Solubility study and selective purification of HMX explosive in organic electrolyte solution of zinc acetate/diethylene glycol
2023-12-07JavadGhorbaniSajjadDamiriHamidRezaPouretedal
Javad Ghorbani,Sajjad Damiri,Hamid Reza Pouretedal
Faculty of Applied Sciences, Maleke-ashtar University of Technology, P. O. Box 83145/115, Iran
Keywords: Organic electrolyte Purification Solubility HMX RDX Diethylene glycol
ABSTRACT In this study,an organic electrolyte solution based on zinc acetate/diethylene glycol (ZA/DEG) is introduced for the selective purification of cyclotetramethylene tetranitramine(HMX)high explosive from its identical homologue cyclotrimethylene trinitramine (RDX).The dielectric constant of various organic solutions were investigated through Electrochemical Impedance Spectroscopy(EIS)in the range of 1.0 Hz-30 MHz.and some quantum-chemical descriptors of RDX and HMX dissolutions in the ZA cosolvent were analyzed using Density Functional Theory(DFT).The results show dielectric constant and solubility of RDX is higher than that of HMX,and by increasing of ZA concentration in DEG solvent,the values of dielectric constants were enhanced.Furthermore,the presence of ZA cosolvent on the solubility of two explosives was statistically investigated by Central Composite Design (CCD) of experiment,and some solubility parameters including activity coefficient,dissolving enthalpy,and mixing enthalpies were determined.The experimental results indicate that the weight ratio of RDX to HMX solubility in the proposed organic electrolyte changes up to 30 times,which provides a selective and sequential separation method to separate two materials with similar chemical properties with a separation efficiency>98% and HMX purity>99.8%.The X-Ray Diffraction (XRD) analysis,High-Performance Liquid Chromatography (HPLC),Laser-Induced Breakdown Spectroscopy (LIBS),and Fourier Transform Infrared Spectroscopy (FT-IR) approves the acceptable quality of the separated materials.The proposed method makes the efficient and safe purification of high-quality HMX for application in oil and gas well perforating gun charges,using a nonvolatile and inflammable organic electrolyte.
1.Introduction
1,3,5,7-tetranitro-1,3,5,7-tetra-azacyclo-octane or cyclotetramethylene-tetranitramine (HMX),usually known as Octogen or HMX (High Melting eXplosive),is one of the most powerful military explosives,which has many applications in manufacturing various ammunition and missile warhead due to its higher detonation rate and thermal stability than its homologue cyclotrimethylene trinitramine (RDX,Royal Demolition Explosive)[1-3].The Bachmann process,using hexamine nitration in ammonium nitrate,nitric acid,acetic anhydride,and acetic acid,is the common process for HMX production [4,5].Usually,both nitramine materials of RDX and HMX,with similar chemical properties,are simultaneously and competitively produced in their chemical processes.The two types of HMX used in military applications are type A (with a maximum of 7.0 wt% of RDX) and type B (with a maximum of 2.0 wt% of RDX) [6].Also,a purer type of HMX,with more than 99.8% purity (insensitive HMX),found many applications in perforating guns in oil and gas wells and insensitive munitions as since it has more stable thermal properties than impure HMX.The presence of negligible amounts of RDX and HMX impurities has reduced the crystalline density,and therefore,increased the shock sensitivity of HMX in explosive charges [7-9]and also decreased thermal resistance of HMX[10-12].So,the production of highly pure HMX through a cheap and safe method is of great importance in military and commercial;applications.Normally,RDX separation from HMX is rather hard since they have similar chemical properties and molecular structures.Up to now,various volatile solvents have been used for the evaluation of solubility,crystallization,and purification of HMX including acetonitrile and propylene carbonate [13],acetone and methyl ethyl ketone[11,14-16],ethyl acetate[17],dimethylformamide(DMF),dimethyl sulfoxide (DMSO),N-methyl-2-pyrrolidone (NMP),gammabutyrolactone [13,18,19].There is a negligible difference in the weight ratio of RDX/HMX solubility in common solvents such as acetone,methyl ethyl ketone,acetonitrile,DMF,DMSO,and gamma-butyrolactone that are used for purification and recrystallization of HMX.On the other hand,most of the solvents used for purification are not feasible in case of cost,availability,hygiene,flammability,and environmental issues.The LD50lethal dose index for methyl ethyl ketone,DMSO,and propylene carbonate solvents are 2737,14500,29100[20]mg/kg,flashpoints are 9,95,132°C[20],respectively.Also,the vapor pressures of these solvents are 12.71,0.081,0.003 kPa[20],respectively.
In the past decade,a significant reduction in energy consumption is reported by the application of various nonvolatile solvents such as ionic liquids(ILs)in separation processes aiming to increase efficiency and selectivity [21-25].ILs are ion pairs based on some organic cations with organic/mineral anions and melting points<100°C.These solvents have low vapor pressures,high thermal stability,adjustable viscosity,and suitable extraction capacity,solubility,and miscibility with many organic and mineral compounds.However,their production cost is generally very high and they do not have hygienic specifications due to their novel and diverse molecular structures [21,26,27].Today,using inflammable organic electrolytes is prevalent in different types of commercial supercapacitors and batteries[28-30].These electrolytes usually consist of conducting salts which are dissolved in organic solvents and are able to be operated at high voltage windows due to their hydrolysis resistance.Organic electrolytes containing Li-ions (e.g.,LiPF6,LiClO4) are usually used due to their smaller ion size and hence facilitate ion intercalation/deintercalation [31-33].The salt used should provide high ion mobility,and thus,high ionic conductivity,chemical inertness towards all cell components,and oxidative and reductive stability.In addition,it should completely dissociate in the solvent.
