A one-pot method to prepare a multi-metal sulfide/carbon composite with a high lithium-ion storage capability
2023-12-20ZHANGWeicaiYANGChaoweiHUShuyuFANGYaweiLINXiaominXIEZhuohaoZHENGMingtaoLIUYingliangLIANGYeru
ZHANG Wei-cai,YANG Chao-wei,HU Shu-yu,FANG Ya-wei,LIN Xiao-min,XIE Zhuo-hao,ZHENG Ming-tao,LIU Ying-liang,*,LIANG Ye-ru,*
(1.Maoming Branch, Guangdong Laboratory of Lingnan Modern Agriculture, Maoming 525000, China;2.Key Laboratory for Biobased Materials and Energy of Ministry of Education, College of Materials and Energy,South China Agricultural University, Guangzhou 510642, China)
Abstract: Because of their high electrochemical activity,good structural stability,and abundant active sites,multi-metal sulfide/carbon (MMS/C) composites are of tremendous interest in diverse fields,including catalysis,energy,sensing,and environmental science.However,their cumbersome,inefficient,and environmentally unfriendly synthesis is hindering their practical application.We report a straightforward and universal method for their production which is based on homogeneous multi-phase interface engineering.The method has enabled the production of 14 different MMS/C composites,as examples,with well-organized composite structures,different components,and dense heterointerfaces.Because of their composition and structure,a typical composite has efficient,fast,and persistent lithium-ion storage.A ZnS-Co9S8/C composite anode showed a remarkable rate performance and an excellent capacity of 651 mAh·g-1 at 0.1 A·g-1 after 600 cycles.This work is expected to pave the way for the easy fabrication of MMS/C composites.
Key words: Multi-metal sulfide/carbon composites;Facile synthesis;One-pot route;Lithium-ion battery;Anode
1 Introduction
Lithium-ion batteries (LIBs) have widely applied in our daily lives over the past few decades,such as portable electronics and electric vehicles[1–4].Considering the escalating challenges on the safety and energy densities of LIBs,developing advanced functional materials,including higher-performance anode materials,is one of the core topics[5–8].Basically,an usable anode material for LIBs requires high electronic conductivity and ionic permeability in both its bulk and surface.The structural stability is another important issue[9–11].Metal sulfides (MSs) have been applied as anode materials due to their good thermodynamically stability and high activity[12–18].Recently,there are an increasing number of studies around multi-metal sulfides (MMSs),as they could provide rich active sites and synergetic effect of multiple components and phases,resulting in enhanced electrochemical performances[19–26].Nevertheless,the great volume change and low intrinsic electronic conductivity of MMSs substantially limit their wide application in the field of LIBs.To this end,MMSs are usually composited with carbon (C) materials with high electronic conductivity and structural stability to realize MMS/C composites with synergistic advantages to overcome these defects[27–33].
Up until now,plentiful MMS/C composites have been synthesized by exploiting various fabrication routes[34–35].Taking bimetallic sulfide/C (BMS/C)composites as an example,fabrications of BMS/C composites usually utilize post-synthesis strategy,which mainly includes pre-synthesis of carbon matrixes,amalgamation of bimetallic oxides (BMOs),and follow-up transformation of BMOs to BMSs by sulfurization (Fig.1a)[19–20,33].Nevertheless,this strategy not only requires laborious,verbose,and multi-step processes,but also easily lead to uneven distribution and poor active sites since the BMOs/BMSs are primarily generated on the surface of the carbon matrixes[28,36].Although the pre-synthesis of carbon matrixes and the combination of BMOs could be simplified to one-step carbonization by using the organic-inorganic hybrid assemblies as precursors,the use of sulfur sources is inescapable during the sulfurization(Fig.1b)[30–31,37–38].Plenty of sulfur sources (normally more than 2 times the amount of MO/Cs) are usually needed to ensure complete conversion,which not only increases additional costs but also causes environmental damage.Additionally,macroscopic solid-solid physical mixing between MOs and sulfur sources often causes low sulfurization efficiency,resulting in the uneven and insufficient distribution of MSs in composites.Moreover,the existing synthetic routes have no significant advantages for the universal fabrication of MMS/C composites.Consequently,the development of facile and general approaches for synthesizing MMS/C composites is a considerable challenge.
