miR-155通过调控PKG1影响滋养细胞生物学功能并参与子痫前期的机制探讨
2023-11-08岳巾晶曾莹郭晓珮董越姬若楠彭瑞罗晓华
岳巾晶 曾莹 郭晓珮 董越 姬若楠 彭瑞 罗晓华
摘要:目的 探究miR-155和環磷酸鸟苷依赖性蛋白激酶1(PKG1)在子痫前期患者胎盘组织中的表达及miR-155在核因子(NF)-κB的介导下通过抑制PKG1对滋养细胞HTR-8/SVneo功能的影响。方法 收集剖宫产分娩的正常产妇(NPE组)以及子痫前期产妇(PE组)的胎盘各20个,体外培养滋养细胞HTR-8/SVneo,分为NC组、mimics组、inhibitor组、siRNA NC组、PKG1 siRNA组、siRNA+inhibitor组;用NF-κB抑制剂PDTC处理细胞,分为Control组、PDTC组、PDTC+NC组、PDTC+mimics组。采用qPCR检测胎盘和滋养细胞HTR-8/SVneo中miR-155和PKG1 mRNA的表达;Western blot检测PKG1蛋白的表达;分别采用CCK-8法、Transwell法、流式细胞术检测细胞的增殖、迁移、凋亡能力。结果 PE组胎盘组织中miR-155的表达升高,而PKG1的表达降低(P<0.05)。体外实验表明,与NC组相比,miR-155 mimics组中PKG1的表达降低,滋养细胞迁移、增殖能力减弱,凋亡能力增强,miR-155 inhibitor组中PKG1表达则升高,滋养细胞迁移、增殖能力增强,凋亡能力减弱(P<0.05);与Control组相比,PDTC组miR-155的表达降低,滋养细胞迁移、增殖能力增强,凋亡能力减弱(P<0.05)。结论 miR-155在子痫前期中表达上调,并且可在NF-κB的介导下通过下调PKG1影响滋养细胞的增殖、迁移和凋亡。
关键词:先兆子痫;细胞运动;细胞增殖;细胞凋亡;NF-κB;miR-155;PKG1;滋养细胞
中图分类号:R714.244文献标志码:ADOI:10.11958/20221869
MiR-155 affects the biological functions of trophoblastic cells through regulating
cGMP-dependent kinase 1 and is involved in the mechanism of preeclampsia
YUE JinjingZENG Ying GUO Xiaopei DONG YueJI Ruonan PENG Rui LUO Xiaohua
1 Department of Gynaecology and Obstetrics, 2 Department of Scientific Research Office, the Third Affiliated Hospital of Zhengzhou University, Zhengzhou 450000, China
Corresponding Author E-mail: Luoxiaohua620@163.com
Abstract: Objective To investigate the expression levels of miR-155 and cGMP-dependent protein kinase 1 (PKG1) in placental tissue of patients with preeclampsia, and the effect of miR-155 on the function of trophoblasts HTR-8/SVneo by inhibiting PKG1 under the mediation of nucleus factor kappa B (NF-κB). Methods Twenty placentas were collected from normal pregnant women and pre-eclampsia pregnant women who delivered by cesarean section. In vitro trophoblasts HTR-8/SVneo were cultured and divided into the NC group, the mimics group, the inhibitor group, the siRNA NC group, the PKG1 siRNA group and the siRNA+inhibitor group. Cells were treated with NF-κB inhibitor PDTC and divided into the control group, the PDTC group, the PDTC+NC group and the PDTC+mimics group. Real-time quantitative polymerase chain reaction (qPCR) was performed to detect the expression of miR-155 and PKG1 mRNA in placentas and HTR-8/SVneo cells. Western blot assay was performed to measure the level of PKG1 protein. The cell proliferation, migration and apoptosis were assessed by CCK-8 assay, Transwell assay and flow cytometry. Results The expression of miR-155 was significantly upregulated in placental tissue of the PE group, while the expression of PKG1 decreased significantly (P<0.05). The vitro experiments showed that compared with the NC group, the expression of PKG1 was significantly reduced in the miR-155 mimics group (P<0.05). The migration and proliferation ability of trophoblast was significantly weakened, the apoptotic ability was significantly enhanced (P<0.05). The expression of PKG1 was significantly increased in the miR-155 inhibitor group, the migration and proliferation ability of trophoblast was significantly enhanced, and the apoptotic ability was significantly weakened. Compared with the control group, the expression of miR-155 was significantly reduced in the PDTC group, the migration and proliferation ability of trophoblast was significantly enhanced, and the apoptotic ability was significantly weakened (P<0.05). Conclusion Results indicate that the expression of miR-155 is upregulated in preeclampsia, and can affect proliferation, migration and apoptosis of trophoblast cells by down-regulating PKG1 mediated by NF-κB.