In this study,a highly selective,inflammable and safe method,based on organic electrolyte of zinc acetate/diethylene glycol (ZA/DEG),is introduced for solid-liquid separation of RDX from HMX explosives,and the solubility parameters of the mentioned explosive materials,such as activity coefficient,solution enthalpy,mixing enthalpy,and separation efficiency,are investigated.Also,the effects of two essential parameters like temperature and cosolvent content,on RDX and HMX solubility in the electrolyte,are optimized,modeled,ad statistically assessed using central cubic design(CCD) of experiments.Then,a safe and efficient leaching method was proposed for HMX purification or RDX separation and the product quality was evaluated through different analytical techniques.There are many limitations in selecting a salt to be used as a cosolvent in an organic electrolyte,like suitable solubility at different temperatures,no resedimentation at low temperatures,acceptable viscosity at the applied temperature,chemical compatibility of the salt with the solvent and explosive material,acceptable safety at high temperatures,low cost,and low toxicity of the salt.zinc acetate is an edible salt with numerous applications in the food,pharmaceutical,and sanitary industries [34].Furthermore,diethylene glycol (DEG) is a low-cost and inflammable solvent with low vapor pressure,good availability,and lower toxicity than the other solvents that have been used for the separation and purification of HMX so far.NFPA health rating and NFPA reactivity rating of diethylene glycol and zinc acetate are 1 and 0,respectively,which are much more suitable for HMX purification than common volatile solvents such as acetone and dimethyl ketone from the hygienists,flammability,and reactivity points of view.The LD50 index,flash point,and vapor pressure are 12565 mg/kg,138°C,and 0.001 kPa for diethylene glycol [35].
2.Experimental
2.1. Materials and instrumentation
The RDX and HMX explosives with >99.8% purity and HMX containing RDX impurity (Type A),in accordance with MIL-DTL-45444C military standard,were supplied by Iran Defense Industry,and diethylene glycol and acetate salts were supplied from Sigma Aldrich Company.All experiments were carried out in a double-walled glass crystallizer equipped with a temperature controller and a mechanical stirrer.Electrochemical impedance spectroscopy technique by a Princeton Applied Research PARSTAT 2273 apparatus equipped with a Frequency Response Analyzer was used to measure the capacity and dielectric constant of the solvents.A high-performance liquid chromatography (HPLC),Shimadzo model LC6A,with a 300×3.9 mm C18 column was used in the experiments.A mixture of methanol/water with a 40/60 composition constituted the mobile phase with 1.2 mL/min flow velocity,and a UV detector at 230 nm was used for the analysis of HMX and RDX mixtures.A scanning electron microscope (SEM)model Philips XI30 was utilized for the investigation of particles’morphologies.Also,polymorph of materials was determined using X-ray spectroscopy,model D8 ADVANCE,Bruker (Germany),and the X-ray spectrum was analyzed by X'Pert HighScore Plus software.The LIBSCAN100 system,manufactured by Photonic Applied,was used to record the LIBS spectrum;this system was equipped with Nd:YAG laser with 1064 nm wavelength,100 mJ output energy,tap width of 7 ± 2 ns,and pulse repetition frequency of 1-20 Hz.To increase the safety of Nd:YAG tests against sparks,HMX explosive material samples were mixed and diluted with KCl salt with 75 wt%;then they were pressed at pressure of 20.0 bar in a hydraulic press in the form of tablets with 0.8 cm diameter to be used in the experiments.
Caution: All explosives,such as HMX and RDX,are dangerous and must be carefully handled and used following approved safety procedures either by or under the direction of experienced persons in accordance with all applicable laws and regulations.
2.2. Measurement of RDX and HMX solubility
Similar to the previous reports [17],the HMX and RDX solubilities were isothermally measured at different weight ratios of ZA/DEG solution and the temperature range of 30-90°C.For this purpose,a little amount of HMX or RDX was added to 100 g ZA/DEG in a double-walled 200.0 mL glass vessel,equipped with a temperature control system,thermometer,and a magnetic stirrer at a constant temperature and in a 30 min time interval.Then,the mixture was left so that the materials were dissolved at a relative equilibrium.The required weight of the material to reach the supersaturation was recorded with 0.1 mg accuracy,and the solubility of HMX and RDX was calculated based on dissolved amount of each substance per 100.0 g of organic electrolyte.
2.3. Measurement of dielectric constant
To carry out this experiment,a 20-mL test tube was filled with 10.0 mL of the solvent.An electrical conductivity electrode,a pair of platinum plate having 1.0 cm2surface area and 1.20 cm distance from each other,was placed in the solution,thereafter the electrochemical impedance spectroscopy (EIS) of the sample was recorded at 1.0 Hz-30.0 MHz,and the dielectric constant (ε′) and dielectric loss (tan δ) were calculated from impedance data using the below-mentioned expression [36]:
where,A=Cross-section area of the electrodes,t=Thickness of the pallet,ε0=Permittivity of free space,Z'=real part of impedance,andZ”=imaginary part of the impedance.
2.4. Experimental design for determination of solubility
In this study,MINITAB 19 software and the central composite design (CCD) of experiment method were used for statistical modeling RDX and HMX solubility data in organic electrolyte solutions.The solubility of these two materials in 100.0 g of solution was measured by the two factors of solution temperature(X1)in the range of 30.0 ≤X1≤90.0°C and zinc acetate cosolvent wt%(X2)in the range of 5.0 ≤X2≤20.0 wt%.According to Table 1,fourteen experiments were designed by the CCD method to investigate the effect of temperature and cosolvent wt% on each explosive material solubility;the responses of RDX solubility (Y1,g/100 g) and HMX solubility (Y2,g/100 g) in ZA/DEG organic solution were recorded.Then,the responses were statistically evaluated and modeled by multivariate linear regression.
