Influence of liquid bridge force on physical stability during fuel storage and transportation
2023-12-07ChiZhngChunhuBiJifnRenJinYo
Chi Zhng ,Chun-hu Bi ,Ji-fn Ren ,Jin Yo
a State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing,100081, China
b School of Mechanical Engineering, Nanjing University of Science and Technology, Nanjing, 210094, China
Keywords: Liquid bridge force Fuel physical stability Multiliquid bridge resultant forces Mixed fuel stratification
ABSTRACT The physical stability of solid-liquid fuel is a factor that needs to be considered for fuel product practicability for storage and transportation.To determine the Influence of liquid bridge force on physical stability,two detection devices were designed.The laws obtained from microscopic experiments are used to verify the physical stability of fuel under different component ratios.The liquid bridge force is found to increase with the droplet volume.Multiliquid bridges above one critical saturation can generate significant resultant forces compared to single-liquid bridges of the same volume.There exist four states of solid-liquid mixed fuel with increasing liquid saturation rate.The liquid bridge force between the solid and liquid plays a dominant role in the physical stability of the first three states.There may be two stages involved in the stratification process for state 4 fuel,and the liquid viscosity is another factor that cannot be ignored.In the process of selecting a fuel ratio,a larger liquid bridge force between the components can be obtained by properly improving the wetting effect so that the fuel shows better physical stability.
1.Introduction
Solid-liquid mixed fuel uses metal powder as the continuous phase,and the liquid components are filled in.The explosive properties of such fuels have been extensively studied[1-5,26].In the 1960s,during the development of FAE(Fuel air explosive)fuel,the United States chose to mix magnesium powder (or aluminum powder) with liquid fuel to produce a liquid-based liquid-solid mixed fuel[6].The existence of solid-liquid two-phase mixing has been proved to bring a great challenge for the physical stability of fuel.In the process of storage and transportation,solid-liquid mixed fuel will inevitably be affected by gravity and vibration loads.The liquid components cannot maintain the adsorption of solid particles because unreasonable proportions will result in solidliquid separation.This result not only affects the performance of the fuel but also leads to a higher safety hazard during storage and transportation.
To solve the problem of physical stability,scholars have carried out research from different aspects.Zhang et al.investigated the physical stability of solid-liquid fuel based on the mesoscopic structure and stacking characteristics [7-9].The research results indicated that the saturation degree of liquid fuel filling in the fuel pores has an important influence on the physical stability.Chen and Hu combined the influence of vibration load on the physical stability during transportation and detected the precipitation volume and density distribution of a solid-liquid mixed fuel under a series of vibration frequency gradients [10,11].They found that the air porosity between solid particles is affected by the wetting ability.The lower the interstitial ratio,the less the fuel density distribution is affected by the oscillation.Wu used an additive to prevent the sedimentation of spherical particles and improve the stability for liquid-solid FAE fuel [12].According to the experimental results,when the additive ratio reaches 3%,although the sedimentation phenomenon of solid particles still persists,the sedimentation time is greatly extended.They hypothesized that the settling time for the solid particles can tend to infinity when the additive ratio is further increased to an appropriate range.
From a microscopic point of view,the solid particles in the solid-liquid mixed fuel will undergo a series of dynamic interactions via connection of the liquid between the pores.Among these interactions,the liquid bridge force has been widely studied as a nonnegligible factor in the hydrodynamic interaction force.It is an attractive force due to the Laplace pressure between particles and the surface tension of the liquid.Under the same particle size,the effect of the liquid bridge force on component particles is much greater than that of van der Waals forces and electrostatic forces[13].The research of Rabinovich et al.showed that the capillary force(liquid bridge force)generated by the condensation of water vapor on the surface between particles can result in agglomeration[14].The corresponding formula for calculating the liquid bridge force was proposed under different ranges of the liquid bridge stretching distance.De Souza et al.used simulation software to divide a single-liquid bridge of a fixed volume into different numbers of equal-volume multiliquid bridges and established a function for the resultant force[15].The resultant force for multiple liquid bridges increases with increasing liquid bridge number.The liquid bridge will appear in four forms:pendulum-like,cyclin-like,capillary-like and serous-like with different liquid saturations[16].The liquid bridge adhesion also increases until the saturation is greater than 100%.Liquid bridge forces play a very significant role in maintaining the physical stability of solid-liquid mixed fuels.
Numerous studies have demonstrated [17-20]that particles adsorbed by liquid bridges result in particle agglomeration,and very few studies have linked this adsorption to the anti-settling properties of solid-liquid fuels.While paying attention to chemical stability [21],enough attention should be paid to the physical stability of multicomponent fuels.In this paper,two sets of devices are designed to discuss the contribution of a liquid bridge force in improving the anti-stratification ability of solid-liquid mixed fuel during storage and transportation.Under microscopic conditions,the liquid bridge force variation trend for a single liquid bridge and multiple liquid bridges was investigated based on the use of a liquid bridge force detection device (device 1) after mixing liquid ether and nitromethane in different proportions.Then,the abovementioned liquid fuel was mixed with aluminum powder,and the physical stability detection device (device 2) was used to investigate the anti-settling ability of the fuel with different ratios.Combining the microscopic experimental laws obtained for device 1 with the phenomena presented by device 2 is expected to provide a valuable reference for the study of the physical stability of solidliquid mixed fuels.