Key words: pre-eclampsia; cell movement; cell proliferation; apoptosis; NF-kappa B; miR-155; PKG1; trophoblast
子痫前期(preeclampsia,PE)是妊娠期特有的疾病,其特征是妊娠20周后出现蛋白尿和高血压。PE的发病率为2%~8%[1],是导致孕产妇及新生儿死亡的主要原因之一,占全球孕产妇死亡的10%~15%[2]。目前普遍认为胎盘滋养层细胞侵袭异常、血管内皮功能受损与PE的发病密切有关[3],但具体发病机制仍不清楚,缺少有效的治疗措施。微小RNA(microRNA,miR)是一类小分子非编码RNA,长度约22个核苷酸[4]。研究表明,miRNA的表达失调可能与PE的发生有关[5]。本团队前期研究发现,miR-155在子痫前期患者胎盘组织中高表达,并且可抑制滋养细胞的增殖、迁移、侵袭,促进其凋亡和炎性反应[6]。已有研究表明,miR-155可通过与一氧化氮(NO)/环磷酸鸟苷(cGMP)通路的主要下游分子环磷酸鸟苷依赖性蛋白激酶1(cGMP-dependent protein kinase 1,PKG1)的3′-非翻译区(UTR)互补结合,从而负向调节血管平滑肌细胞(VSMC)中PKG1的表达,进而导致VSMC表型转换和功能障碍[7]。然而,该通路在PE和滋养细胞中的作用尚未被证实,笔者推测miR-155可能通过调节PKG1参与PE的进展。本研究旨在探究miR-155/PKG1轴对PE的影响以及对滋养细胞生物学功能的调节机制。
1 材料与方法
1.1 材料 选择2020年6月—2021年12月于郑州大学第三附属医院剖宫产分娩的PE产妇20例作为PE组,选择同期剖宫产分娩的正常产妇20例作为NPE组。PE的诊断标准参考《妊娠期高血压疾病诊治指南(2020)》[8]。2组产妇均排除双胎及多胎妊娠、慢性肝肾疾病、妊娠期糖尿病、传染性或代谢性疾病等。本研究经本院伦理委员会批准(伦理号:2022-238-01),所有研究对象均签署知情同意书。
1.2 主要试剂与仪器 人绒毛膜滋养层细胞HTR8/SVneo购自上海中国科学院细胞库;Trizol试剂购自日本TAKARA公司;Lipofectaimine 2000、Transwell小室购自美国Thermo Fisher Scientific公司;miR-155 mimic、inhibitor、NC、PKG1 siRNA以及PKG1 siRNA NC购自上海吉玛制药技术有限公司;PDTC购自英国Abcam公司;荧光定量PCR(qPCR)试剂盒购自德国DBI公司;PKG1抗体购自美国Boster公司;BCA蛋白定量试剂盒购自南京凯基生物发展有限公司;CCK-8试剂盒购自上海碧云天生物技术有限公司;Annexin V-FITC/PI试剂盒购自南京诺唯赞生物科技股份有限公司;PCR扩增仪(Mx3000P)购自杭州晶格科学仪器有限公司。
