A highly selective and sensitive electrochemical Cu(II) detector based on ion-imprinted magnetic carbon nanospheres
2023-12-20LIRuizhenQINLeiFUDongjuWANGMeilingSONGXingfuBAIYonghuiLIUWeifengLIUXuguang
LI Rui-zhen,QIN Lei,FU Dong-ju,WANG Mei-ling,SONG Xing-fu,BAI Yong-hui,LIU Wei-feng,,*,LIU Xu-guang,*
(1.College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China;2.Key Laboratory of Interface Science and Engineering in Advanced Materials, Ministry of Education,Taiyuan University of Technology, Taiyuan 030024, China;3.College of New Materials and New Energies, Shenzhen Technology University, Shenzhen 518055, China;4.National Engineering Research Center for Integrated Utilization of Salt Lake Resource, East China University of Science and Technology, Shanghai 200237, China;5.State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, Ningxia University, Yinchuan 750021, China)
Abstract: An electrochemical sensor for Cu(II) based on ion-imprinted polymers was prepared by combining surface imprinting with electrochemical polymerization deposition.The sensor was modified by ion-imprinted magnetic carbon nanospheres with a specific selectivity and sensitivity for Cu(II).The morphology and structure of the materials were characterized and analyzed.Sensors with the imprinted electrode had a stronger selectivity and higher sensitivity towards Cu(II) compared with their original counterparts.Within relative concentrations of Cu(II) from 10-6 to 10-10 mol L-1,the detection limit of the sensor was as low as 5.138×10-16 mol L-1 (S/N=3).The sensor is resistant to interference,and has good reproducibility,and stability,making it excellent for the electrochemical detection of metal ions.
Key words: Ion imprinted polymer;Electrochemical sensor;Cu(II);Magnetic carbon nanospheres;Selective and sensitive
1 Introduction
Cu(II) is one of the essential micronutrients for the human body[1–2].Insufficient intake of Cu(II) leads to anemia,neutropenia and skeletal abnormalities[3].However,excessive Cu(II) would inhibit the activity of biological intracellular enzymes,and further affect the normal metabolism of organisms[4].Besides,excess Cu(II) would also damage the self-purification ability of water environment,resulting in water quality deterioration[5–6].The U.S.Environmental Protection Agency requires that the concentration of Cu(II)in drinking water lower than 20 μmol L-1[7].Therefore,a rapid and efficient Cu(II) detection technology is very important.Currently,methods for Cu(II) detection mainly include inductively coupled plasma mass spectrometry[8–9],high-performance liquid chromatography[10]and electrochemical methods[11–14].Among these methods for Cu(II) detection,electrochemical detection is of considerable interest due to its advantages of low cost,easy preparation,good stability and short response time[15].In order to improve the analytical performance of electrochemical sensors,the modification of the electrode surface has been the focus of many researchers[16–17].In our early work,An et al.[18]prepared a selective ion-imprinted electrochemical sensor based on the ion-imprinted polymer-modified electrode.The detection of Cu(II) ions in water can be achieved with an extremely small amount of Cu(II)-IIPs.It is demonstrated that the ion-imprinted polymer-modified electrode is effective for the detection of heavy metal ions.
Ion imprinting technology has been proved to be one of the most effective modification technologies to improve the selectivity and sensitivity of electrochemical sensor.The sensor selectivity could be achieved by ion-imprinted polymers (IIPs) with imprinted holes which match the shapes,sizes and recognition sites of target ions[19].Owing to their good chemical and mechanical stabilities,excellent specific recognition ability,and highly sensitivity,IIPs have been successfully adopted in the fields of enantiomeric separation[20],solid phase extraction[21],and the chemical and biological recognition in sensors[22–23].
Though bulk IIPs are limited in their adsorption and desorption efficiency by the mass transfer kinetic limitations and the inaccessibility of binding sites,these drawbacks could be overcome by surface imprinting technology,of which an imprinted polymer is coated onto the surface of a porous inorganic substrate[24–25].The recognition cavities of surface imprinted material are distributed on or close to its surface,which could effectively ensure easy elution of and rapid affinity to target ions.
IIPs could be constructed through a variety of ways including bulk polymerization,emulsion polymerization,electropolymerization,chemical grafting and electropolymerization[26].Among these methods,electropolymerization is a facile and safe way to fabricating IIP membranes directly on electrode surface.The thickness and morphology of electropolymerized membrane could be regulated by changing the voltage and the cycle number.In addition,the polymer membrane could be used for improving conductivity and adhesion of electrodes without using additional binders[27].
