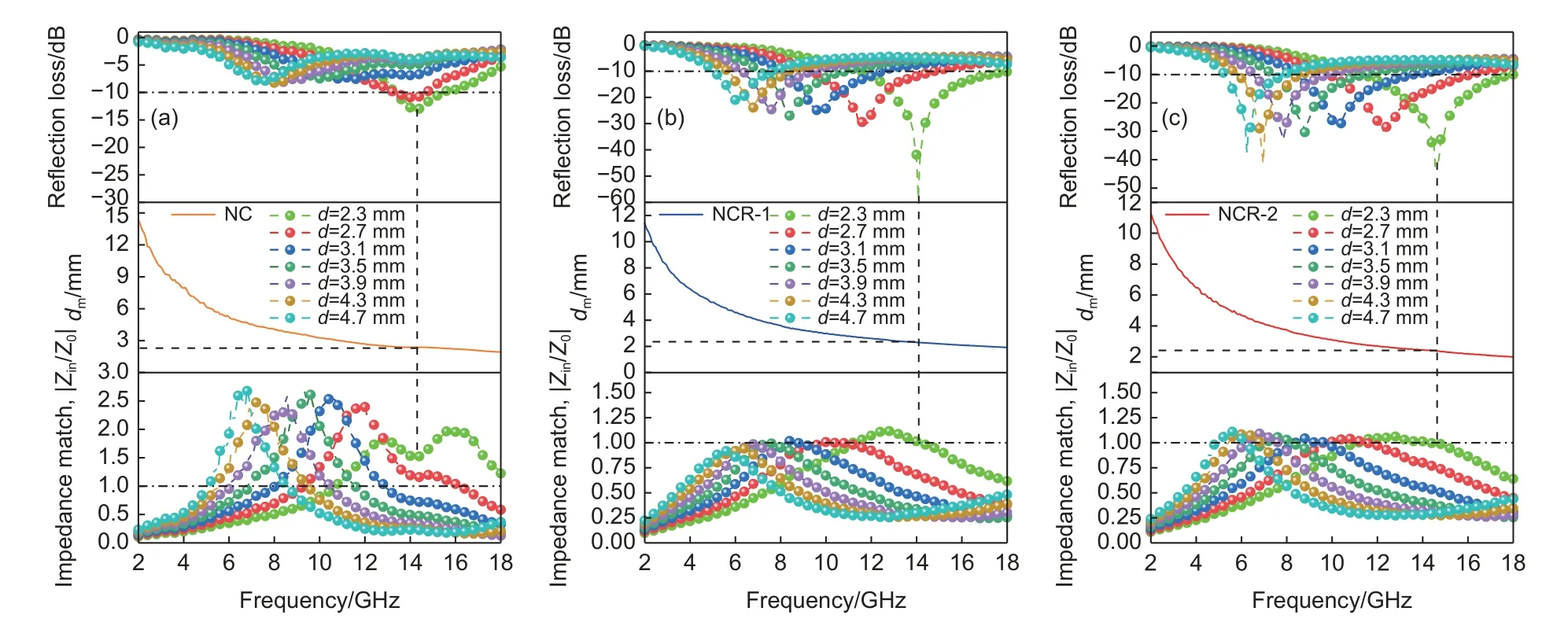
Fig.6 (a,b,c) RLmin,1/4 λ curve,and impedance matching of NC,NCR-1 and NCR-2 at frequency and different thicknesses
TheRLminof NC,NCR-1 and NCR-2 in the 2-18 GHz range first decreased and then increased with the increasing thickness.The effective absorption range shifted to the low frequency region with the increase of thickness,which was consistent with the 1/4 wavelength theoretical model[53–55].The value of impedance matching (|Zin/Z0|) of NCR-1 and NCR-2 is approximately equal to 1.And the |Zin/Z0| of NCR-1 is closer to 1.The |Zin/Z0| value of NC is much greater than 1.The incident electromagnetic waves are fully absorbed and the reflectivity will decrease when the value of |Zin/Z0| is approximately equal to 1.It indicates that the better the match,the better the performance of the sample.The main source of electromagnetic wave absorption of granular NC and 3D mesh foundation structures is dielectric loss,and the polarization relaxation loss is mainly caused by dipole relaxation polarization or interfacial polarization[56–57].