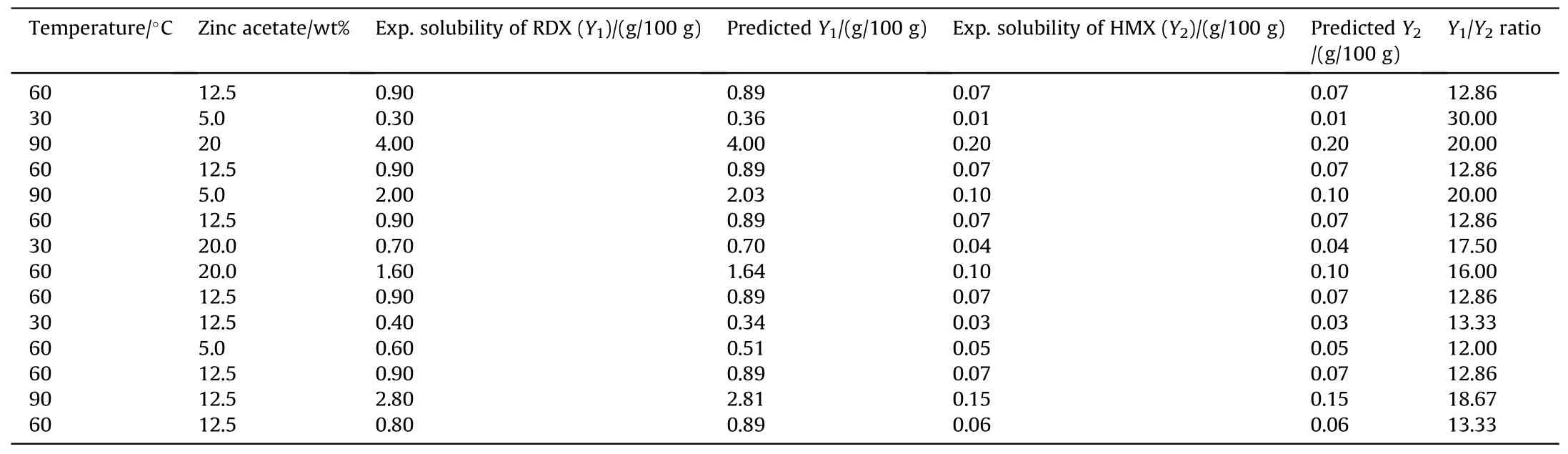
Table 1 Central composite design of experiments for the determination of RDX and HMX solubility in 100.0 g ZA/DEG solution.
2.5. Solid-liquid extraction of RDX from HMX in the organic electrolyte solution
For the solid-liquid extraction or leaching of RDX impurity from solid HMX (containing 20.0 wt% RDX),20.0 g of the prepared mixture by Bechmann process (as the feed) was added to 200.0 g ZA/DEG organic solution containing 20.0 wt% zinc acetate,as the leaching solvent.The mixture was stirred for 60.0 min with a mechanical stirrer at 90.0°C.Then,the hot suspension containing HMX particles(and RDX solution)was filtered using a vacuum filter with a Whatman cellulose filter papers with 20 μm pores at the temperature range of 70-90°C.The obtained solid material (Raffinate) was pure HMX was washed with pure water;then,it was dried in a 50°C oven and weighed.The RDX content of HMX was analyzed by HPLC-UV method and the separation efficiency was calculated.The filtered solution(extract),contain RDX impurity on organic electrolyte was cooled down to near 5.0°C so the dissolved material in the organic solution was precipitated;the obtained white solid material (RDX) was washed with water and dried.The organic electrolyte was sequentially used for HMX purification to evaluate its performance in subsequent purification processes.
2.6. Recovery of diethylene glycol from organic electrolyte
The efficiency of organic electrolyte decreases after repeated use.Here,a vacuum distillation at -0.6 bar pressure was used for evaporation and separation of DEG from the salts.For this purpose,the extract from which the RDX has been removed was indirectly heated to 180°C with silicon oil to separate its pure DEG content.The rest of the components,including the remained zinc acetate,which is a cheap material,was burnt and the zinc oxide was collected.
3.Results and discussion
3.1. Selection of organic electrolyte components
Using nonvolatile and inflammable solvents for improving safety and sanitary indexes is valuable in the purification processes of explosive material.Here,the inflammable solvents of ethylene glycol (MEG),diethylene glycol,triethylene glycol (TEG),and zinc acetate hygiene salt,as a cosolvent,were investigated in an organic electrolyte.Metal acetate salts have good solubility in the mentioned solvents and their chemical compatibility with explosive material is also acceptable.The vapor pressure of MEG,DEG,and TEG is 0.0107,0.0013,and 0.0013 kPa,and,their flash points were reported 119.0,143.0,and 177.0°C with lethal doses of 4700,1265,and 17000 mg/kg,respectively[35].
Firstly,the difference in RDX and HMX solubilities in MEG,DEG,and TEG were investigated.The solubility of RDX 90.0°C is equal to 0.45,1.20,0.85 g/100 g solvent,respectively.Also,the solubility of HMX in these three solvents is less than 0.05 g/100 g solvent.Therefore,DEG selectivity for the dissolution of RDX and HMX explosives is superior to MEG and TEG.However,the total dissolution of the explosive materials,especially RDX,is rather low.Experimental studies indicate that using metal acetate cosolvent in DEG-based electrolytes can improve RDX dissolution.
The aim of this work is to separate RDX from HMX by solidliquid separation or leaching method.To determine a suitable salt for RDX and HMX dissolution in a diethylene-based organic electrolyte,the solubility of both materials was measured at 90.0°C in a solvent containing 10.0 wt% of metal acetate salts including potassium acetate,magnesium acetate,zinc acetate,nickel acetate,calcium acetate,and lead acetate in DEG.Results revealed that RDX is dissolved in the range of 1.1-2.6 g/100 g and HMX in the range of 0.05-0.12 g/100 g,with sequence of magnesium acetate >zinc acetate>nickel acetate>potassium acetate>lead acetate salts.A near twice increase was observed in RDX solubility in DEG by using zinc acetate in DEG to make an organic electrolyte.