Magnetic carbon nanospheres of Fe3O4@C possess a large specific surface area and various functional groups,allowing for strong interactions with the target ions.Therefore,as an imprinting carrier,Fe3O4@C could be constructed with the imprinted cavities on its surface for Cu(II) ions detection.The technique enables high sensitivity and selectivity of Cu(II) ions.In this study,surface imprinting and electrochemical polymerization deposition were combined with magnetic carbon nanospheres (Fe3O4@C)as carrier matrix,Cu(II) as the target ion,and pyrrole as the functional monomer.Potentiostatic polymerization was used to prepare highly sensitive carbon-based surface Cu(II) ion-imprinted polymers (Cu(II)-IIPs).Theoretical simulation has been employed to reveal the interaction mode between Cu(II) and pyrrole molecules.By combining with the calculation of activation energy and frontier orbital level-energy difference,the specific interactions between Cu(II) and pyrrole have been confirmed.Moreover,the imprinting effect has been determined.Finally,through differential pulse cyclic voltammetry (DPV),the electrochemical performance,anti-interference,and stability of the imprinted electrode were evaluated.The sensor with the ion-imprinted polymer electrode (IIPs/GCE)showed an enhanced responsiveness and selectivity towards Cu(II).
2 Experimental
2.1 Materials
Ferrocene (Fe(C5H5)2),acetate buffer (pH=7),anhydrous cupric sulfate (CuSO4),α-Al2O3polishing powder,acetone (CH3COCH3),nitric acid (HNO3),hydrogen peroxide (H2O2) and potassium chloride(KCl) were purchased from Qingyuan Chemical Factory,Gu’an County (Hebei,China).Anhydrous ethanol (C2H5OH),pyrrole (C4H5N) were purchased from Shanghai Maclean Biochemical Technology Co(Shanghai,China).Concentrated sulfuric acid (H2SO4,95%-98%) was purchased from Luoyang Chemical Reagent Factory (Luoyang,China).KH2PO4,Na2HPO4,Na2SO4,Cu(NO3)2,Pb(NO3)2,Ni(NO3)2and K3[Fe(CN)6] were purchased from Tianjin Fuchen Chemical Reagent Factory (Tianjin,China).All chemicals were of analytical purity.All solutions were prepared with ultrapure water.
2.2 Characterization
Field scanning electron microscopy (FESEM,JEOL JSM-6700F,Japan),transmission electron microscopy (TEM,JEOL JEM-2100,Japan) and Fourier transformation infrared spectroscopy (FTIR,BRUKER FTS-165,Germany) were used to characterized the morphologies and structures of the obtained products.The electrochemical experiments were carried out on a electrochemical workstation(Wuhan Coster CS350,China).
2.3 Preparation of ion-imprinted electrode
Magnetic carbon nanospheres (Fe3O4@C) were synthesized by hydrothermal method according to our previous work[28].Fe3O4@C (2.5 mg) was added in 1 mL ethanol under ultrasound for 30 min.The glassy carbon electrode (GCE) was then obtained by dropping 10 L of the suspension onto the surface of a bare glassy carbon electrode and allowing it to dry naturally.Then,imprinted electrode was fabricated by using electrochemical polymerization in a freshly prepared electrolyte solution (30 mL) containing 0.1 mol L-1pyrrole,0.02 mol L-1cupric sulfate,and 0.2 mol L-1potassium chloride.The electrochemical polymerization process was performed under potentiostatic condition of 0.7 Vvs. Hg/HgO for 5 min.All electrochemical behavior tests were conducted at room temperature.Finally,the obtained electrode was washed with ultrapure water and dipped into phosphate buffer (pH=6.86).Template Cu(II) ions were removed with cyclic voltammetry with a scan range from -0.2 to 1.3 V at 100 mV s-1.The prepared electrode is denoted as IIPs/GCE.As a comparison,nonimprinted electrode (NIPs/GCE) was prepared under the same preparation conditions without the addition of template Cu(II).The scheme illustrates the preparation of Cu(II)-IIPs composite and the electrochemical detection of Cu(II),as shown in Fig.1.

Fig.1 Schematic illustrating the preparation of the Cu(II)-IIPs composite for detecting Cu(II)
2.4 Electrochemical measurements
2.4.1 Detection of Cu(II) peak potential
DPV current response of bare electrode was measured separately in 10-3mol L-1CuSO4acetic acid buffer,10-3mol L-1Na2SO4acetic acid buffer and 10-3mol L-1Cu(NO3)2acetic acid buffer.The peak position of Cu(II) was determined according to the curve comparison.