The polarization relaxation phenomenon of the absorber can be determined by the Col-Cole curve,and each semicircle on the curve corresponds to a Debye relaxation process.Therefore,NCR-1 aerogels have multiple polarization relaxation processes.These relaxation processes may result from the defects and residual functional groups in aerogel.The value can be evaluated by the equation[58–59]:
whereε∞andεsare the permittivity at the high-frequency limit and the static permittivity,respectively.Fig.7a-c show the Cole-Cole curve of NC,NCR-1 and NCR-2.The curve of NC has 2 inconspicuously small circles (Fig.7a).It indicates that the polarization relaxation loss of NC is relatively low.The circles of NCR-1 are significantly increased (Fig.7b),which indicates that the incident EMW was effectively dissipated by multiple Debby relaxations.Although the number of circles in NCR-2 does not increase compared with NC,its radius increases slightly(Fig.7c).It reflects the greater degree of Debye relaxation in NCR-2.The interfacial polarization and dipole polarization are enhanced because of the residual functional groups of GO and the assembled 3D mesh structure.It is well known that,RGO is a non-magnetic dielectric loss medium,and the dielectric loss of absorbing materials can be divided into conductive and polarization loss.For conductive loss,the value is closely related to dielectric conductivity.It can be found that conductive loss is important for dielectric loss.Conductivity measured from electrochemical impedance spectroscopy (EIS) in Fig.7d manifests that the conductivity of NC,NCR-1 and NCR-2 increases with the increase of graphene content.

Fig.7 (a-c) Cole-Cole plots of NC,NCR-1 and NCR-2.(d) EIS spectra
Fig.8 shows the EM absorption properties of granular NC and the 3D mesh foundation structure NCR with different graphene content.In general,an ideal absorber should have a wide EAB,small absorption,thin thickness (d),etc.The EMA performance of single-component and weakly polarized NC absorbers is negligible (Fig.8(a,b)).With the addition of reduced graphene oxide,EMA ability is observably enhanced by reduction-enhanced polarization and assembled 3D mesh structure (Fig.8(c,d)).TheRLminvalues of NCR-1 and NCR-2 can reach -41.9 and-58.5 dB,respectively,and EAB of 6.3 and 6.5 GHz.The weak EMA performance of NCR-2 aerogel is attributed to their relatively low interface polarization loss and impedance matching,which