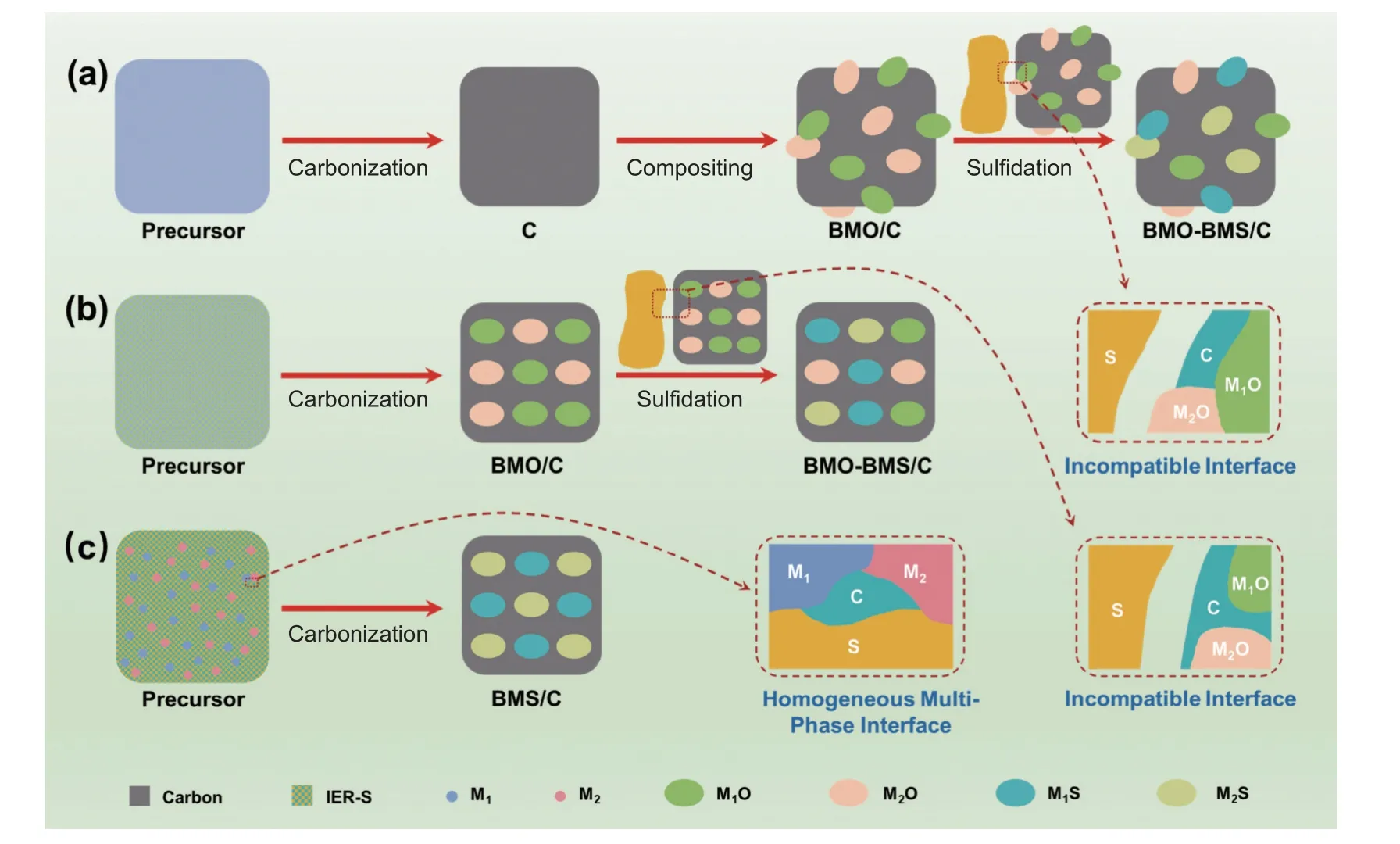
Fig.1 Synthetic scheme for preparing MMS/C composites by taking BMS/C composites as example.(a) Traditional post-synthesis strategy and (b) simplified post-synthesis strategy.(c) One-pot synthetic route by using a homogeneous multi-phase interfacial engineering
Here,a one-pot and general route to MMS/C composites as anodes for LIBs are presented.The key to this synthetic route is the coupling of metal cations to ―SO3-anion groups of sulfonic acid ion exchange resins (i.e.,IER-S) to create a hybrid assembly that integrates multi-metal ions,carbon and sulfide sources.The robust coordinate-covalent bonds between IER-S and multi-metal ions could realize the formation of uniform multi-phase interface,which ensures theinsitugeneration of MMSs evenly scattered into the carbon skeleton after one-pot heat-treatment (Fig.1c).Compared with traditional preparation strategies,our route opens up a straightforward and efficient way for the one-pot synthesis of MMS,thus possessing several advantages.First of all,the carbonization,amalgamation of MOs,and their transformation to MMSs are achieved in one step,then considerably simplifying the synthetize procedures and being suitable for mass production.Furthermore,this synthetic route is versatile and universal.For example,by changing the combination or quantity of multi-metal ions categories,a variety of MMSs can be expediently incorporated into the composite architecture.In addition,the uniform distribution of sulfur sources at the nanoscale greatly increases the sulfidation efficiency,which minimizes the sulfur content demand and corresponds with the pressing demand of energy conservation and environment governance.Last but not least,the evenly distributed MMSs within the carbon framework result in dense heterointerfaces,which creates abundant active sites for speedy electron/ion transport.By combining the advantages of high conductivity of carbon skeleton,impressive electrochemical activity of MMSs and profitable synergistic effect of heterointerfaces,the typical MMS/C composite shows notable lithium-ion storage performances.For example,the ZnS-Co9S8/C composite anode revealed a reversible capacity (651 mAh·g-1after 600 cycles at 0.1 A·g-1) and a remarkable rate performance (capacity retention of 54% with a 20-fold enhancement of current density).
2 Experimental
2.1 Synthesis of IER-S-M1/M2 or IER-S-M1/M2/M3 hybrid assembly