1.3 研究方法
1.3.1 标本的收集与处理 所有标本均在剖宫产胎盘娩出后5 min内收集,避开钙化灶及出血区域,于胎盘母面不同位置剪取胎盘组织,置于无菌的EP管中,并迅速置于-80 ℃冰箱中保存备用。
1.3.2 细胞分组转染 将体外培养的HTR-8/SVneo细胞分为6组:NC组(转染miR-155 NC)、mimics组(转染miR-155 mimic)、inhibitor组(转染miR-155 inhibitor)、siRNA NC组(转染PKG1 siRNA NC)、PKG1 siRNA组(转染PKG1 siRNA)、siRNA+inhibitor组(转染PKG1 siRNA和miR-155 inhibitor)。取对数期生长至密度为90%的细胞接种至6孔板(3?105/孔),并更换为无血清无双抗的培养基,按照Lipofectaimine 2000試剂盒说明书对各组细胞分别进行转染,于37 ℃、5%CO2、饱和湿度培养箱中培养4 h后更换为含10%胎牛血清的培养基继续培养48 h。
1.3.3 核因子(NF)-κB抑制剂PDTC处理 PDTC是国际公认的NF-κB抑制剂,可以在多种细胞中抑制NF-κB的激活。将细胞分为4组:Control组(常规培养)、PDTC组(PDTC处理)、PDTC+NC组(PDTC与miR-155 NC共处理)、PDTC+mimics组(PDTC与miR-155 mimics共处理)。PDTC组使用含PDTC 30 μmol/L的培养基培养48 h,PDTC+NC组与PDTC+mimics组中,miR-155 NC或miR-155 mimics转染后更换为含PDTC 30 μmol/L的培养基再培养48 h。
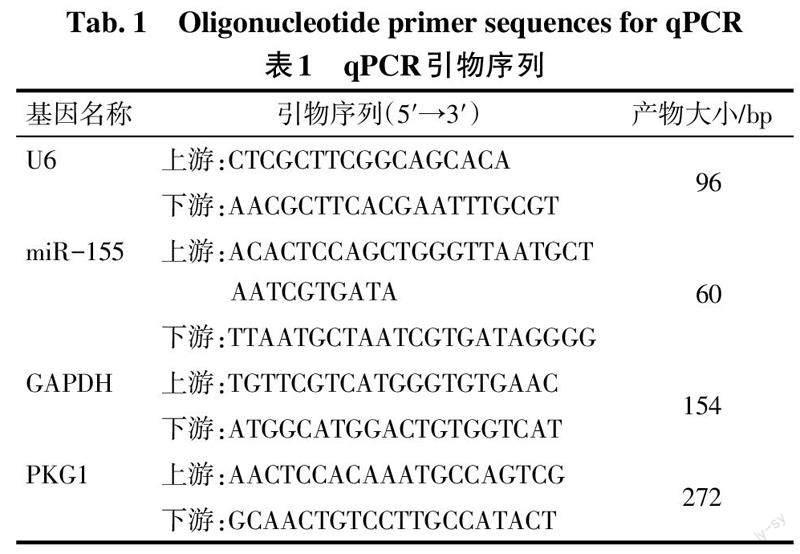
1.3.4 总RNA提取及qPCR检测 将所保存的胎盘组织从冰箱中取出,采用Trizol法进行胎盘组织和各组HTR-8/SVneo细胞中总RNA的提取,质检后继续-80 ℃保存。按照逆转录试剂盒说明书将RNA合成cDNA,随后用Agilent Stratagene荧光定量PCR仪进行荧光定量, PCR反应条件:95 ℃ 2 min;94 ℃ 20 s,58 ℃ 20 s,72 ℃ 20 s,40个循环。miR-155以U6为内参,PKG1以GAPDH为内参。引物序列见表1。采用2-ΔΔCt法计算miR-155和PKG1的相对表达量。
1.3.5 Western blot检测各组PKG1的表达 采用RIPA裂解液提取胎盘组织和各组HTR-8/SVneo细胞中的总蛋白,按照BCA试剂盒检测蛋白浓度,用10%十二烷基硫酸钠-聚丙烯酰胺凝胶电泳分离蛋白样品,并转移到PVDF膜上。用5%脱脂奶粉封闭2 h后,加入一抗(1∶1 000)孵育过夜,然后加入二抗(1∶2 000)室温下孵育2 h,之后进行ECL显影,各条带的灰度值采用Image Pro Plus Ver.6.0进行分析,目的蛋白相对表达量=目的条带灰度值/GAPDH灰度值。