2.Experimental method
2.1. Solid-liquid two-phase liquid bridge force detection device and experimental grouping
As shown in Fig.1,the liquid bridge force detecting device was designed by referencing the literature [22,23].It consists of a robotic arm,a microscope(GP-50,GAOPIN,China),and an electronic balance(0.1 mg precision,SUPO,China).The working principle for device 1 is shown in Fig.2.The robotic arm descends slowly under program control.The droplet contact is magnified by the horizontal microscope.When the upper metal sheet comes into contact with the liquid to form a bridge,the balance reading changes accordingly.At this point,the robotic arm will start to slowly lift,and the latest reading for the balance will be recorded under the set stretching distance.We calculate the liquid bridge adhesion forceFlbby using Eq.(1).
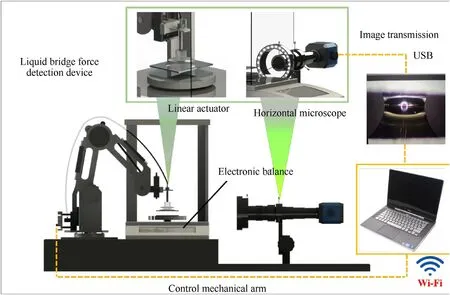
Fig.1.Schematic diagram of the image transmission and the liquid bridge force detection device.

Fig.2.The working principle for the solid-liquid two-phase liquid bridge force detection device.
where mdry is the sum of the masses of the droplet and lower metal sheet(when the upper metal plate is not in contact with the droplet),mwet is the balance reading after the upper metal sheet contacts the droplet forming a liquid bridge,and g is the local acceleration due to gravity.Parameters such as the stretched length of the droplet,the contact diameter with the metal plate,and the contact angle can be directly measured by using the supporting software.Each experimental condition was repeated three times,and an average value was taken.
The liquid fuels (ether and nitromethane) used in this experiment were analytical reagents with a purity of 99.5%.A surface tensiometer (K100,Kruss,Germany) was used to measure the surface tension of the mixed liquid fuel (nitromethane/ether).Other experimental conditions and groupings are shown in Table 1.In the multiliquid bridge experiment,single liquid bridges with volumes of 20 μL and 40 μL were divided into 4×5 μL and 4×10 μL liquid bridges(4 small liquid bridges of equal volume).In addition,combined with the fact that the liquid fuel is filled in the limited space between the particles in actual production,the contact surface of the four equal-volume liquid bridges will be controlled within a limited range.
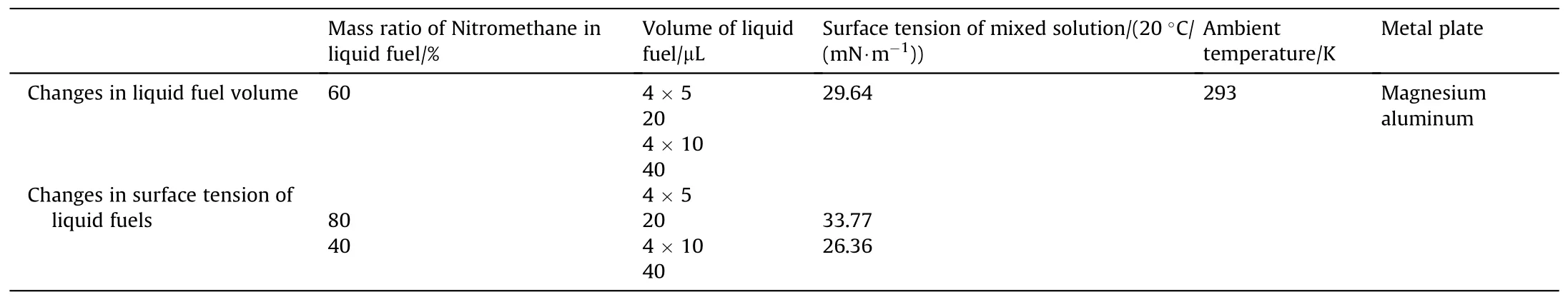
Table 1 Liquid bridge force detecting experiment grouping.
2.2. Solid-liquid mixed fuel physical stability detection device and experimental grouping
The metal used in this article was customized after a certain scaling based on the actual production fuel storage container.The inner diameter was 70 mm,the height was 100 mm,and the material was 45-gauge steel.The tank body and cover were connected by threads.A rubber ring was added to increase the airtightness of the container.The reserved interface of the tank cover was connected to a liquid level gauge and pressure gauge.The liquid level gauge contained a pressure sensor and a thermometer for monitoring the internal liquid level and temperature changes.This paper refers to the relevant Ref.[24]and selected the JX-W190 electronic pressure gauge as the monitoring tool.Its measurement range was 0-60 kPa,with an accuracy level of 0.25%,and the sampling frequency was 3 times/second.In this paper,pressure gauges and liquid level gauges were mainly used to exclude the influence of fuel volume expansion and liquid volatilization on the experimental results.
The metal powder used in this experiment was composed of flake aluminum powder with a median particle size of 14.28 μm[25].It was mixed with the liquid components in specific proportions in a container and placed in an air bath oscillation device,as shown in Fig.3.The device was retrofitted with custom-sized slots to ensure that the metal container did not drift during vigorous shaking.The vibration amplitude of the oscillating device was set at a fixed value of 20 mm,and the ambient temperature was 293 K.The mixed fuel showed different apparent states,as shown in Fig.4,with increasing proportion of liquid components.We hypothesize that mixed fuels with different morphologies need to be discussed individually in the process of analyzing the fuel stability,and the final grouping is shown in Table 2 below.