The IER-S was put into an aqueous solution including predetermined concentration of bimetallic ions or trimetallic ions (e.g.,Zn2+/Co2+,Cd2+/Co2+,Ni2+/Cu2+,Co2+/Mn2+,Zn2+/Co2+/Cu2+,Ni2+/Co2+/Mn2+,etc.).Coordination reaction was carried out for 30 min.After being washed and dried,the IER-S-M1/M2or IER-S-M1/M2/M3hybrid assemblies were obtained.
2.2 Synthesis of BMS/C or trimetallic sulfide/carbon (TMS/C) composites
In a typical procedure,the BMS/C or TMS/C composites by using IER-S-M1/M2or IER-S-M1/M2/M3hybrid assemblies as precursors were obtained by one-pot heat-treat at 800 °C for 4 h under an N2flow.The heating rate was 5 °C·min-1.
2.3 Structural characterization
The microstructures were characterized by scanning electron microscopy (SEM) (SU8220,Hitachi)and high-resolution transmission electron microscopy(HRTEM) (Talos F200S,FEI).The content of sulfur was determined by an elemental analyzer (Vario EL cube,Elementar).The content of metal ions was determined by flame atomic absorption spectrometer(220FS,Varian).Fourier transform infrared spectroscopy (FTIR) tests were carried out with an infrared spectrometer (Avatar 360,Nicolet),using the KBr disk method.X-ray diffraction (XRD) was tested by using a powder X-ray diffractometer (Ultima IV,Rigaku).Thermogravimetric analysis (TG209F1LibraTM,Netzsch) was performed with a heating rate of 10 °C·min-1.The surface elements and chemical components of samples were analyzed by using X-ray photoelectron spectroscopy (Nexsa,Thermo Fisher Scientific).
2.4 Electrochemical tests
Electrochemical measurements were performed by using coin-type half-cell configuration.Electrode slurries were obtained by uniformly blending the active material (80% by weigh),polyvinylidene fluoride(10% by weigh),and Super-P (10% by weigh) in Nmethyl pyrrolidone.Then,the resultant slurries were evenly coated on the copper foils and dried at 100 °C for 12 h.The electrodes were cut into discs with a semidiameter of 6 mm.The coin cells were fabricated by using Li as reference/counter electrode.The electrolyte was composed of 1 mol·L-1LiPF6in a 1∶1 (v/v)mixture of diethyl carbonate and ethylene carbonate.The electrochemical performances were performed on a Neware battery cycler (CT4008-5V10mA-164).Electrochemical impedance spectroscopy (EIS) were executed by an electrochemical workstation (CHI 660E,Chenhua) with a frequency range from 1×106to 1×10-2Hz and an amplitude of 5×10-3V·s-1.