is due to the reduction in the number of interfaces formed by NCR-2.The lightweight NCR can cover 4.5-18 GHz of the frequency band (Fig.8e,f),and the stable 3D mesh structure can avoid the agglomeration of traditional microscopic particles,providing tortuous space and rich polarization position.In addition,the electromagnetic absorption performance of NCR-1 and NCR-2 with good component coupling is greatly improved by introducing some dissipation methods.However,the constituent quantity of graphene cannot be arbitrarily large.It is found that the load ratio of NCR-1 shows the best EMA performance.
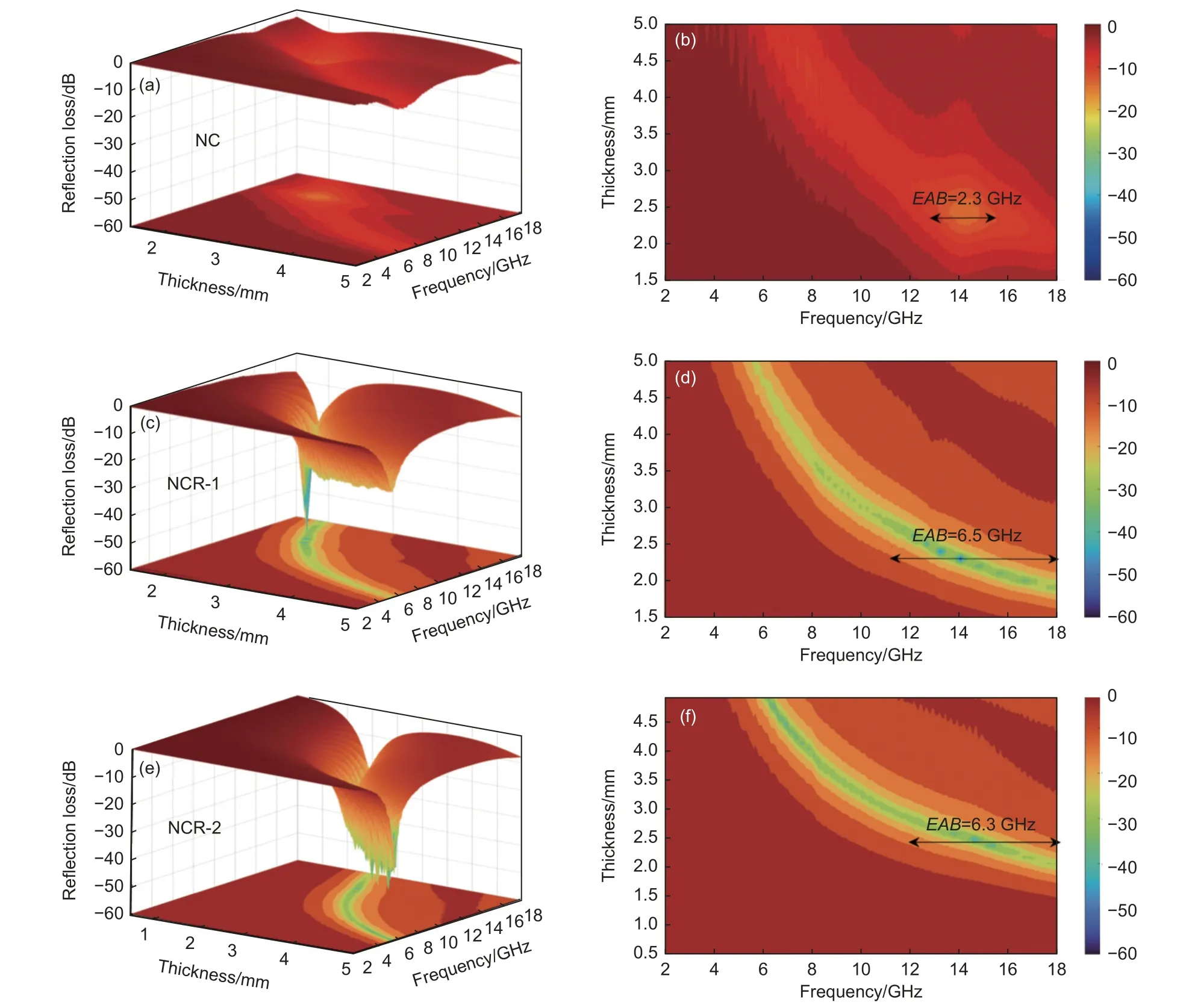
Fig.8 Calculated theoretical RL value of NC,NCR-1,NCR-2: (a) three-dimensional images and (b) two-dimensional projection images
Fig.9a shows the comprehensive comparison of the EMA performance based onRLmin,EAB of NC,NCR-1,NCR-2 and RGO.The excellent EMA performance of NCR-1 aerogels.Compared with other previous work,the EMA behavior of NCR-1 is listed in Table S1 and Fig.8b.The results indicate that composite aerogels can be used as excellent EAMs.As shown in Fig.10,a schematic illustration of EM wave absorption for NCR.It indicates that the lack of abundant heterogeneous interfaces and pore structure led to the poor EMA ability of NC.The regulation of the supporting component graphene should be adjusted to promote NCR absorption ability.However,the EMA properties of NCR-2 with more graphene deteriorated because of the reduction of the heterogeneous interface.From the above analysis,it can be seen that the excellent absorption performance of NCR-1 is due to the good impedance matching brought by the electromagnetic parameter balance adjusted by graphene,and the strong attenuation ability brought by a variety of electromagnetic wave absorption methods.The NiCo2(CO3)3particles were embedded in the graphene to form a compact 3D porous and interfacial matrix,providing multiple interfaces and rich polarization.The incident electromagnetic waves are reflected several times inside the 3D porous structure aerogel and dissipated due to dielectric and magnetic loss.The conductivity loss and dipole polarization relaxation are provided by graphene.NiCo2(CO3)3provides dipole polarization relaxation and magnetic loss,including natural resonance,exchange resonance,and eddy current loss.The interfaces of the two components contribute to the interface polarization relaxation.