1.3.6 Transwell检测细胞迁移能力 在Transwell小室的上室接种细胞(3?104/孔),下室加入含10%胎牛血清的完全培养基,培养24 h后采用4%多聚甲醛固定,结晶紫染色,随机抽取3张图片,显微镜下观察迁移细胞数。
1.3.7 CCK-8检测细胞增殖活性 将细胞接种于96孔培养板(3?103/孔)中培养12 h,分别于0 h、24 h、48 h、72 h时取出培养板,加入100 μL CCK-8工作液,再培养2 h,然后检测450 nm处的吸光度(A)值。
1.3.8 流式细胞术检测细胞凋亡 按照Annexin V-FITC/PI试剂盒说明书进行操作。首先收集培养48 h的细胞,制备细胞悬液,然后加入凋亡检测工作液,避光孵育15 min。用FlowJo软件测定细胞凋亡率。
1.4 统计学方法 采用SPSS 26.0及Graphpad Prism 9.0软件进行数据分析。正态分布的计量资料以x±s表示,2组间比较采用独立样本t检验,多组间比较采用单因素方差分析,组间多重比较采用LSD-t检验。不符合正态分布的计量资料以M(P25,P75)表示,2组间比较采用Mann-Whitney U检验。以P<0.05为差异有统计学意义。
2 结果
2.1 受试者一般资料比较 与NPE组相比,PE组年龄、体质量指数(BMI)和孕周的差异无统计学意义(P>0.05),收缩压(SBP)及舒张压(DBP)升高,而胎儿出生体质量降低(P<0.05),见表2。
2.2 2组胎盘组织中miR-155及PKG1的表达比较 与NPE组相比,PE组miR-155的表达水平升高(P<0.01),而PKG1的mRNA及蛋白[0.15(0.07,0.32) vs. 0.88(0.77,1.03),n=4,Z=16.000]水平均降低(P<0.05),见表3、图1。
2.3 miR-155对滋养细胞HTR-8/SVneo中PKG1表达的影响 与NC组相比,miR-155 mimics组miR-155水平升高,miR-155 inhibitor组降低(P<0.05),miR-155 inhibitor组PKG1 mRNA和蛋白水平升高,而miR-155 mimics组降低(P<0.05)。见表4、图2。
2.4 miR-155对滋养细胞HTR-8/SVneo迁移、增殖、凋亡的影响 与NC组相比,miR-155 inhibitor组细胞迁移、增殖能力增强,细胞凋亡率减少(P<0.05),而miR-155 mimics组细胞迁移、增殖能力减弱,细胞凋亡率增加(P<0.05),见表5,图3、4。
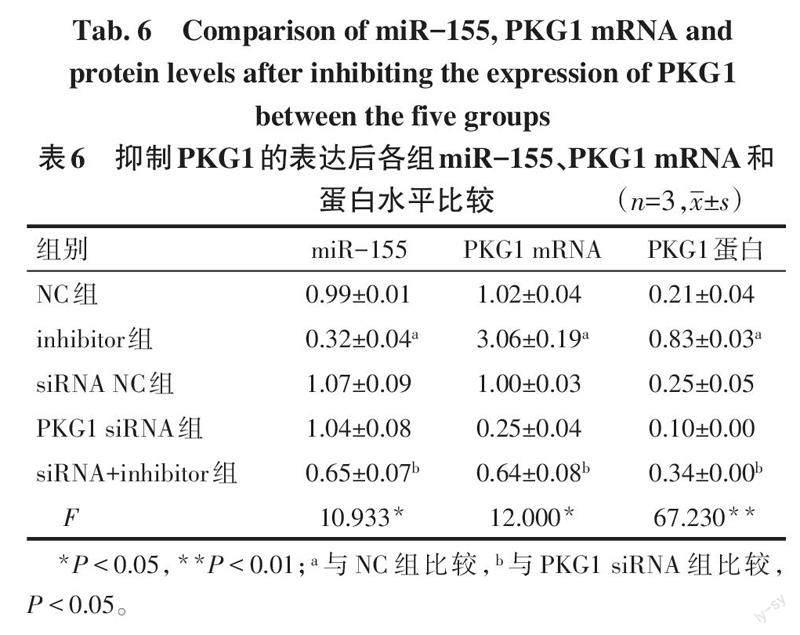
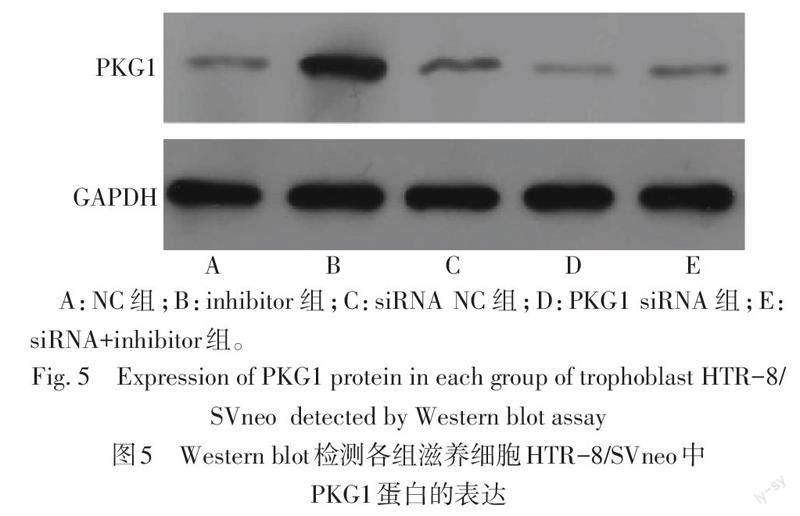