3 Results and discussion
3.1 Fabrication of MMS/C composites
IER-S is capable of combining with different metal ions to form hybrid assemblies with multi-phase interfaces[39–41].The ―SO3-groups of IER-S could couple with metal ions through coordination bonds(Equ.1,2 and 3),thus forming a stable multi-phase organic/inorganic interface structure.In addition,the functional ―SO3-groups are able to act as sulfur sources during carbonization to support thein-situgrowth of MS nanoparticles,which ensures the uniform distribution of MS nanoparticles in the carbon matrix[42].Based on this,we proposed a general strategy for synthesizing MMS/C composites by constructing carbon source-sulfur source-multi-metal ions hybrid assemblies with the homogeneous multi-phase interfaces as precursors.
As a verification of concept,the IER-S and Zn2+/Co2+were employed for fabricating the ZnSCo9S8/C composite.First of all,the IER-S was soaked in an aqueous solution containing Zn2+/Co2+.Then the―SO3-groups of IER-S were combined with Zn2+and Co2+through coordination,resulting in the formation of IER-S-Zn/Co hybrid assembly.It could be seen that the color of IER-S changes from yellow-brown to purple-red after coordination reactions (Fig.2a).In IER-S-Zn/Co hybrid assembly,each Zn2+and Co2+were combined with two ―SO3-groups by coordination bonds,forming stabile organic/inorganic interface structures (Fig.2b).Fig.2c shows the Zn,Co,S and C elements are uniformly distributed throughout the hybrid assembly,confirming that IER-S-Zn/Co possesses homogeneous multi-phase interface.The FTIR spectra display that after coordination with Zn2+/Co2,the characteristic peaks assigned to the―SO3-groups shift from 1 040 and 1 128 cm-1to 1 037 and 1 120 cm-1,respectively (Fig.2d).[43–45]This result affords a proof that the ―SO3-groups are involved in bonding the Zn2+/Co2+into IER-S.X-ray photoelectron spectroscopy (XPS) of IER-S-Zn/Co was performed to further investigate coordination structures.Fig.S1 confirms the existence of C,S,O,Zn and Co elements in IER-S-Zn/Co.The S 2p spectrum was contributed by 3 peaks at 167.7,169.0 and 169.9 eV (Fig.2e),which were assigned to S=O,S―O and S―C bonds,respectively[46].The O 1s spectrum indicates that the Zn2+and Co2+combine with ―SO3-groups by the Zn―O and Co―O bonds(Fig.2f),respectively[47].The S 2p and O 1s XPS spectra confirmed the coordinate bond between multimetal ions and IER-S rather than physical mixing.

Fig.2 (a) Digital photos of IER-S before and after coordination.(b) Schematic illustration of Zn2+ and Co2+ combined with IER-S.(c) EDS images,(d) FTIR spectra of IER-S before and after coordination.(e) S 2p and (f) O 1s spectra of IER-S-Zn/Co.The inset in (f) shows a schematic diagram of the structure of 2 benzenesulfonates coupling to a divalent metal ion
The contents of various elements in IER-S-Zn/Co were measured by flame atomic absorption spectrometer and elemental analyzer.As displayed in Fig.3a,the mass contents of Zn,Co and S elements are 1.7%,4.9% and 12.9% with the corresponding substance contents of 0.3,0.8 and 4.0 mmol·g-1,respectively.After one-step carbonization at high temperature in an inert gas atmosphere,the IER-S-Zn/Co hybrid assembly was transformed into the ZnS-Co9S8/C composite.To understanding the phase transition process of IER-S-Zn/Co hybrid assembly during carbonization,ex-situXRD tests were executed.Fig.3b shows XRD patterns of the samples obtained at various heattreatment temperatures.The sample obtained at 300 °C exhibits a broad characteristic peak corresponding to C,which implies that no MSs have been formed at this temperature.By rising the temperature to 350 °C,the as-formed sample exhibited only characteristic peaks corresponding to Co9S8,indicating the formation of Co9S8.As the temperature was increased to 400 °C,typical peaks ascribed to ZnS emerged,implying the formation of ZnS.Evidently,the higher heat-treatment temperature we used,the more pronounced the characteristic peaks of ZnS we could observe.The phase transformation process was further determined by the thermogravimetric analysis (TGA)test under an N2atmosphere.Fig.3c displays the weight loss process that is separated into several typical regions.In the temperature range of 30-100 °C,the weight loss is ascribed to the removal of interlayer moisture.The IRE-S was slowly pyrolyzed and gradually transformed into a carbonaceous skeleton within the temperatures of 100-300 °C,while some―SO3-were transformed into SO2(Equ.4).With the further increase in temperature,SO2reacts with C to generate S and CO2gas (Equ.5),and the CO2leads to the aggravation of weight loss.At the same time,some Co2+react with S to form Co9S8(Equ.6).Starting from 350 °C,Zn2+participates in the reaction with S and gradually converts to ZnS (Equ.7),and Co9S8continues to be formed in this process.All the transitions from metal ions to MSs are completed at around 450 °C,which are consistent with those obtained fromex-situXRD patterns.