2.4.2 Detection of sensitivity
DPV was performed in 10-3mol L-1CuSO4acetic acid buffer with the prepared imprinted polymer electrode (Cu(II)-IIPs/GCE),non-imprinted polymer electrode (NIPs/GCE),and Fe3O4@C modified electrode,separately.Subsequently,the sensitivities of these 3 electrodes were compared by corresponding response currents.
2.4.3 Electrochemical responses of sensor
IIPs/GCE was soaked in acetic acid buffer with different CuSO4concentrations,and DPV was used to record the current potential curves.The detection limit was determined by linear fitting of the current and Cu(II) concentration.
2.4.4 Specific detection of Cu(II)
Several other heavy metal ions are selected for interference test since the existence of interfering ions may have a certain influence on Cu(II) detection.DPV curve were obtained by immersing the ion-imprinted polymer electrode into acetic acid buffer containing 10-3mol L-1Cu(II),10-3mol L-1Ni(II) and 10-3mol L-1Pb(II).The Cu(II) specific detection was analyzed by the response peaks.
2.4.5 Cyclic stability test
The prepared Cu(II)-IIPs/GCE was subjected to DPV in Cu(II) buffer solution to detect Cu(II).Afterwards,the Cu(II)-IIPs/GCE was eluted in phosphate buffer solution (pH=6.86).The above steps were repeated for 7 times to compare the peak current value changes and calculate the ratio of each response current to the initial response current.
The Cu(II) imprinted polymer membranes were prepared parallelly on two other electrodes.The DPV current response in acetic acid buffer of 10-3mol L-1CuSO4was measured to evaluate the regenerability of the imprinted membranes.
In addition,the freshly prepared Cu(II)-IIPs/GCE was stored at 25 °C for 3 days.The changes of DPV current response in 10-3mol L-1CuSO4acetic acid buffer and the changes of electrode current response were detected to assess the stability of the Cu(II)-IIPs/GCE.
3 Results and discussion
3.1 Structure and composition of IIPs/Fe3O4@C
The microstructures of Fe3O4@C and Cu(II)-IIPs were characterized by FESEM.From Fig.2 (a-b),the Fe3O4@C nanoparticles have smooth surfaces and uniform spheric shape in size of about 100 nm with good grain size dispersion.As shown in Fig.2 (c-d),Cu(II)-IIPs presented as larger particles and the surface becomes rougher.The morphology differences are resulted from the introduction of polymer in the imprinting process.
The TEM images show clearer microstructures of Fe3O4@C and Cu(II)-IIPs.Both Fe3O4@C and Cu(II)-IIPs exhibit core-shell structures.The Fe3O4nanoparticles are coated by a layer of carbon or carbon and polymer.The average diameters of the Fe3O4@C and Cu(II)-IIPs particles are 100 and 120 nm,respectively,as shown in Fig.2 (e-h).The thicknesses of the encapsulating layers on Fe3O4nanoparticles from Fe3O4@C and Cu(II)-IIPs are about 10 and 20 nm,respectively.Compared with Fe3O4@C,Cu(II)-IIPs show an increase in the encapsulating layer thickness caused by the extra imprinted polymer layer.
The functional groups of Fe3O4@C and Cu(II)-IIPs nanospheres were characterized by FT-IR analysis.From Fig.3,the absorption bands at 3 441,1 630,1 385 and 600 cm-1correspond to ―O―H,―C=O,―C―H and Fe―O―Fe bonds of Fe3O4@C nanospheres,respectively.Compared with Fe3O4@C,Cu(II)-IIPs exhibit different absorption bands at 3 422 and 1 171 cm-1,which is attributed to the ―N―H and―C―N of polypyrrole (PPy),respectively.Meanwhile,a new absorption band appears near 1 510 cm-1,which is attributed to the ―C―N and ―C=C in the imprinted polymer layer.The occurrence of ―N―H,―C―N and ―C=C bands indicate that PPy is electrodeposited onto the surface Fe3O4@C.Consequently,the composite is rich in ―OH and ―NH2groups which are specific recognition sites that promote Cu(II) adsorption[29].The vibrational band of pyrrole rings typically lies in 1340-1600 cm-1.For a single pyrrole ring,there is a strong absorption band at 1480-1500 cm-1.Moreover,overlapping pyrrole rings will affect the intensity and shape of these absorption bands.When two adjacent pyrrole rings exist,the intensity of the absorption band at 1 394 cm-1decreases while the intensity of the band at 1 510 cm-1increases.Therefore,two pyrrole rings could be indentified by analyzing the FTIR spectra.