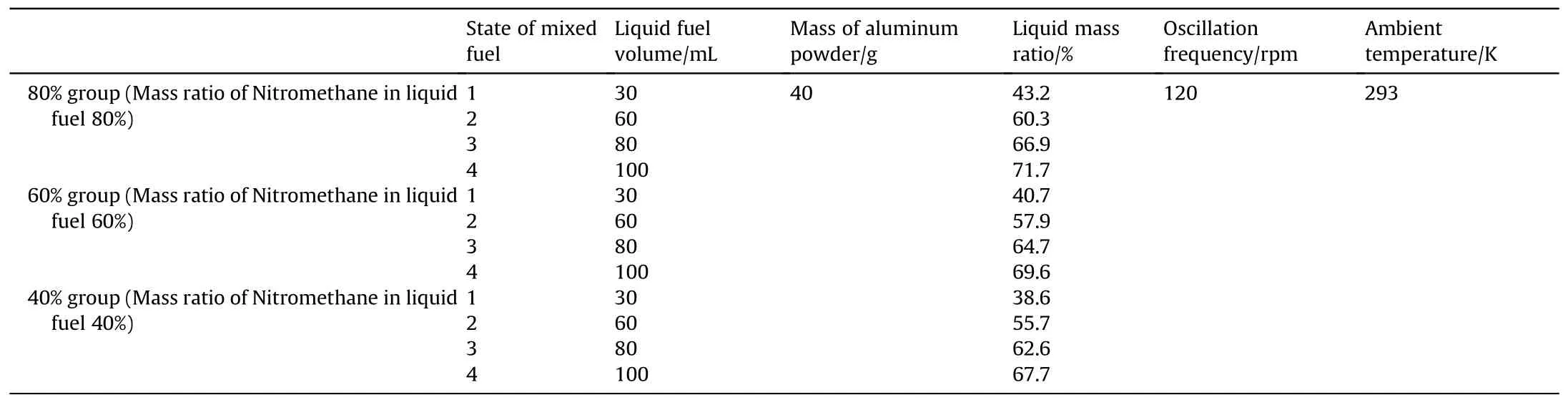
Table 2 Mixed fuel stability experimental groups.
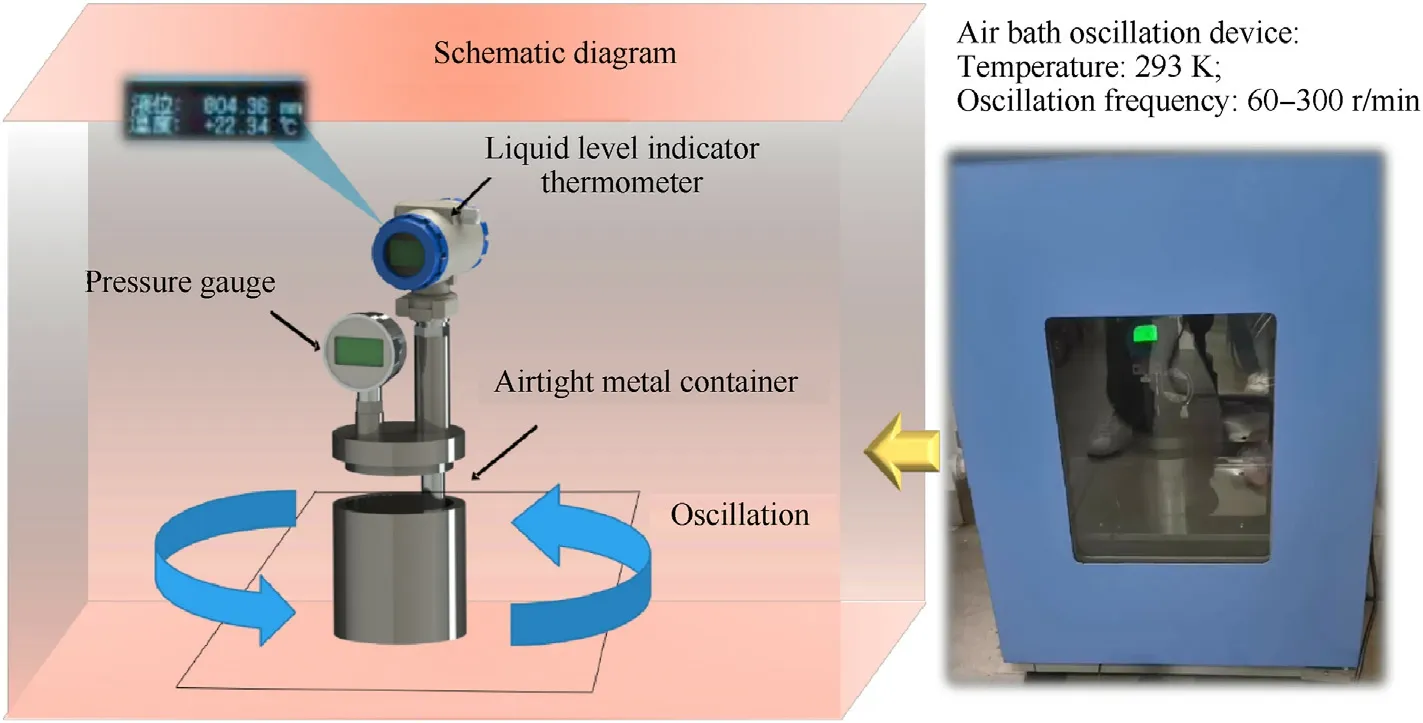
Fig.3.Physical stability detection device and working principle for the solid-liquid mixed fuel.