According to Fig.1(a),the measurement of the dielectric constant by EIS technique (see subsection 2.3),for various metal acetate cosolvent,approved the above-mentioned changing trends.When the solution dielectric constant increases,the solubility of RDX and HMX explosives increases as well.Here,zinc acetate cosolvent was selected for the preparation of the organic electrolyte due to its suitable sanitary properties,chemical compatibility with explosives,and good solubility in DEG.Zinc acetate is an edible salt with low toxicity that has many applications in the food,pharmaceutical,and sanitary industries.Additional investigations according to Fig.1(b) show that with increasing zinc acetate concentration in DEG,the dielectric constant of solution also increases.Dielectric constant is high in low frequency region,but when the frequency increases the dielectric constant decreases because of inability of electric dipole to comply with variation of applied AC electric field[36].Furthermore,as shown in Fig.2,the study of the solutions’conductivities indicated that an enhanced concentration of zinc acetate salt increases the solution conductivity due to the enhanced ion concentration in the solution.On the other hand,after the addition of different RDX amounts,the electric conductivity was decreased;this indicates that the interactions between zinc acetate ions and RDX and their enhanced solubilities in the organic electrolyte decrease the ion movements [37].
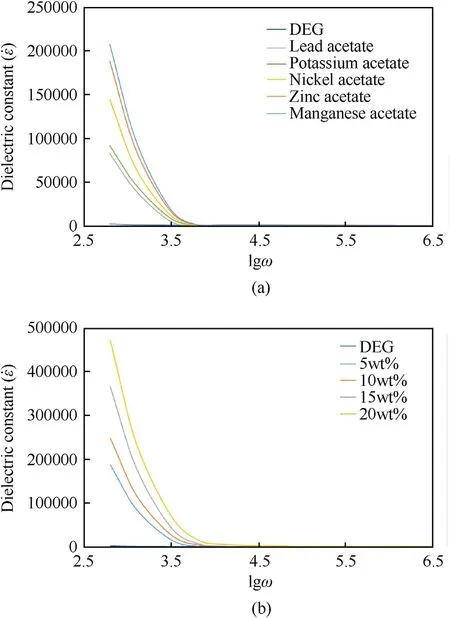
Fig.1.Dielectric constant of (a) Some DEG solutions containing various metal acetate salts (10.0 wt%);(b) DEG with various concentration of zinc acetate cosolvent.
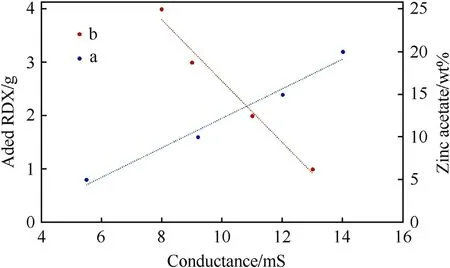
Fig.2.Electric conductivity of ZA/DEG solution at different concentrations of zinc acetate at 90°C and ZA/DEG solution containing 20.0 wt% zinc acetate by the addition of various amounts of RDX at 90°C.
Here,some quantum-chemical descriptors including the dielectric constant (έ),polarizability (α) and Gibbs free energy(ΔGsolv)properties of two explosive compounds(HMX and RDX)in different organic electrolyte solvents were studied via density functional theory(DFT)using LC-BLYP density functional and AUGcc-pVDZ basis sets[38].The results are presented in Table 2.As it is observed,the dielectric constant of RDX is higher than that of HMX.Also,by increasing of zinc acetate concentration in DEG solvent,the values of dielectric constant and polarizability of two substances in the solution increases and their differences were enhanced,confirming the solubility trends of RDX and HMX in the presence of cosolvent.
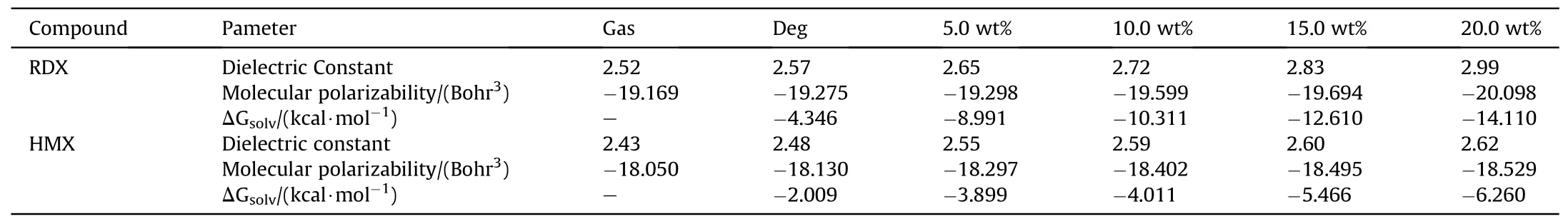
Table 2 Dielectric constant,molecular polarizabilities and Gibbs free energy in gas phase for dissolving of RDX and HMX explosives in different ZA/DEG concentrations calculated at LCBLYP/AUG-cc-pVDZ.
Calculations by the density functional theory(DFT)and ab-initio approaches indicated that HMX dielectric constant is less than RDX[39];thus,the RDX solubility is higher in more polar solutions.
Generally,RDX solubility in rather polar solvents like ethyl acetone and methyl ethyl ketone [11,14-16],ethyl acetate [17]or others is higher than HMX.Compounds with higher dielectric constants have higher dipole moments,too.When the polarity and dipole moment of a solvent increases,the species with higher dipole moments are more affected and will have greater interactions with the components of the organic electrolyte;this will enhance the Gibbs free energy of dissolution and increase the solubility [40-42].
Generally,increase of cosolvent concentration in DEG causes an increase in the ionic strength and dielectric constant of the solution.It seems that it also increases the polarizability of RDX and HMX organic molecules due to the increased polarity of the solution;therefore,the solubility of RDX and HMX increases [43].Diethylene glycol contains two hydroxyl functional groups and one ether oxygen;1.0 mol of DEG can interact with at least 1.0 mol of zinc acetate.FT-IR spectroscopy,presented in Fig.3,shows the O-H bond peak at 3330 cm-1wave number.After the preparation of the ZA/DEG organic electrolyte containing 20.0 wt% zinc acetate,this peak shifts toward higher frequencies (3430.0 cm-1).This phenomenon can be due to the presence of hydrogen bonds between zinc ions and diethylene glycol or its ability in weakening intermolecular hydrogen bonds in diethylene glycol [44,45].