3.2 The influence of pH value
Phosphoric acid buffer of appropriate pH value was selected to ensure that Cu(II) ions could be completely eluted without incluencing the conductivity of PPy.As shown in Fig.4(a),the current intensity detected in the phosphoric acid buffer showed a weak decreasing trend as the pH value increases from 5.5 to 6.1.From pH=6.1,the current response presented a rapid increasing trend,and after pH=6.86,it began to weaken rapidly.Therefore,phosphoric acid buffer with pH=6.86 is the best choice for eluent.The peak current change of Cu(II) ion adsorption at different pH values (3-7) is shown in Fig.4(b).With the increase of pH value,the adsorption capacity of Cu(II) ions increases.At lower pH values,the N atom of pyrrole molecule was protonated,so that the Cu(II) ion interaction with the N atom on pyrrole was weakened[30–31].According to the formulaKsp=C(Cu2+) ·C2(OH-) (whereKsp=2.2×10-20mol3L-1,C(Cu2+)=10-3mol L-1),when value pH is higher than 7,Cu(II) hydroxide precipitates could be formed.The formation of Cu(II) hydroxide reduced the amount of free Cu(II) ions adsorbent on the polymer cavity.Therefore,alkaline environments are not considered for pH selection.On the basis of these results,pH=6.86 is selected for the following investigation.
3.3 Detection of Cu(II) peak potential
The polished bare glassy carbon electrode was immersed in 10-3mol L-1CuSO4acetic acid buffer,10-3mol L-1Na2SO4acetic acid buffer,and 10-3mol L-1Cu(NO3)2acetic acid buffer,separately.The current responses in DPV curves of 3 electrolyte buffers were measured.As shown in Fig.5,the peaks of CuSO4and Na2SO4are different.Specifically,CuSO4buffer shows a sharp peak at -0.1 V,while Na2SO4buffer has a peak at -0.6 V.The peak intensity measured in the Na2SO4buffer is much lower than that measured in the CuSO4buffer.For CuSO4buffer and Cu(NO3)2buffer,the contained cations are the same,while the anions are different.Both of them show sharp response peaks at -0.1 V,which exclud the influence of anions.According to the comparison of these three curves,it could be confirmed that the peak at -0.1 V could be assigned to the interaction with Cu(II) while SO42-has no influences on detection.
3.4 Detection of sensitivity
The electrochemical properties of Fe3O4@C and Cu(II)-IIPs were evaluated by DPV in acetate buffer solution containing 1×10-3mol L-1CuSO4.As shown in the DPV curves in Fig.6,a well-defined DPV signal with different peak currents for Cu(II) could be observed at a potential range of -0.7 to 0.75 V of all the tested electrodes.IIPs/GCE showed the largest current response of -1.8×10-4A,followed by NIPs/GCE of -6.5×10-5and GCE of -8.6×10-6A.The weak current response peaks at -0.1 V could be assigned to the reduction of Cu(II) by GCE.Although NIPs/GCE surface was not specially designed to recognize Cu(II),it exhibited a large current response to Cu(II) due to the coordinate effects of PPy with Cu(II).In addition to the coordination effect,IIPs/GCE owed its strong specific recognition effect towards Cu(II) mainly to the imprinted cavities.When Cu(II) was located near the imprinted cavities,the exposed N absorb Cu(II),which led to further electrocatalytic reduction.Such cavities are densely distributed on the surface of the membrane,endowing a strong current response signal.The imprinted cavities produced by ion imprinting technology could specifically adsorb Cu(II).