2.5 抑制PKG1的表达后对miR-155、PKG1表达的影响 与NC组相比,inhibitor组miR-155水平降低,PKG1 mRNA和蛋白的水平升高(P<0.05);与PKG1 siRNA组相比,siRNA+inhibitor组miR-155水平降低,PKG1在mRNA和蛋白水平均升高(P<0.05),见表6、图5。
2.6 抑制NF-κB的表达对滋养细胞HTR-8/SVneo中miR-155和PKG1水平的影响 与Control组相比,PDTC组miR-155水平降低,PKG1 mRNA和蛋白表达水平升高(P<0.05);与PDTC+NC组相比,PDTC+mimics组miR-155的水平升高(P<0.05),PKG1 mRNA和蛋白水平均降低(P<0.05),见表7、图6。
2.7 抑制NF-κB表达对滋养细胞HTR-8/SVneo生物学功能的影响 与Control组相比,PDTC组滋养细胞的迁移、增殖能力增强,凋亡率下降(P<0.05);与PDTC+NC组相比,PDTC+mimics组滋养细胞的迁移、增殖能力减弱,凋亡率增加(P<0.01)。见表8,图7、8。
3 讨论
PE是一种严重威胁母婴健康的妊娠特异性疾病,伴有炎症反应和内皮细胞功能障碍[9],如不及时治疗会导致脑卒中、肾功能衰竭、肺水肿、肝破裂、子痫等严重并发症[10]。目前PE的病因和发病机制尚不清楚,治疗旨在减缓病理进展以延长孕周,然而可用于治疗该疾病的临床策略有限,只有胎盘娩出才可彻底解除母体的症状,因而寻找有效的治疗靶点是当前围产医学领域中的研究热点。
3.1 miR-155在PE中的表达及作用 miRNAs在基因的表達调控中发挥着重要的作用,其通过与靶基因mRNA的3′-UTR反向互补,参与细胞分化、增殖、凋亡、代谢等多种生物学过程[11]。有学者研究miRNAs的基因表达谱发现,一些miRNAs,如miR-335、miR-424、miR-18a、miR-155可以通过作用于多种靶点导致滋养层细胞功能障碍[12-13]。miR-155是保守性较好的多功能miRNA之一,由位于21号染色体上的miR155宿主基因(MIR155HG)编码,可以在包括PE在内的多种疾病中表达。本课题组前期研究发现,miR-155在PE患者中高表达,并与其启动子区域DNA甲基化水平呈负相关[6],但其具体机制尚不清楚。本研究通过对PE患者和正常产妇胎盘中相关因子的检测发现,PE患者胎盘组织中miR-155的表达水平显著升高,与Wu等[14]研究结果一致,而PKG1的表达明显降低,提示miR-155的高表达与PE的发生密切相关。