Fig.4.Apparent state of mixed fuels under different ratios.
To determine the shaking time for the experiment,different ligand fuels were shaken in each environment,and the volume of the precipitated liquid was measured with a syringe every half an hour until it reached the plateau phase.The fuel in states 1 to 3 was shaken for 24 h,and no liquid was precipitated.The state 4 fuel appeared as a precipitated liquid,and the time to reach the plateau was approximately 4 h.Therefore,the fuel oscillation time for all groups in this part was uniformly set as 4 h.
This part of the experimental detection content was divided into two aspects: measurement of the precipitated liquid volume and the distribution of the fuel density.(1) Precipitated liquid measurement: After the oscillation was over,if there was liquid precipitation,a syringe was used to remove the liquid to determine the volume.(2)Density measurement(shown in Fig.5):For the mixed fuel without liquid precipitation,the top was first leveled,then the maximum height H of the fuel stack was measured,divided into three equal parts and defined as the top,middle and bottom.The three parts of the fuel were taken out in turn,and the mass of this fuel was weighed using a balance to obtain the top mass,middle mass,and bottom mass.The three-part fuel volume was obtained by multiplying the inner vessel bottom area byH/3.Finally,the top density,middle density,and bottom density were obtained with their corresponding mass/volume
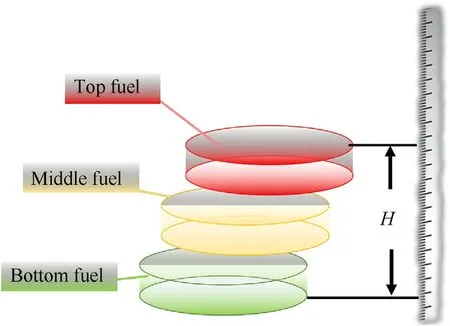
Fig.5.Schematic diagram of the experimental method used to determine the fuel density distribution after oscillation.
2.3. Experimental error analysis
(1) Detection error for the liquid bridge force
The maximum random error for the 5-50 μL pipette system was 0.3%,resulting in a maximum volume error of approximately 0.15 μL.The maximum error for the balance was 0.1 mg,and the repeatability error of the robotic arm was 0.1 mm.
(2) Fuel physical stability error
In the process of dividing the fuel height into three equal parts,since the top of the mixed fuel in states 1-3 was not completely horizontal,an error of 2-3 mm was generated in the measurement.
3.Results and discussion
In the actual fuel mixing process,the liquid components are not completely present in the form of a complete single liquid bridge.Liquid components can come into contact with surrounding solid components in the form of multiple liquid bridges due to the presence of air bubbles.Therefore,combined with the actual problems that may be encountered in production,we designed an experiment with multiple liquid bridges,and the single liquid bridge group was set as the control group(see Fig.6).
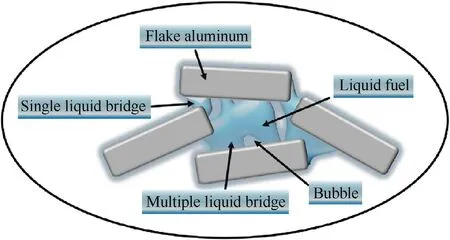
Fig.6.Schematic diagram of a single-liquid bridge and a multiliquid bridge formed between components of a solid-liquid mixed fuel.
3.1. Influence of droplet volume on liquid bridge force
Fig.7 shows the variation trend for the liquid bridge force with the stretching distance for a liquid bridge volume of 20 μL,4×5 μL,40 μL,and 4 × 10 μL (the mass ratio of nitromethane in liquid components: 60%).The liquid bridge force decreases with the stretching distance.As the total liquid fuel volume increases,there will be two different outcomes for the multiple liquid bridge group.When the total volume of liquid fuel is 20 μL,the liquid bridge force generated by multiple liquid bridges at different stretching distances is not significantly different from the(corresponding)single liquid bridge condition.Compared with the single liquid bridge(under the same conditions),the 4×5 μL multiliquid bridge group exceeds the critical stretching distance and fractures at 2.7 mm due to the reduction of the liquid bridge volume.However,as the total liquid volume is increased to 40 μL,the liquid bridge force of the multiliquid bridge group increases significantly compared with its control group.The 4 liquid bridges produces a 1.25 times greater liquid bridge force than that for their control group.
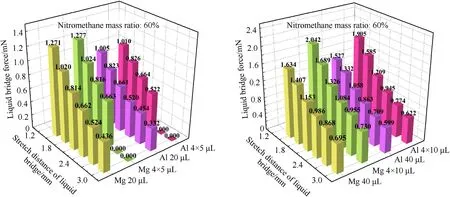
Fig.7.Variation trend for liquid bridge force with stretching distance under different volumes (Liquid bridge volume: 20 μL,4 × 5 μL,40 μL,4 × 10 μL;Fixed liquid ratio:nitromethane mass ratio of 60%;Solid metal: metal magnesium plate,metal aluminum plate).