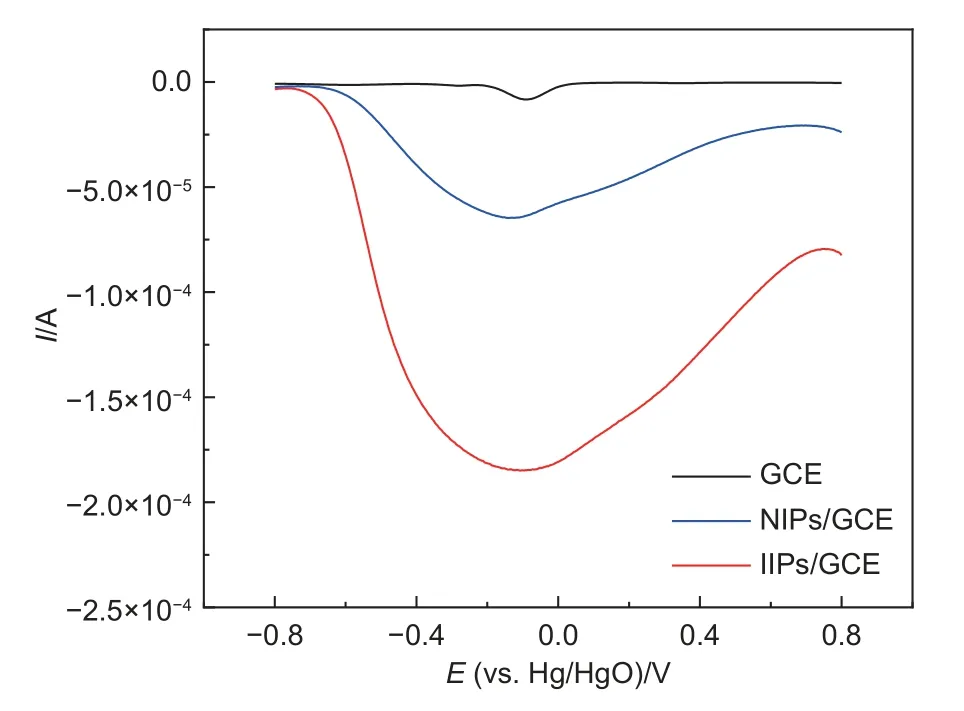
Fig.6 Comparison for the DPV responses of GCE,NIPs/GCE,and IIPs/GCE in acetate buffer
3.5 Electrochemical responses of the sensor
To evaluate the sensitivity,Cu(II)-IIPs were prepared under optimized experimental conditions and the DPV responses to different concentrations of Cu(II) were measured.As illustrated in Fig.7 (a),Cu(II)-IIPs exhibited an increased current response with the increase in Cu(II) concentration,which confirmed the recognition capability.The calibration curve for Cu(II)-IIPs sensor was fitted by plotting the relationship between the peak current (A) and the logarithm of Cu(II) concentration.As depicted in Fig.7 (b),within the range of 10-6to 10-10mol L-1,the peak current is linearly related to the logarithm of Cu(II) concentration,and the regression equation isI(A)=-1.55175×10-5lgC(mol L-1) -2.59311×10-4,with a correlation coefficientR2of 0.999.Limit of Detection (LOD) was calculated to be 5.138×10-16mol L-1,with a signal-to-noise (S/N) ratio of 3.The prepared sensor Cu(II)-IIPs offered an ideal linear range and a low detection limit,which is attributed to the strong affinity of the electropolymerized PPy to Cu(II).The ion imprinting technology provides a great improvement on sensitivity.

Fig.7 (a) Typical DPV curves of Cu(II)-IIPs in acetate buffer with different Cu(II) concentrations (1.0×10-6,1.0×10-7,1.0×10-8,1.0×10-9 and 1.0×10-10 mol L-1).(b) Linear relationship between the peak current and Cu(II) concentration logarithm
3.6 Evaluation of the selectivity of Cu(II)-IIPs modified electrodes
The selectivities of the prepared Cu(II)-IIPs and Cu(II)-NIPs sensors were investigated by measuring the sensor responses to potential interfering ions.The response of IIPs and NIPs to the mixture solution of acetate buffer containing 10-3mol L-1Cu(II),10-3mol L-1Ni(II) and 10-3mol L-1Pb(II) was evaluated.Each response current was compared with that in the acetic acid buffer containing Cu(II) only.Ni(II) was selected as interfering ion here for its size similar to that of Cu(II),while Pb(II) represented a class of heavy metal ions with a larger size than Cu(II).Both of them were representative,which enabled a deeper understanding of the properties and functions of imprinted electrode.Indeed,as shown by Fig.8(a),the current response value for Cu(II) IIPs/GCE was 2.86 times higher than that for Cu(II) NIPs/GCE.For Cu(II)-IIPs/GCE,there was no significant difference in the current response peak positions of Cu(II) and the peak current.From Fig.8 (b),the deviation of Cu(II) response between Cu(II) solution and the mixture was less than 10% in the presence of other heavy metal ions.However,for Cu(II)-NIPs/GCE,the deviation of Cu(II) response between Cu (II) solution and mixture was about 35%.These results indicated a poor selectivity of the NIPs/GCE.The heavy metal ions did not interfere with the determination of Cu(II)by Cu(II)-IIPs/GCE.This phenomenon could be attributed to the specific recognition sites of the polymer and the different oxidation potentials of Cu(II)[32].