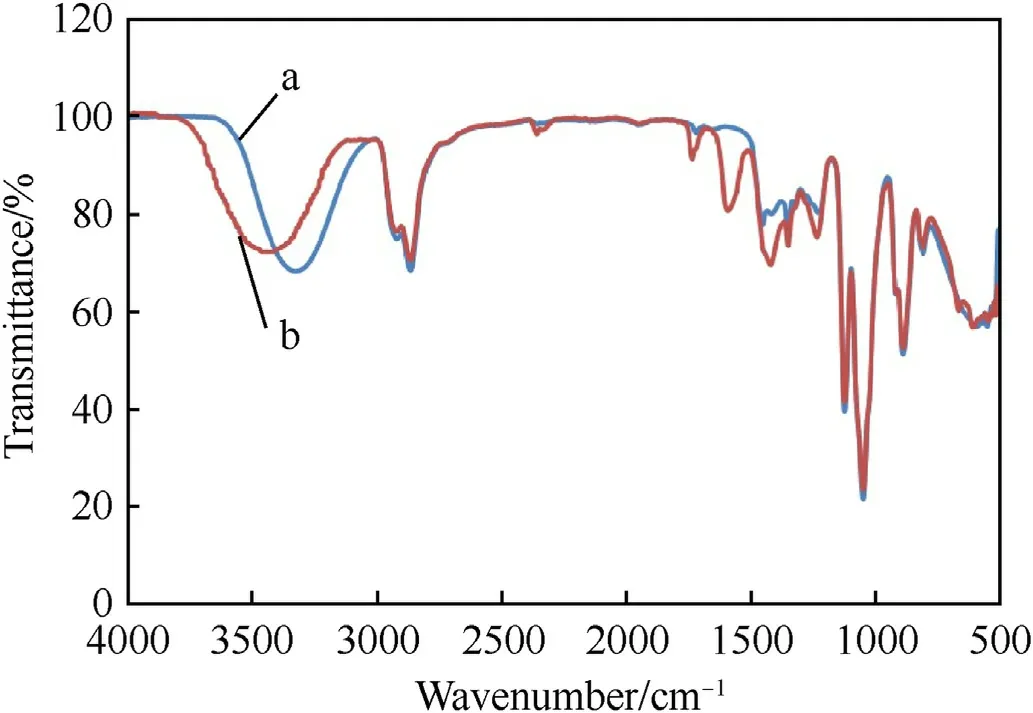
Fig.3.IR spectrums of DEG and ZA/DEG electrolyte solution containing 20.0 wt% zinc acetate.
3.2. Calculation of activity coefficient, enthalpy of solution, and enthalpy of mixing for RDX and HMX solubility
According to Table 1,the difference in the solubility of HMX and RDX explosives in ZA/DEG electrolyte is rather considerable at 30-90°C,and RDX solubility in the electrolyte solution can reach more than 30.0 times greater than HMX.HMX solubility is in the range of 0.01-0.3 g HMX/100 g ZA/DEG solution.The RDX impurity can be separated from HMX particles by the adjustment of experimental conditions such as amount of zinc acetate in DEG and the process temperature.It can be observed that increasing of temperature and zinc acetate concentration in DEG results in an improvement in HMX and RDX solubilities.Similar to the previous reports [14,17],solubility data can be used to estimate the activity coefficient at equilibrium conditions.The equation of state for solid-liquid equilibrium calculations is presented in Eq.(2).
In whichx1is HMX molar ratio in the solvent,γ1is HMX activity coefficient,ΔHfus,1is HMX fusion enthalpy,Tm,1is the melting point,Tis the absolute temperature,andRis the gas constant.HMX melting point is close to 558.15 K and its fusion enthalpy was 70154.38 J/mol;RDX melting point was 478.65 K and its fusion enthalpy was 35647.68 J/mol [30].
According to Eq.(2) and the results presented in Table 2,the activity coefficient γ1can be calculated with the experimental data of solubility,and melting temperature and enthalpy of HMX and RDX.Furthermore,Eq.(2) can be transformed into Eq.(3) to calculate the dissolution enthalpy of explosive materials crystals[14,17].
wherex1is the solute mol fraction and ΔHsol,1is its dissolution enthalpy.In ideal systems,ΔHfus,1is equal to ΔHsol,1,which is obtained by setting the activity coefficient=1.Also,for non-ideal systems we have ΔHsol,1=ΔHfus,1+ΔHmix,1in which ΔHmix,1is the mixing enthalpy and shows the solvent-solute interaction;while,the fusion enthalpy is not dependent on the solvent.According to Eq.(3),dissolution enthalpy can be calculated from the slope of ln(x1) as a function of the T-1curve.
As seen in Table 2,when temperature raises,RDX solubility coefficient experiences more increase than HMX.Also,zinc acetate wt% enhancement from 0.0 to 20.0 wt% increases the solubilities of RDX and HMX;this shows the effect of increasing the ionic strength of an electrolyte solution and the role of zinc acetate as a cosolvent on increasing the solubilities of mentioned explosives.Here,the solubility parameters such as activity coefficient (γ),mole fraction(x),dissolution enthalpy(ΔHsol,1),and mixing enthalpy (ΔHmix,1)were calculated using the previous correlations and equations,as reported in Table 3.The more negative values of HMX and RDX mixing enthalpy,by zinc acetate cosolvent concentration raise in the organic electrolyte,shows that the system moves toward the miscibility easier at higher concentrations of the solvent and HMX and the solvent have more interactions [13,15].