The reasons for the above differences will be analyzed by comparing the morphological features of multiple liquid bridges with different volumes.In a limited area,when the total volume of liquid fuel is small,four relatively independent small liquid bridges are formed,as shown for the 4 × 5 μL group in Fig.8.The morphology of the multiliquid bridge group composed of the 4 × 5 μL group in this paper is very similar to that of the 4 × 3 μL multiliquid bridge reported in Lu's research (Lu Z.P.,2016).Lu speculated that the equipment cannot control the three-phase line of the liquid bridge,resulting in the phenomenon that the resultant force does not increase significantly when multiple liquid bridges form.However,when the volume limit of the liquid bridge increases to the stretched length,as shown in Fig.8 (4 × 10 μL at 1.5 mm),we find that the liquid bridges will contact each other during the stretching process and finally form four liquid bridge packages in the shape of the central gaseous space.However,when the liquid bridge volume is increased to 4×10 μL,as shown in Fig.8(stretching length of 1.5 mm),we find that the liquid bridges contact each other during the stretching process.Four thicker liquid bridges are formed to wrap the central gas phase space.This can be approximated as the multiliquid bridge group with the same total volume actually forming a “single liquid bridge” with a volume larger than that of its control group.According to the law obtained from the single liquid bridge experiment,the liquid bridge force increases with increasing droplet volume.
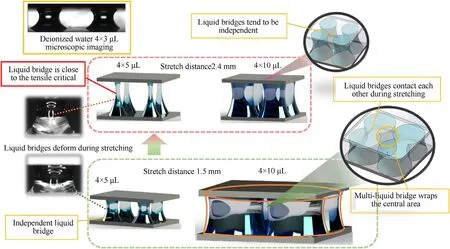
Fig.8.Analysis of the mechanism for the liquid bridge force generated by different volumes of multiliquid bridges (4 × 3 μL deionized water microscopic image [23]).
3.2. Influence of droplet ratio on liquid bridge force
To explore the influence of the liquid surface tension,this section investigates the variation trend for the liquid bridge force with the stretching distance for a nitromethane ratio of 80% and 40%(as shown in Fig.9).When the total liquid volume is 20 μL,the change in the liquid surface tension does not result in the multiliquid bridge group resultant force being significantly different from that of the control group.As the nitromethane proportion is decreased,the liquid bridge force for the 40% group becomes slightly lower than that of the 80% group with decreasing surface tension.In addition,the critical distance for liquid bridge breakage is reached earlier in the 40% group than in the 80% group due to the reduced liquid viscosity.However,when the total liquid volume reaches 40 μL,the resultant force for the multiliquid bridge forces in the two ratio groups is significantly improved compared with their control group.Compared with the 40% group,the resultant force of the liquid bridge force in the 80% group is more obviously increased under the same stretching distance.When the stretching distance is 1.5 mm,the resultant force between the two magnesium plates is increased by 35% compared with the control group.

Fig.9.Variation trend for the liquid bridge force with stretching distance under different liquid ratios(liquid bridge volume:4×5 μL,4×10 μL;liquid ratio:nitromethane mass ratio of 80%,40%;solid metal: metal Magnesium plate,metal aluminum plate).
The multiliquid bridge force results suggest that there is a critical condition for the liquid saturation rate in a limited space resulting in a larger resultant force (compared to the same condition with a single liquid bridge).This resultant force increases with increasing liquid viscosity.However,the fuel as the 80% group may not reach the wetting state of the 4×10 μL group in the experiment resulting from its high liquid surface tension in the actual mixing process.The effect of droplet surface tension change on the wetting presents a trend as shown in Fig.10.The contact angle of the droplet increases with the increased liquid surface tension.The wettability of the 80% liquid is inferior to that of the 40% group.This may make it difficult to produce a better wetting effect when 80% of the liquid components are filled in the gaps between solid components.
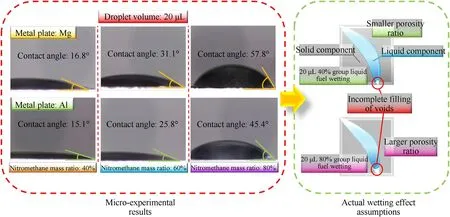
Fig.10.Results of droplet wetting on metal plates and assumptions between actual filled solid components.
Therefore,it is necessary to appropriately reduce the surface tension of the liquid fuel,ensuring that more liquid components can be filled into the gaps to effectively enhance the liquid bridge force and improve the physical stability of the fuel.Therefore,proper reduction of the liquid surface tension to ensure that enough liquid components are filled can effectively enhance the liquid bridge force and improve the physical stability.
3.3. Effect of liquid fuel ratio on the physical stability of mixed fuel
In this part,the physical stability of the solid-liquid mixed fuel with different ratios in the (293 K) oscillation process was investigated.The results obtained for liquid fuel precipitation after vibration at a frequency of 120 rpm for 4 h are shown in Fig.11.When the fuel is maintained in states 1-3,no liquid precipitation phenomenon occurs after 4 h of vibration.The fuel in state 4 shows a solid-liquid stratification phenomenon due to its supersaturated state.The mixed fuels for state 1 and state 2 exist in the form of powder and have a lower liquid content.The dense packing of the liquid fuel by the aluminum powder effectively prevents the liquid fuel from flowing out of the pores.The fuel (state 3) has a higher liquid saturation rate and a larger liquid bridge volume.According to the microscopic experimental law,the larger the volume,the greater the liquid bridge force between the solid and liquid.In addition,the fragmentation of the interparticle bubbles under the vibration action also provides space for the liquid fuel,avoiding the appearance of solid-liquid stratification.