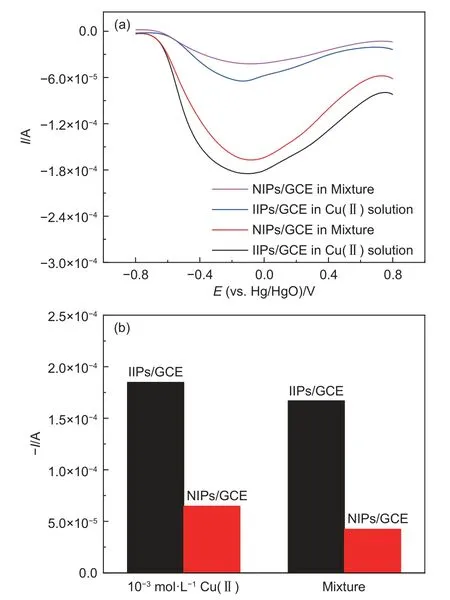
Fig.8 (a) Current response of Cu(II)-IIPs/GCE in acetate buffer to 10-3 mol L-1 Cu(II) solution and mixture (containing 10-3 mol L-1 Cu(II),10-3 mol L-1 Ni(II),and 10-3 mol L-1 Pb(II)) and (b) the corresponding histogram of peak current
3.7 Stability of Cu(II)-IIPs modified electrodes
Regenerability and stability of Cu(II)-IIPs sensor are important factors in evaluating detection performance for practical application.As illustrated in Fig.9 (a),the detection performance of Cu(II)-IIPs sensor remained 46.3% of the initial value after 3 days.The newly prepared imprinted polymer electrode was stored at 25 °C for 3 days to detect the DPV current response in acetic acid buffer containing 10-3mol L-1of Cu(II).The current response of the fresh one was compared to evaluate the electrode stability.From Fig.9 (b),the electrode current of the stored sample maintained more than 91% of the current response of the fresh one.The slight decrease in adsorption capacity was probably due to the residual Cu(II),and destruction of pores during elution process[33–34].The above results showed a good stability of the ion-imprinted electrochemical sensor.
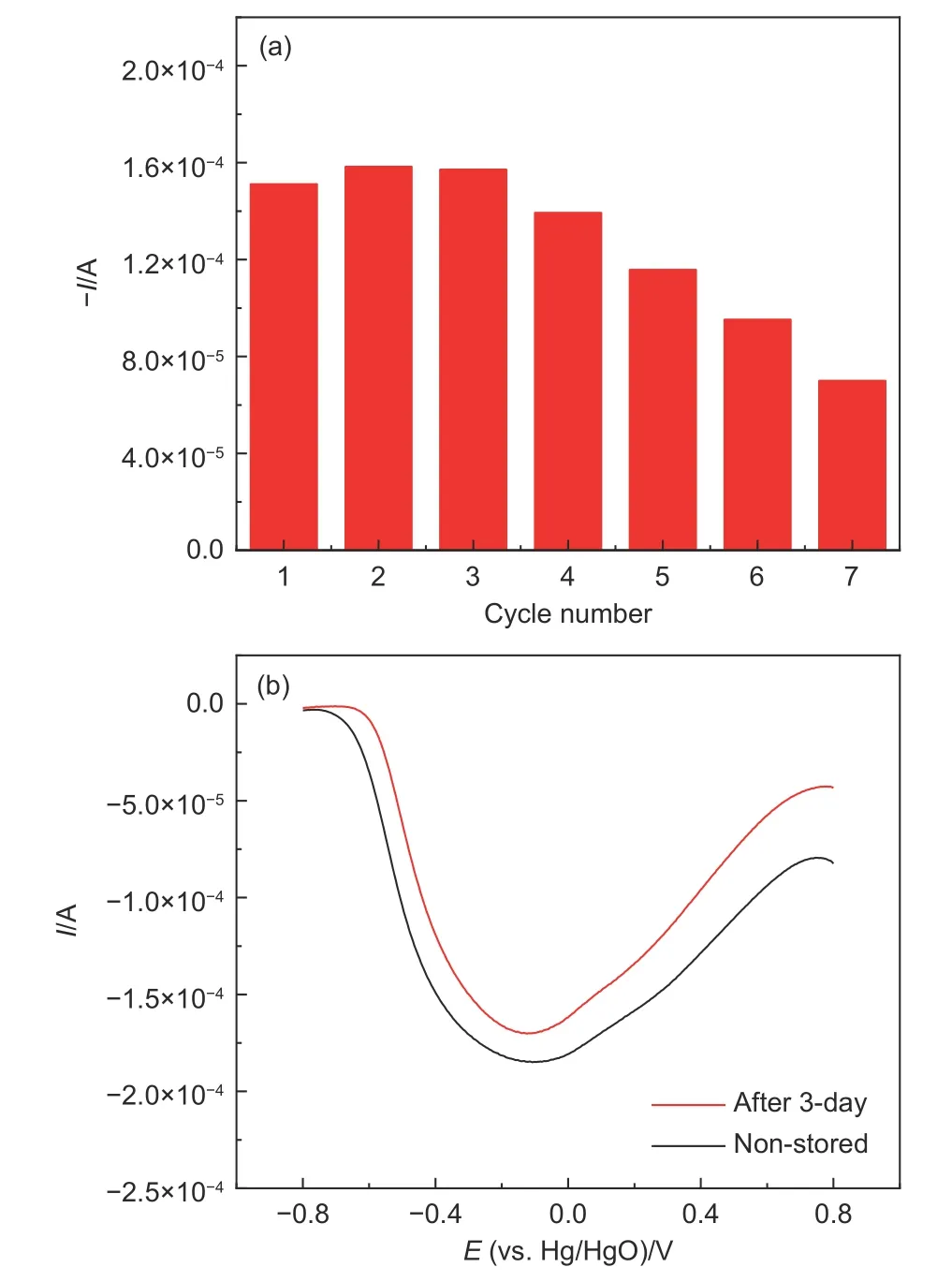
Fig.9 (a) Regeneration of Cu(II)-IIPs sensor.(b) DPV current response compared for the fresh and 3-day stored electrodes in 10-3 mol L-1 Cu(II)acetic acid buffer
3.8 Theoretical calculation