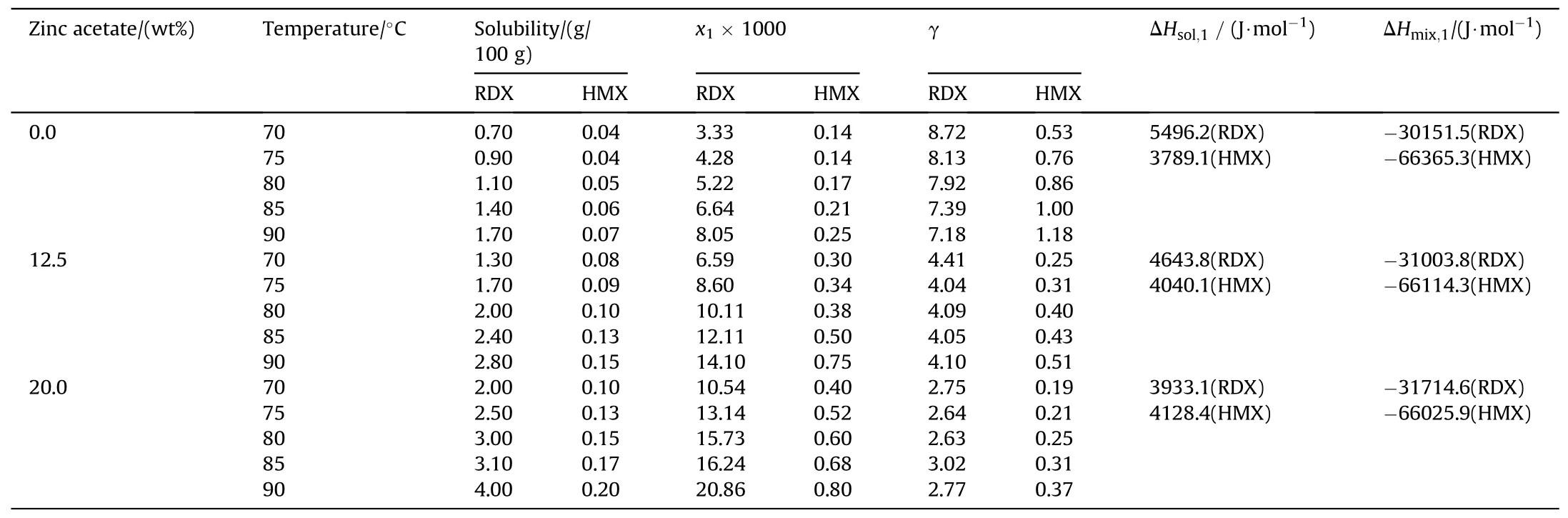
Table 3 Mole fraction,activity coefficient,dissolution enthalpy,and mixing enthalpy of RDX and HMX in ZA/DEG solution.
3.3. Modeling RDX and HMX solubility in the organic electrolyte using the CCD design method
According to the design of experiments in subsection 2.4,the solubility of RDX (Y1) and HMX (Y2) was measured against the solution temperature in the range of 30.0 ≤X1≤90.0°C and zinc acetate wt% in the range of 30.0 ≤X1≤90.0°C at 100 g ZA/DEG solution and was modeled and statistically evaluated using multiple linear regression method in Minitab software.The approved mathematical equations for RDX and HMX solubilities were determined as below.
whereX1is the temperature in°C,X2is zinc acetate wt% in the solution,andY1andY2are the RDX and HMX solubility in g per 100 g ZA/DEG solution.
To evaluate the models,the obtained statistical parameters show a high correlation between the experimental and predicted data.ForY1response,R2=99.80% and adjustedR2=99.62%,and forY2response,R2=99.29% and adjustedR2=98.68%.Also,in the residue curves (not shown) the distribution of the residues was normal for the responses and they have a random distribution.The analysis of variance (ANOVA) was used for the determination of statistical parameters of the model in the confidence limit of 95% according to Table 3.The values of theFparameter were 574.34 and 163.25 forY1andY2,respectively and thep-value was close to zero,and lack-of-fitpvalues approved the precise modeling.
The application of the presented solubility models can help predict the optimum conditions for RDX separation from HMX.The experimental conditions that maximize the RDX solubility and minimize the HMX solubility are appropriate for the separation process.Similar to the digestion or leaching method presented in previous reports [14,17],complete dissolution of HMX is not needed.Fig.4 shows the response surface plot of temperature and ZA salt wt% in DEG effects on RDX and HMX solubilities(Y1andY2).It is evident that increasing the temperature and salt wt% increases RDX and HMX solubilities.However,HMX solubility is negligible even at 90°C.