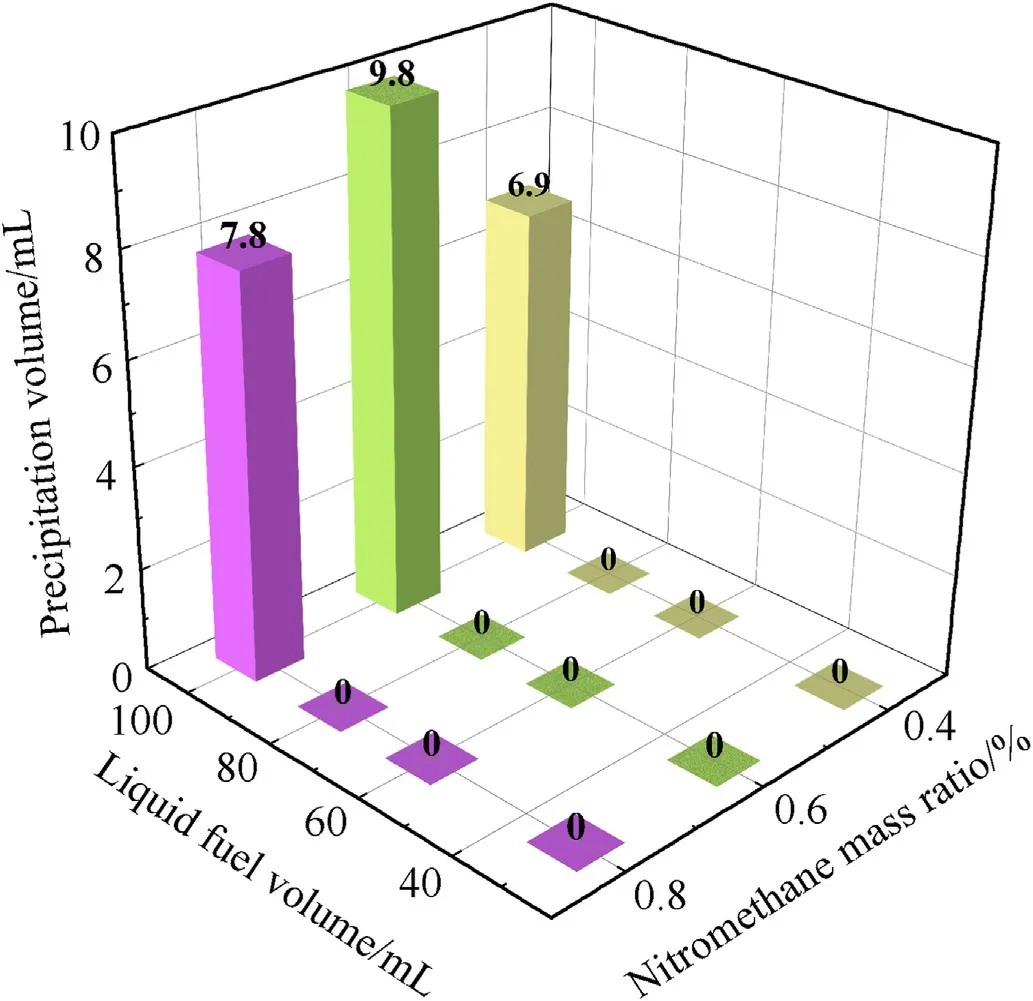
Fig.11.Volume of precipitated liquid fuel after oscillating for 4 h with different ratios(Ambient temperature 293 K;amplitude: 20 mm;Oscillation frequency: 120 rpm(2 Hz);Constant mass of the solid component: 40 g flake aluminum powder).
The state 4 fuel is in a supersaturated fluid state before oscillation,and the surface is covered by a silver-white liquid film.We collected the precipitation liquid from the upper layer through a needle and found that the volume of the precipitation liquid for the three fuel ratios(the mass ratio of nitromethane was reduced from 80 to 40%) shows a trend of first increasing and then decreasing.The apparent morphology of the stratified fuel(as shown in Fig.12)suggests that two stages are involved in the whole process(Fig.13).In stage 1,the main obstacle that hinders the supersaturated fuel from being affected by vibration is the interaction force between liquids.The greater the viscosity of the liquid components,the greater the interaction force between the liquid and liquid.In the second stage,when part of the liquid fuel is precipitated,the mixed fuel in the middle and lower parts will reach a state close to that of state 3.At this stage,the main factor for ensuring physical stability is the liquid bridge force between the solid and liquid components.The lower the liquid surface energy,the better the solid infiltration effect and the greater the liquid bridge force.

Fig.12.Display of the supersaturated fuel stratification state.
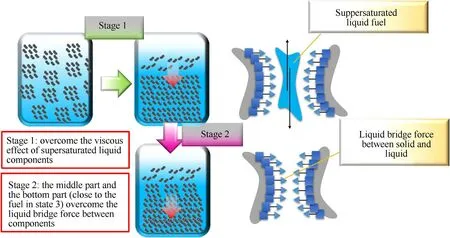
Fig.13.Conjecture of two stratification stages for supersaturated fuel.