To further validate the specific binding interaction between IIPs and Cu(II),Dmol3 module based on density functional theory was used to calculate the energies of Cu(II),pyrrole and their complex.The interaction energies could reflect the interaction strength between the template ion and the capture agent.Thus,the interaction mode could be inferred.The results of the energies calculation are shown in Table 1.
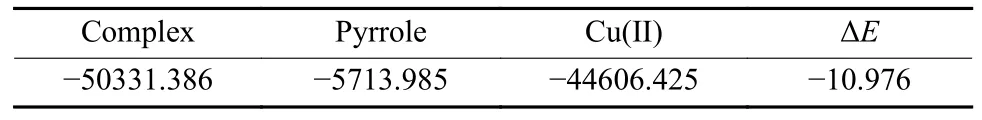
Table 1 Energy (eV) calculations for Cu(II),pyrrole,and their complex
The interaction energies ΔEbetween the template ion and the functional monomer is calculated according to the following formula:
whereE(template)is the energy of Cu(II),E(functionalmonomer)is the energy of pyrrole.
From the calculation results,the energy value of the formation of the complex was negative.It is clear that the positively charged Cu(II) could interact with the lone pair electrons of N atom from pyrrole.The complex exhibited a high binding energy,corresponding to specific binding.
In addition,the energy level difference of frontier orbital and the absolute value of HOMO orbital energy could reflect the stability of molecules.Fig.10 is the schematic diagram of LUMO and HOMO orbital formed by Cu(II) and pyrrole.The calculation results showed that the energy of the highest occupied orbital (EHOMO) was -0.627 eV,the energy of the lowest unoccupied orbital (ELUMO) was -0.593 eV,and the energy gap of the front-line orbital (ELUMO-EHOMO)was 0.035 eV.The results showed that the complex of Cu(II) and pyrrole could exist stably.