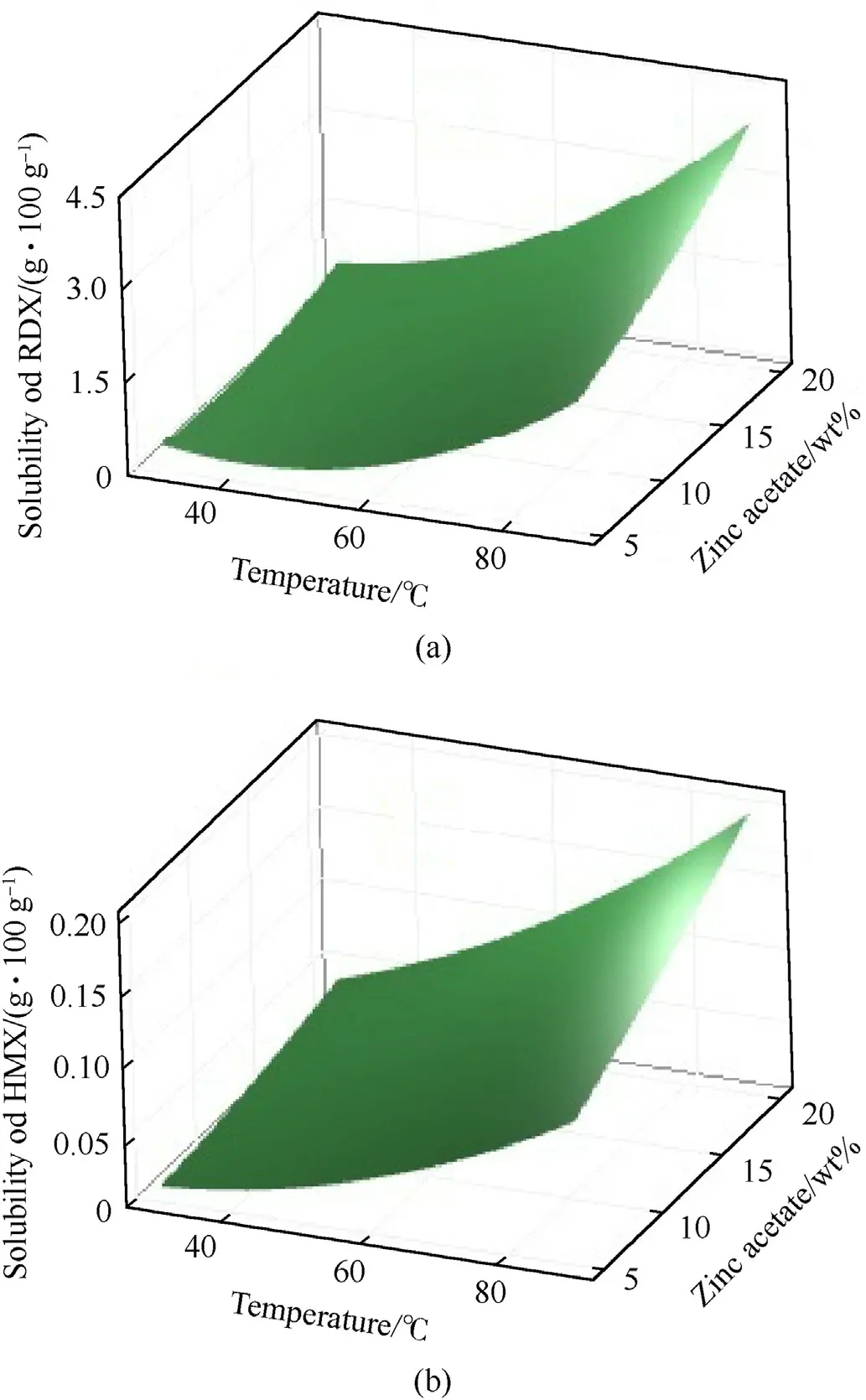
Fig.4.Response surface plot of (a) RDX solubilities and (b) HMX solubilities at different temperatures and zinc acetate concentrations.
The quantitative results presented in Table 1 indicate that the RDX to HMX solubility ratio changes from 12 to 30.Considering this fact,an efficient and safe selective separation method can be presented for the separation of HMX and RDX mixtures using inflammable ZA/DEG solution.The diethylene glycol flash point is 147°C,which is safer than other common solvents such as acetone,methyl ethyl ketone,and ethyl acetate[20].
In solid-liquid extraction for HMX purification,the RDX solubility should be maximized and that of HMX should be minimized.Also,the total material solubility must have an acceptable value to increase the purification weight capacity.Optimization of responses with Minitab software indicated that the maximum response for RDX (4.00 g/100 g) and the minimum response for HMX(0.20 g/100 g)are obtained at the experimental conditions of 90°C temperature and 20.0 wt% zinc acetate;the RDX to HMX solubility ratio is near 20.0 times in these conditions,which shows that these conditions are suitable for this separation process.It should be mentioned that Table 1 also shows the solubility ratio of 30 for RDX to HMX,but the total solubility of the two materials is rather low.Considering that the solubility experiment was done for the two separate RDX and HMX crystals,diethylene glycol can be used for the separation of the two materials.However,in real conditions,RDX and HMX crystals are produced simultaneously in the Bechmann process [46]and form semi cocrystals.Therefore,considering the purpose of this study for HMX purification,in the leaching process the crystals should be broken to separate RDX from HMX particles.This is better achieved at higher temperatures where the total solubilities are higher since extraction kinetics and physical crystals’ breakage is more probable.
3.4. HMX purification
Based on the presented method in subsection 2.5 for the separation of 20.0 wt% RDX from HMX crystals using solid-liquid separation or leaching process at 90°C,impure HMX crystals were dissolved in an organic electrolyte and the solid materials,pure HMX crystals,were filtered at 70.0-90.0°C.The materials should be hot-filtered to prevent RDX recrystallization.The weight of obtained solid materials at 90°C and 70°C filtration was 15.8 g (with 98.0% separation efficiency) and 17.85 g (with about 80.0% efficiency).According to Fig.5,the component analysis with HPLC indicated that HMX purity is 99.8% and 91.0%,respectively,in other words,HMX containing 20.0 wt% RDX was purified efficiently.It can be seen that the reduction in filtration temperature reduces the purity of recovered HMX.Comparison of the experimental results with the predicted ones by the models and Eqs.(4)and(5)indicates that the accuracy errors of the predicted models are 1.97% and 9.0% at 90°C and 70°C,respectively.
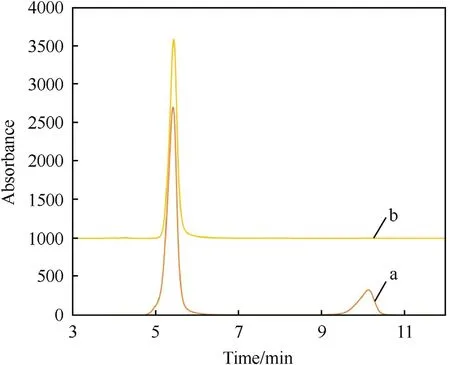
Fig.5.HPLC chromatograms for RDX-HMX mixture (20 wt%/80 wt%) and purified HMX in organic electrolyte of ZA/DEG containing 20.0 wt% zinc acetate at 90.0°C.