The viscosity of liquid nitromethane (293 K) is 0.648 mPa s,while the viscosity of liquid diethyl ether is 0.225 mPa s.The viscosity of the liquid fuel decreases with decreasing nitromethane proportion,and the anti-settling ability of the first stage is weakened,which leads to the 60% (nitromethane) mass ratio group to precipitate more liquid than the 80% group.As the proportion of nitromethane is reduced to 40%,the reduction in the liquid surface tension results in an enhanced wetting effect.This increases the contact area between the liquid fuel and the aluminum powder and increases the liquid bridge force between the solid and liquid.The ability of the liquid component to adhere to the solid particles in the middle and lower layers is increased.The stratification of the 40% group fuel in stage two is more effectively suppressed.In addition,we found that the volume of precipitated liquid in the 40% group is less than that in the 80% group.Combined with the previous conjecture for the multiple liquid bridges resultant force,this is most likely because the 80% group does not reach the wetting critical condition,resulting in an inability to generate a larger liquid bridge force.
Fig.14 shows the density distribution of the solid-liquid mixed fuel in four states after oscillation for 4 h (120 rpm).In this experiment,the fuel was divided into three equal parts from top to bottom,and the difference in density of the three parts was evaluated based on their standard deviation (SD).The fuel density shows a transformation from top to bottom (as shown in Fig.15).The fuels in state 1 and state 2 are in a natural stacking state before oscillation,and there are gaps between the particles.During the oscillation process,the aluminum powder or the smaller powder particles that are not adhered to the liquid fuel are filled in by gravity action,sinking from the top,and the density of the three parts is,thus,different.The gaps for the fuel in state 3 are not completely filled with liquid components before the oscillation,and bubbles still exist.During the oscillation process,as the bubbles between the components are broken,the liquid components further penetrate into the solid particle gaps,and the overall density changes accordingly.The liquid in state 4 shows a density difference due to the appearance of two-stage stratification.Under the same liquid fuel ratio,the solid-liquid mixed fuel in state 3 presents the most uniform density distribution and the best physical stability.
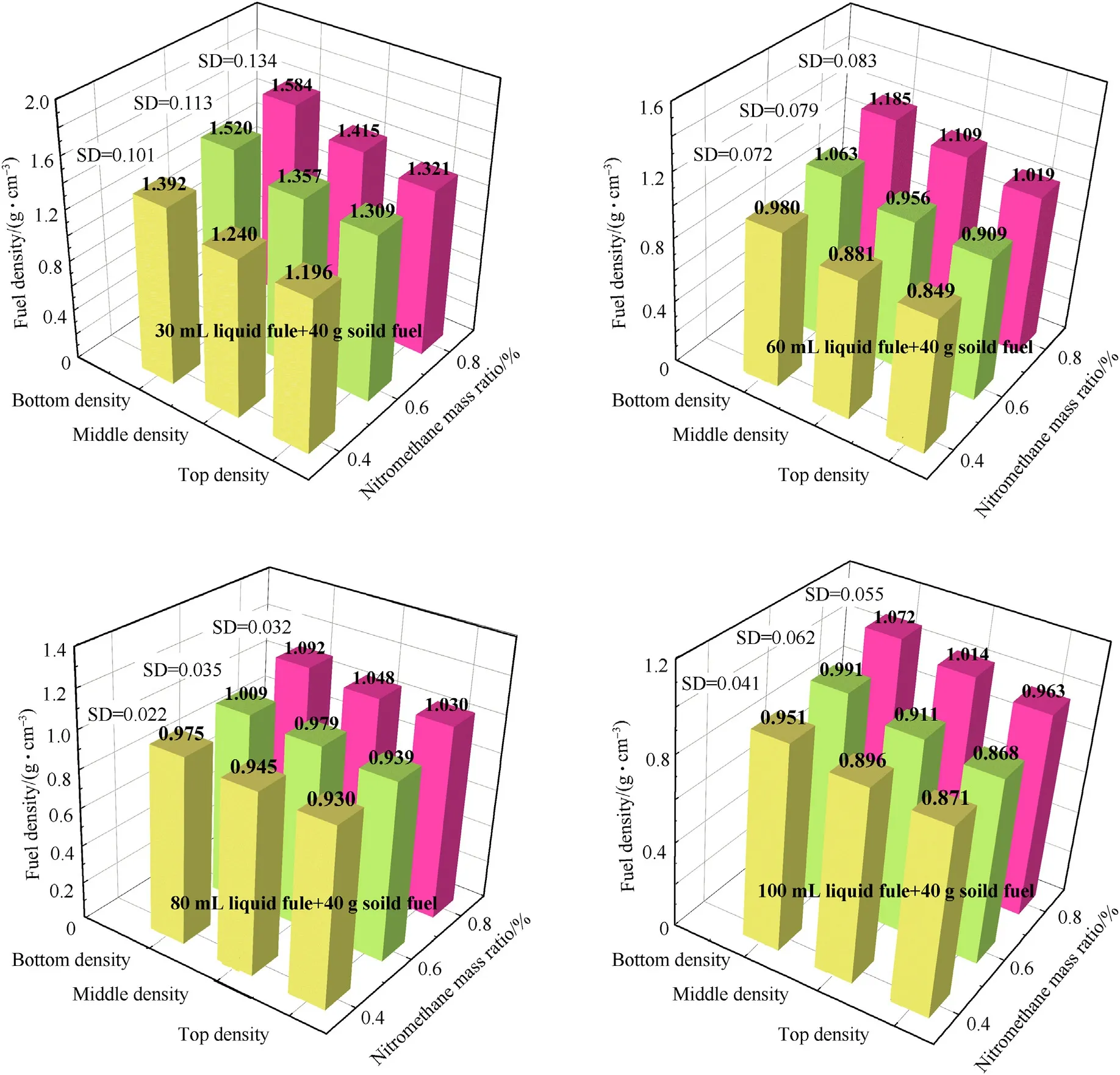
Fig.14.Density distribution of solid-liquid fuel after oscillation under different ratios(Ambient temperature 293 K;Amplitude 20 mm;Oscillation frequency 120 rpm(2 Hz);40 g flake aluminum powder).