Fig.3 (a) Mass content and relative amount of substance of Zn,Co,and S elements in IER-S-Zn/Co.(b) XRD patterns of distinct samples obtained by heating IER-S-Zn/Co under different temperatures.(c) TGA curve obtained by heat treatment IER-S-Zn/Co under N2 flow.(d) Schematic of the evolution for IER-SZn/Co during the heating process.(e) SEM image,(f) EDS mapping images,(g) HRTEM image,(h) TGA curve,(i) XRD pattern and (j) S 2p spectrum of ZnS-Co9S8/C
According to the above discussion,a formation and evolution mechanism of the multi-phase interface for IER-S-Zn/Co is proposed (Fig.3d).At room temperature,Zn2+,Co2+and ―SO3-groups in IER-S were tightly combined by coordination bonds to form IERS-Zn/Co.When the temperature was risen to a certain extent,Co9S8started to be generated first.The ZnS was gradually formed by increasing the temperature,while the Co9S8continued to be generated.After reaching 450 °C,the Co9S8and ZnS have been converted completely and distributed uniformly in the carbon matrix.
The target ZnS-Co9S8/C composite could be derived by one-pot heat-treat IER-S-Zn/Co hybrid assembly at 800 °C.The SEM image of ZnS-Co9S8/C composite displays two kinds of MS nanoparticles with different crystal types which were evenly embedded or encapsulated in the carbonaceous matrix(Fig.3e).In addition,the EDS mapping images of ZnS-Co9S8/C (Fig.3f) further confirmed the even dispersion of Zn,Co,C and S elements.The HRTEM image of ZnS-Co9S8/C in Fig.3g evidenced the welldefined lattice fringes of multicomponent nanocrystal phases.The distinct lattice fringes with interplanar spacings of 0.292 and 0.313 nm are matched to the(101) and (002) planes of ZnS,respectively.The(400),(311) and (200) planes of Co9S8are assigned interplanar distances of 0.249,0.299 and 0.497 nm,respectively.Moreover,the dense heterointerfaces observed between distinct components of ZnS-Co9S8/C could cause an inner electric field and enlarge the number of active reaction sites,thereby improving the fast electronic/ionic diffusion kinetics[19–20,27,48–49].According to the TGA,the weight percentage of ZnS and Co9S8in ZnS-Co9S8/C is about 45.4% (mass fraction),of which ZnS is about 11.6% and Co9S8is about 33.8% (mass fraction) (Fig.3h and S2).XRD pattern(Fig.3i) showed the positions of characteristic peaks are basically the same as the standard peaks of ZnS(JCPDS card No.36-1450) and Co9S8(JCPDS card No.19-0364) crystals,authenticating the coexistence of ZnS and Co9S8.The surface chemical constituent of ZnS-Co9S8/C composites was further determined by XPS.Fig.S3 signals the full XPS spectrum of ZnSCo9S8/C,which clearly reveals the existence of Zn and Co elements.The peaks of S 2p spectrum at 164.2,162.9 eV and 163.8,161.7 eV correspond to the Zn-S bonds and Co-S bonds,respectively (Fig.3j).It should be noted that the peaks at 165.9 and 165.1 eV pertain to the C–S bonds[36],illustrating that BMSs and C bonded by C–S bonds,which facilitates the construction of inseparable and even carbon/non-carbon interfaces in the obtained composites.