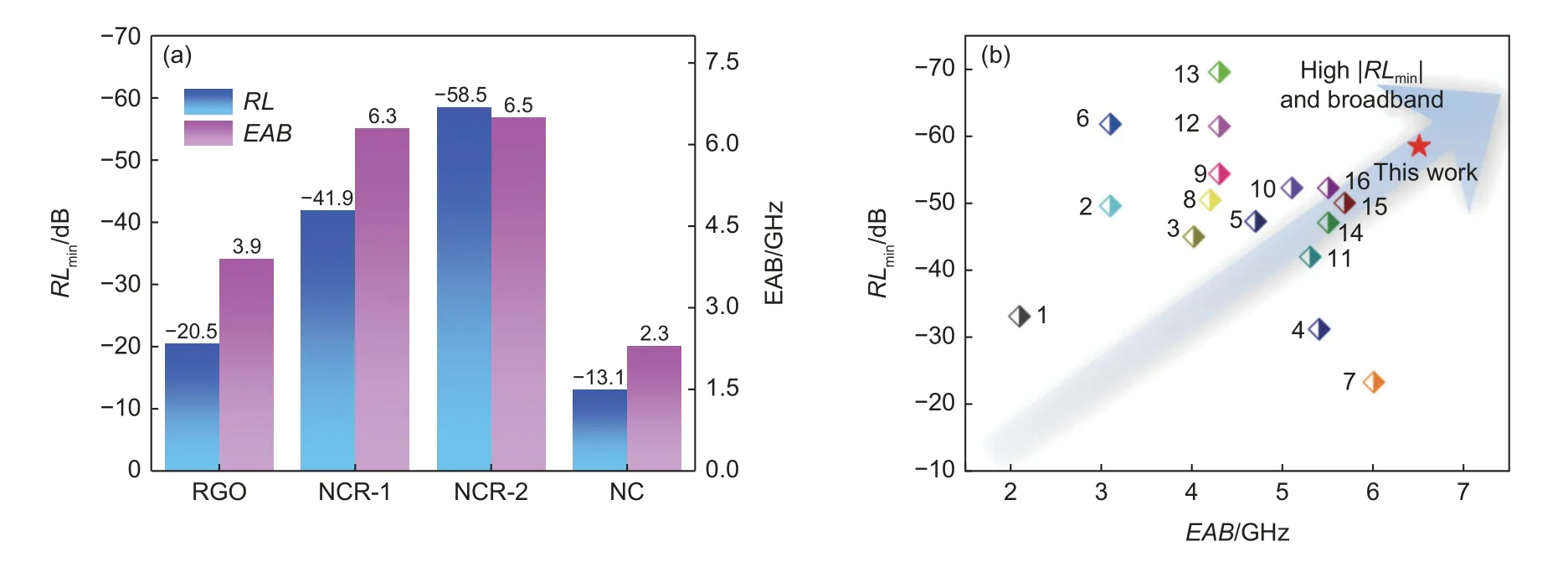
Fig.9 (a) Comprehensive comparison of the EMA performance based on RLmin and EAB of NC,NCR-1,NCR-2 and RGO.(b) Comprehensive comparison of the EMA performance given RLmin, and EAB with reported EMA materials
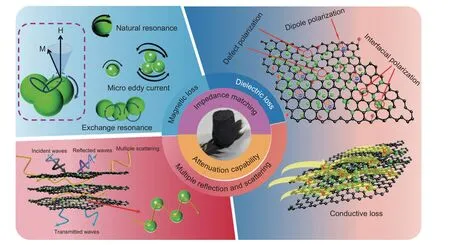
Fig.10 Schematic illustration of EM wave absorption for NCR
4 Conclusion
In summary,the 3D mesh structure of NiCo2(CO3)3/RGO aerogel was prepared by a simple hydrothermal reduction and freeze-drying method.The strategy of assembling multicomponent materials in a rationally designed structure solves the problem of physical agglomeration of NiCo2(CO3)3particles and regulates electromagnetic parameters to improve impedance matching and attenuation capability.The synergistic effect of the 3D porous structure and the interfacial matrix imparts excellent EMA performance to NCR-1 and this aerogel with ultra-low density shows enhanced dielectric loss capability in the 2-18 GHz range.TheRLminat 2.3 mm is -58.5 dB and the effective EMA performance of NCR-1 can cover the 4.5-18 GHz range through the adjustment of sample thickness.The broad band,thin film feature,and ultra-light characteristics of NiCo2(CO3)3/RGO aerogel have immense potential for application as advanced electromagnetic absorbers.
Data availability statement
The data that support the findings of this study are openly available in Science Data Bank at https://www.doi.org/10.57760/sciencedb.j00125.00089 or https://resolve.pid21.cn/31253.11.sciencedb.j00125.00089.
Acknowledgements
This work was supported by the Central Government Guides Local Science and Technology Development Special Fund Projects (YDZJSX2022B003);Shanxi Province Science and Technology Major Projects (202101120401008);Key Research and Development Project of Shanxi Province(202102030201006);Natural Science Foundation of Shanxi Province (202203021212205);Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2022L074).
杂志排行
新型炭材料的其它文章
- Carbon-based photothermal materials for the simultaneous generation of water vapor and electricity
- 生物质炭材料作为金属空气电池阴极的研究进展
- 煤基富氧多孔炭纳米片的制备及其超级电容器性能
- Mott-Schottky heterojunction formation between Co and MoSe2 on carbon nanotubes for superior hydrogen evolution
- A 2D montmorillonite-carbon nanotube interconnected porous network that prevents polysulfide shuttling
- A one-pot method to prepare a multi-metal sulfide/carbon composite with a high lithium-ion storage capability