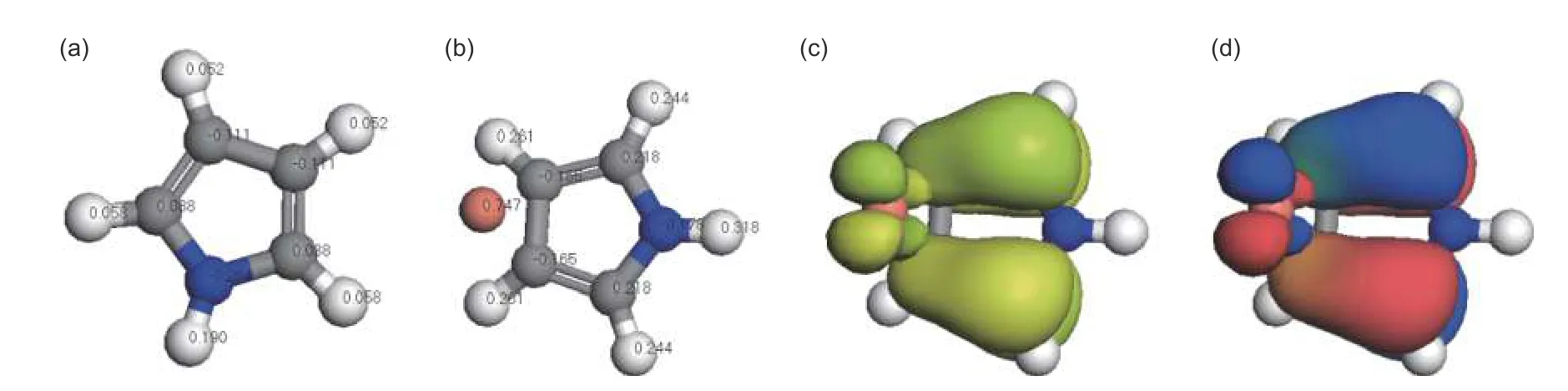
Fig.10 Schematic diagram of (a) the structure of pyrrole and (b) the complex formed by Cu(II) and pyrrole;(c) HOMO and (d) LUMO orbital formed by the interaction of Cu(II) and pyrrole
3.9 Evaluation of the mechanism of Cu(II)-IIPs modified electrodes
The theoretical simulation results showed that the lone pair of electrons on N-atom of pyrrole interact strongly with Cu (II).The interaction energy was-10.976 eV and the energy gap of the front-line orbital was 0.035 eV.Therefore,it is easy for Cu(II)-IIPs to complex with Cu(II) stably.The match cavity was highly matched with Cu(II).Meanwhile,the functional monomer had specific recognition effect towards Cu(II) and thus could complex with Cu(II).As for the elution of Cu(II) from Cu(II)-IIPs/GCE,cyclic voltammetry test was conducted to perform PPy peroxidation,thus weakening the complexation of Cu(II)and releasing Cu(II).Therefore,the prepared Cu(II)-IIPs/GCE exhibited excellent Cu(II) detection performance with respect to previous works (Table 2).A superior Cu(II) detection within a wide linear range of the Cu(II)-IIPs/GCE has been demonstrated.

Table 2 Comparison for the linear ranges and detection limits of the Cu(II) sensors
4 Conclusion
In conclusion,an ion-imprinted polymer electrochemical sensor was prepared to detect Cu(II) by combining unipolar pulse electropolymerization and surface ion imprinting technology.The sensor with the imprinted polymer electrode showed excellent specific selectivity towards Cu(II).The response current shows a good linear relationship with Cu(II) concentration in the range of 1.0×10-6to 1.0×10-10mol L-1.The Cu(II) detection limit has been improved to 5.138×10-16mol L-1(S/N=3).DFT calculation results further verified the specific selectivity between the imprinted polymer electrode and Cu(II).The sensitivity mainly comes from the interaction between the positively charged Cu(II) and the lone pair electrons on N from pyrrole in the imprinted sites.In addition,the sensor with imprinted polymer electrode possesses good reproducibility and stability,suggesting its detecting capability of low concentration heavy metal ions.
Data availability statement
The data that support the findings of this study are openly available in Science Data Bank at https://www.doi.org/10.57760/sciencedb.j00125.00075 or https://resolve.pid21.cn/31253.11.sciencedb.j00125.00075.
Acknowledgements
This work was financially supported by Key R&D Program of Shanxi Province (International Cooperation,201903D421077),Central Leading Science and Technology Development Foundation of Shanxi Province (YDZJSX2022A009),Key Program of Yinchuan science and Technology Bureau(2021ZD08),National Natural Science Foundation of China (51972221,51603142,51902222),Foundation of State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering (2021-K46),Natural Science Foundation of Shanxi Province(20210302124046),Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2019L0255,2020L0097).
杂志排行
新型炭材料的其它文章
- Carbon-based photothermal materials for the simultaneous generation of water vapor and electricity
- 生物质炭材料作为金属空气电池阴极的研究进展
- 3D porous NiCo2(CO3)3/reduced graphene oxide aerogel with heterogeneous interfaces for high-efficiency microwave absorption
- 煤基富氧多孔炭纳米片的制备及其超级电容器性能
- Mott-Schottky heterojunction formation between Co and MoSe2 on carbon nanotubes for superior hydrogen evolution
- A 2D montmorillonite-carbon nanotube interconnected porous network that prevents polysulfide shuttling