3.2 Structural control of MMS/C composites
The synthesis of BMS/C composites based on the homogeneous multi-phase interface strategy has strong customizability and controllability.As demonstration,by one-pot heat treatment diverse hybrid assemblies acquired through coupling IER-S with Mn2+/Zn2+,Cd2+/Co2+,Zn2+/Cu2+,Ni2+/Cu2+,Cu2+/Co2+,Cu2+/Mn2+,Ni2+/Mn2+,Ni2+/Zn2+,Co2+/Mn2+,and Fe3+/Ni2+(Fig.4a and S4),the MnS-ZnS/C,CdSCo9S8/C,ZnS-Cu7.2S4/C,Ni7S6-Cu7.2S4/C,Cu7.2S4-Co9S8/C,Cu7.2S4-MnS/C,Ni3S2-MnS/C,Ni3S2-ZnS/C,Co9S8-MnS/C,and Fe7S8-Ni3S2/C composites could be easily obtained,respectively.Fig.4b presents a general synthesis procedure for BMS/C composites.Firstly,IER-S-M1/M2hybrid assembly has been formed by coupling IER-S and metal ions (i.e.,M1n+and M2m+).After one-step carbonization,BMS/C composites with MS nanoparticles encapsulated in carbon matrixes could be fabricated.As shown in the XRD patterns of Fig.4c,the diffraction peaks of each MS are well-fitted with their standard cards,which demonstrate the successful synthesis of BMS/C composites.It should be noted that there exists a weak peak at about 26°,which corresponds to the (002) diffraction plane of the amorphous carbon after heat-treat originate from IERS.The structural characteristics of these composites could be further characterized by SEM.Fig.4d-g show that MS nanoparticles are tightly embedded or encapsulated in carbon matrixes,indicative of their well-organized structures.

Fig.4 (a) Digital photos of different IER-S-M1/M2 hybrid assemblies.(b) Synthesis schematic of BMS/C composites.(c) XRD patterns and (d–g) SEM images of BMS/C composites
It is worth mentioning our strategy could be used for facile and efficient synthesis of TMS/C composites only by increasing the types of metal ions(Fig.5a).For example,ZnS-Co9S8-Cu8S5/C,Ni3S2-Co9S8-MnS/C,and Ni3S2-Co9S8-Fe7S8/C composites could be prepared by one-pot carbonization of the IER-S-Zn/Co/Cu,IER-S-Ni/Co/Mn,and IER-SNi/Co/Fe hybrid assemblies (Fig.5b and S4),respectively.The EDS mappings determine that the M(M=Zn,Co,Cu,Ni,Mn,Fe),C and S elements are homogeneously distributed in these TMS/C composites (Fig.5c-e),revealing that ZnS/Co9S8/Cu8S5(Fig.5f),Ni3S2/Co9S8/MnS (Fig.5g) or Ni3S2/Co9S8/Fe7S8(Fig.5h) are evenly amalgamated into the carbon skeleton.Besides,the successful transformations of different metal ions into TMSs are certified by the XRD patterns as well (Fig.5i).