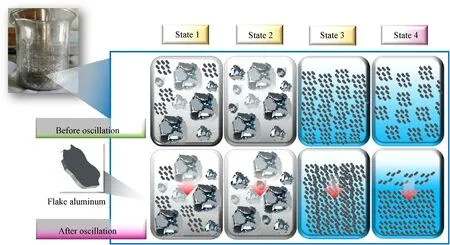
Fig.15.Schematic diagram of mixed fuel density distribution changes in four states during oscillation.
The change in the proportion of liquid components also affects the fuel density distribution after oscillation.The SD value for a mixed fuel composed of state 1 and state 2 decreases with decreasing nitromethane mass ratio.The addition of diethyl ether reduces the liquid surface energy,which enables the fuel to better wet the aluminum powder.More aluminum powder in state 1 fuel is adsorbed by the liquid fuel,and the amount of sinking aluminum powder is reduced.For the fuel in state 2,the diameters of the agglomerates in the stacking state are closer to each other due to the above reasons,and the gaps between the particles are reduced.
The SD value for state 3 and state 4 mixed fuel shows a different trend from the previous two states.With an increase in nitromethane,the SD value first increases and then decreases.This is because the effect of liquid viscosity on the physical stability of the fuel also needs to be considered under these two liquid saturation conditions.For state 3 fuels,the effect of the liquid bridge force on the physical stability is still dominant at this time due to the relatively low proportion of liquid.Therefore,the SD value for the 80% group is closer to 60%.The 40% group shows to a larger liquid bridge force due to the enhanced wetting of the solid particles by the liquid component,resulting in stronger physical stability.The state 4 fuel is a supersaturated fluid,and the influence of liquid viscosity on its physical stability cannot be ignored.The 80% group and 40% group effectively reduce the influence of external factors on the physical stability of the fuel in stage 1 and stage 2 via a strengthening of the liquid viscosity and the liquid bridge force between the components,respectively.Comparing the 40% and 80% groups,we also found that rather than simply increasing the viscosity,appropriately reducing the liquid fuel surface energy to improve the affinity with the metal can more effectively provide physical stability for the fuel.
4.Conclusions
Considering the interaction force between components,a specific device is designed to detect the relationship between the liquid bridge force,droplet volume and droplet ratio.Another device is assembled to explore the physical stability of fuel with different component ratios in an oscillating environment,and the results are evaluated in combination with the interaction force between the components.The main conclusions are given as follows:
(1) For the same proportion,the larger the volume of the liquid bridge,the greater the liquid bridge force generated;for the same liquid volume,the greater the surface tension,the greater the liquid bridge force.
(2) The presence of critical liquid saturation enables multiple liquid bridges to combine with the enclosed central space to form a larger single liquid bridge.This enables multiple liquid bridges to generate a resultant force that is greater than that of a single liquid bridge with the same total volume.
(3) The powdered fuel has high physical stability under the action of the liquid bridge force because the liquid components are tightly wrapped in solid component.With the improvement of the liquid component infiltration,the state 3 fuel shows a larger liquid bridge force and the best physical stability.
(4) The first stage of the supersaturated state 4 fuel stratification process mainly overcomes the liquid-liquid interaction,and the viscosity of the liquid components plays a major role in this process.In the second stage,the precipitation of the liquid components requires one to overcome the solid-liquid interaction force.
In this paper,the adhesion law of liquid bridge force is brought into the discussion of the physical stability for the first time.This provides a valuable reference for improving the physical stability of solid-liquid fuels.However,due to the irregular spatial structure,the mechanical system of the liquid bridge force in the actual solidliquid fuel may be more complicated than the experimental law.This needs to be refined and corrected in future experiments by microscopic imaging instruments or simulations.Optimizing the physical stability of fuel can effectively ensure product quality and avoid accidents.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China(Grant No.12102197).
杂志排行
Defence Technology的其它文章
- High-Velocity Projectile Impact Behaviour of Friction Stir Welded AA7075 Thick Plates
- Dynamic analysis of buried pipeline with and without barrier system subjected to underground detonation
- Adaptive fuze-warhead coordination method based on BP artificial neural network
- Investigation on the ballistic performance of the aluminum matrix composite armor with ceramic balls reinforcement under high velocity impact
- Cooperative multi-target hunting by unmanned surface vehicles based on multi-agent reinforcement learning
- AI-based small arms firing skill evaluation system in the military domain