It should be mentioned that the filtered solutions used in the above experiments were cooled down to about 10°C for 1.0 h and the precipitated white solid was filtered,washed with water,and dried.The obtained solid weight at 90°C and 70°C was 4.15 g(with 96.0% RDX purity)and 2.05 g(with 95.0% RDX purity).This type of RDX complies well with RDX type B,according to MIL-DTL-398D military standards.The filtered solution can be used in the leaching process for further sequential extractions.The organic electrolyte was used 5 more times for the purification of HMX containing 20.0 wt% RDX.However,the separation efficiency decreased slightly as 98.5%,98.3%,98.0%,97.5% and 97.0%,respectively.
In the development of optimal leaching separation strategies,it should also be noted to total solubility of impurities (RDX in this study),selectivity,kinetics,particle size of crystals and required purification capacity(kg of pure solid/kg solvent)of the purification process.Here,solubility ratio of RDX/HMX(and solubility data)was determined in semi-equilibrium state.Although the solubility difference at ambient temperature is more than 90.0°C,the separation kinetic sand capacity is more favorable at high temperatures.Fig.6 presents the SEM images of impure HMX and purified solid HMX.As seen in this figure,the digestion and purification process causes breakage and size reduction of the particles leading to enhancement in purification kinetics.
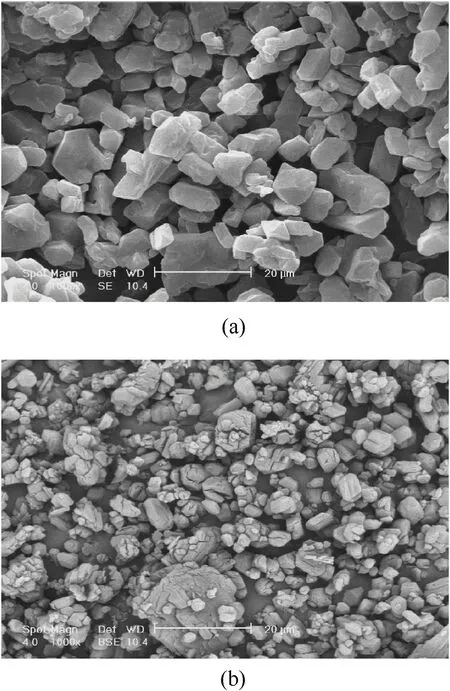
Fig.6.SEM images of (a) impure HMX and (b) purified HMX after digestion in the organic electrolyte ZA/DEG containing 20.0 wt% zinc acetate for 60.0 min at 90°C.
The analysis of X-ray spectrums of the samples (see Fig.S1 in supporting information) with X'Pert HighScore Plus indicated the highly efficient HMX purification with desired β-type polymorph,without the presence of RDX impurity diffraction peaks.Also,the FT-IR spectrums (Fig.S2 in supporting information) revealed the absence of remained impurities such as diethylene glycol and zinc acetate in the purified sample.The peaks that belong to the alcoholic functional group (-OH) of diethylene glycol,and the acetate group(-COO) in zinc acetate are not observed at 1600-1700 cm-1wave numbers.
Also,the sensitive LIBS technique was used to track the trace amounts of zinc acetate in the purified HMX product.To follow the safety procedures and desensitization of explosive material,75 wt% of the tablets were prepared from KCl and the rest were the explosive material.Fig.7 shows the spectrums of the two samples of HMX containing zinc acetate and the purified product.As the LIBS spectrums indicate,zinc component peaks are not observed at 202.55 and 206.2 nm wavelengths in the product (purified HMX),while the HMX sample with less than 1.0 wt% zinc acetate has these two peaks.Therefore,it seems that washing the product with water had enough efficiency to remove the remaining and the absorbed salts from the pure HMX surface [46].

Fig.7.LIBS spectrum for pure HMX and HMX containing zinc acetate/KCl (25 wt%/75 wt%).
4.Conclusions
In this study,the inflammable and safe zinc acetate/diethylene glycol organic electrolyte was used for purification of HMX explosive material.The mixing of DEG with zinc acetate cosolvent causes the wavenumber frequency displacement from 3330 to 3430 cm-1in the O-H bond peak in diethylene glycol,which is due to the formation of hydrogen bond between zinc acetate and DEG.As temperature increases and after the addition of the cosolvent,the RDX and HMX mixing enthalpy with the electrolytes become more negative and the mixture approaches miscibility,so the solubility of these two explosive materials increases in the electrolyte solution.Furthermore,the conductivity and dielectric constant of the electrolyte solution increases after the addition of zinc acetate;it seems that it increases the polarizability of RDX and HMX organic molecules by increasing the solvent polarity and consequently,improves the solubility of the species.Separation results indicate that the weight ratio of RDX solubility in the proposed electrolyte is up to 30 times higher than the HMX solubility;this provides the possibility of selective and sequential separation via temperature control of the solid-liquid process,to prepare HMX with more than 99.8% purity.The XRD and HPLC study approved the good quality of the separated material.Also,the final product assessment with LIBS and FT-IR showed the absence of zinc acetate and ethylene glycol impurities.
Novelty statement
The novelty of this research can be summarized as follows:
· Development of a green and inflammable organic electrolyte solution based on zinc acetate/diethylene glycol.
· A selective purification of HMX high explosive by solid-liquid separation method.
· Preparation of high pure HMX for application in explosive charges of oil and gas perforating guns.
· Dielectric constant of organic electrolyte was estimated using electrochemical impedance spectroscopy.
· Solubility parameters of HMX and RDX explosives in ZA/DEG solution were modeled and calculated.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful for the financial support of this work by Malek-ashtar University of Technology(I.R.Iran).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2023.02.005.
杂志排行
Defence Technology的其它文章
- Measurement of alumina film induced ablation of internal insulator in solid rocket environment
- AI-based small arms firing skill evaluation system in the military domain
- High-Velocity Projectile Impact Behaviour of Friction Stir Welded AA7075 Thick Plates
- Investigation on the ballistic performance of the aluminum matrix composite armor with ceramic balls reinforcement under high velocity impact
- Adaptive fuze-warhead coordination method based on BP artificial neural network
- Influence of liquid bridge force on physical stability during fuel storage and transportation