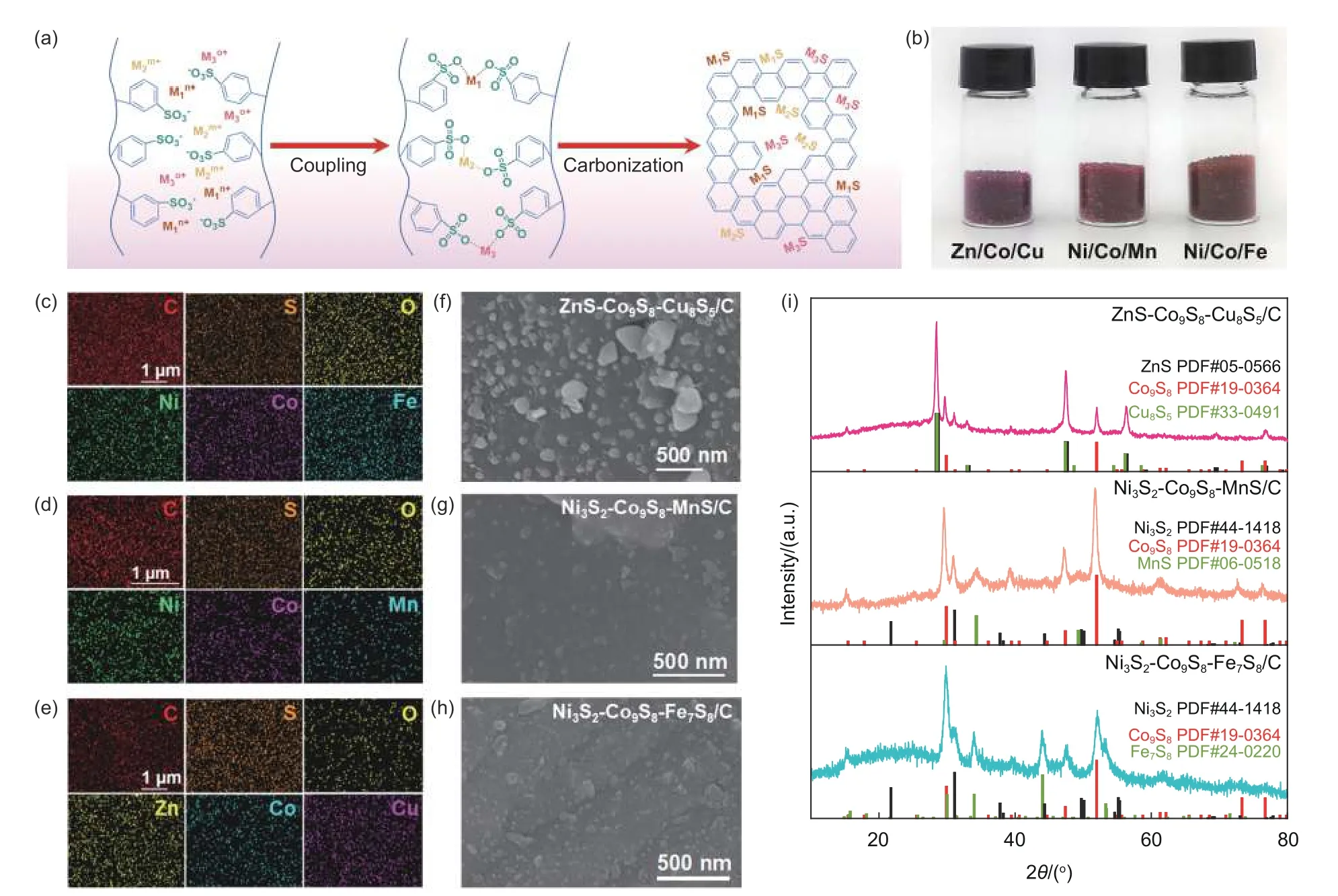
Fig.5 (a) Schematic illustration for fabrication of TMS/C composites.(b) Digital photos of different IER-S-M1/M2/M3 hybrid assemblies.(c-e) EDS mapping images,(f-h) SEM images,and (i) XRD patterns of TMS/C composites
3.3 Lithium-ion storage performances
To take advantage of the well-organized structure and beneficial synergistic effects,BMS/C composites have large application potential in advanced LIBs.As a conceptual demonstration,we evaluated the lithium-ion storage capability of the ZnS-Co9S8/C composite.To demonstrate the advantages of MMSs,the electrochemical properties of Co9S8/C prepared without Zn2+and ZnS/C prepared without Co2+(Fig.S5 and S6) were also studied under identical test conditions.Fig.6a shows the ZnS-Co9S8/C anode parades a discharge capacity of 595 mAh·g-1after 120 cycles at 0.1 A·g-1.This value is higher than the ZnS/C anode (473 mAh·g-1),Co9S8/C anode (526 mAh·g-1),and those of typical metal sulfide-based anodes reported in the literatures (Table S1).In addition,the ZnSCo9S8/C anode delivered a high capacity of 651 mAh g-1over 600 cycles.In contrast,the ZnS/C and Co9S8/C anodes failed at the 123rdand 528thcycles,respectively.The ZnS-Co9S8/C anode showed a better cycling stability than ZnS/C and Co9S8/C anodes.A similar superiority in the ZnS-Co9S8/C anode was observed in the cycling test at 1 A·g-1(Fig.6b).
The rate capability of the ZnS-Co9S8/C composite was assessed (Fig.6c).The ZnS-Co9S8/C anode presented impressive reversible capacities of 592,509,441 and 320 mAh·g-1at the current densities of 0.1,0.2,0.5 and 2 A·g-1,which surpassed those of ZnS/C and Co9S8/C anodes.The corresponding capacity retention rate reached as high as 54% with a 20-fold enhancement of current density,which was superior to those of ZnS/C and Co9S8/C anodes.While the current density turned back to original state,similar capacity values could be recovered,confirming the valuable stability of ZnS-Co9S8/C anode at a wide current range.
To determine the cause for the enhanced lithiumion storage capability,the EIS of ZnS-Co9S8/C,ZnS/C,and Co9S8/C anodes were performed.As indicated in Fig.6d-6e and Table S2,the Ohmic resistance (Rs) of ZnS-Co9S8/C,ZnS/C,and Co9S8/C anodes are 2.8,4.5 and 6.0 Ω,respectively.With similar carbonaceous skeleton,the ZnS-Co9S8/C anode exhibited the lowestRs,indicating a good efficiency of electronic transmission provided by the heterostructures.TheRctof ZnS-Co9S8/C anode (i.e.,34.0 Ω) is minor than that of the ZnS/C (i.e.,67.7 Ω) and Co9S8/C (i.e.,63.3 Ω),manifesting the superior charge transfer efficiency of ZnS-Co9S8/C.The lithium-ion diffusion coefficient (DLi) of the ZnS-Co9S8/C anode is 23.9×10-20cm2·s-1(Fig.S7 and 6f),which is higher than that of the ZnS/C (i.e.,12.8×10-20cm2·s-1) and Co9S8/C (i.e.,9.6×10-20cm2·s-1),confirming ZnSCo9S8/C anode possesses faster ion/charge transfer rates.
The efficient lithium-ion storage capability of ZnS-Co9S8/C composite derives from its favorable combination of heterostructure with an instructive synergistic effect (Fig.7).First,the carbonaceous skeleton enables high electron transfer rate,heightening the holistic conductivity of the ZnS-Co9S8/C anode.Secondly,the ZnS/Co9S8nanoparticles are steadily encapsulated by carbon skeletonviaC–S bonds,that forestalls the deterioration of active material,holds the completeness of the composite,and provides more electron/ion diffusion channels.Last but not the least,the dense and uniform heterointerfaces between distinct components and phases supply more accessible active sites for Li+storage,boost the inherent electronic conductivity of BMSs,and shorten the solid-phase transport length of Li+,thereby realizing high active material utilization and rapid electronic/ionic transportation kinetics.
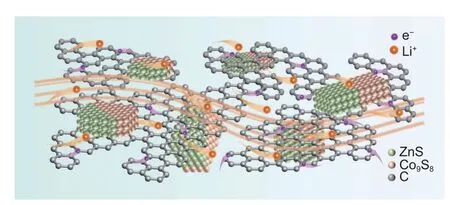
Fig.7 Schematic illustration of fast electron and ion transportation of ZnS-Co9S8/C anode
4 Conclusions
In conclusion,we have developed a one-pot and versatile strategy for preparing MMS/C composite materials including BMS/Cs and TMS/Cs based on homogeneous multi-phase interface engineering.This route enables the efficient and simplified synthesis of various MMS/Cs.Benefiting from the well-organized structure,the typical ZnS-Co9S8/C composite exhibits enhanced electron/ion transport kinetics,high capacity,good cyclic stability,and superior rate capability when used as the anode material of LIBs.This study is expected to offer fresh insight and carve out a valued avenue for fabricating multitudinous MMS/C composites,also expanded to readily incorporate other multi-metal compounds (multi-metal oxide,multimetal phosphides,multi-metal selenide) into the carbon skeleton for widespread application scenarios like electrochemical energy storage,electrocatalysis,and sensors.
Data availability statement
The data that support the findings of this study are openly available in Science Data Bank at https://www.doi.org/10.57760/sciencedb.j00125.00087 or https://resolve.pid21.cn/31253.11.sciencedb.j00125.00087.
Acknowledgements
This work was financially supported by Independent Research Project of Maoming Laboratory(2022ZD002) and National Natural Science Foundation of China (51972121 and 52373074).
杂志排行
新型炭材料的其它文章
- Carbon-based photothermal materials for the simultaneous generation of water vapor and electricity
- 生物质炭材料作为金属空气电池阴极的研究进展
- 3D porous NiCo2(CO3)3/reduced graphene oxide aerogel with heterogeneous interfaces for high-efficiency microwave absorption
- 煤基富氧多孔炭纳米片的制备及其超级电容器性能
- Mott-Schottky heterojunction formation between Co and MoSe2 on carbon nanotubes for superior hydrogen evolution
- A 2D montmorillonite-carbon nanotube interconnected porous network that prevents polysulfide shuttling