3.2 miR-155通过抑制PKG1的表达在PE中发挥作用 PKG1是cGMP的重要效应物,具有多种重要功能,如血管舒张与重建、细胞分化和凋亡等。NO/cGMP信号转导通路在内皮功能和心血管稳态中发挥重要作用[15]。NO由L-精氨酸通过内皮型一氧化氮合酶磷酸化形成,在调节血压方面起重要作用,NO可结合平滑肌细胞中NO的受体可溶性鸟苷酸环化酶(sGC),从而将三磷酸鸟苷(GTP)转化为cGMP,引起cGMP依赖性蛋白激酶(PKG)水平的升高而发挥生物学效应,当这一途径受损时可导致血管内皮功能受损、血管舒张障碍[16]。在哺乳动物中,PKG有2种同工酶,即PKG1和PKG2,由不同基因编码[17]。PKG1主要在心血管系统中发挥作用,而PE是一种以血管功能障碍为特征的疾病[18],这表明在PE中PKG1可能更为重要。Choi等[7]研究认为,miR-155模拟物可降低VSMC细胞中PKG1的水平,从而导致VSMC表型发生改变和舒张功能障碍。为验证miR-155是否可通过抑制PKG1的表达进而影响滋养细胞的生物学功能,本研究采用滋养细胞HTR-8/SVneo进行了体外实验,转染miR-155 mimics或inhibitor建立过表达或干扰细胞模型,结果显示miR-155低表达可以上调滋养细胞PKG1 mRNA和蛋白的水平,还可增强细胞的增殖、迁移能力,诱导细胞凋亡,提示miR-155可通过抑制PKG1引起滋养层细胞的生物学功能发生改变。
3.3 抑制NF-κB表达后对PE的影响 NF-κB是一种细胞内转录因子,可以调节多种细胞功能,如炎症反应、胚胎发生、细胞增殖与凋亡以及对各种有害刺激的应激反应[19]。NF-κB信号转导通路是一种经典的促炎通路,在感染或损伤期间,组织会迅速释放促炎因子,如白细胞介素(IL)-6、IL-8、肿瘤坏死因子α,从而激活NF-κB通路,进而促进炎性因子、趋化因子等的释放[20]。根据Staff[21]提出的改良PE两阶段模型,笔者认为PE的致病机制可能与氧化应激和炎症反应密切相关。在炎性因子的刺激下NF-κB通路被激活,促进机体进一步分泌大量的炎性细胞因子,持续存在的炎症反应可引发血管内皮损伤,从而促进PE的发展[22]。有研究表明,在PE大鼠体内选择性抑制NF-κB的表达可降低大鼠血压以及尿蛋白,并可减缓胎盘损伤[23]。Mann等[24]研究发现,炎症刺激可激活NF-κB,导致miR-155水平升高,进而抑制SHIP1和SOCS1以及其他潜在靶标的表达。本研究发现,与Control组相比,NF-κB抑制剂PDTC处理后滋养细胞miR-155表达降低,PKG1的表达则明显升高,滋养细胞的迁移、增殖能力明显增强,凋亡能力减弱,而转染miR-155模拟物后可逆转PTDC对PKG1水平以及滋养细胞生物学功能的影响。笔者推测NF-κB可能是通过miR-155发挥生物学作用,并影响PE进展的重要信号因子。
综上,PE患者胎盘组织中miR-155呈高表达,而PKG1呈低表达;NF-κB介导的miR-155通过下调PKG1的表达,从而抑制滋养细胞的增殖和迁移,增加细胞凋亡。本研究对miR-155在PE发病中的作用进行了初步分析,为明确PE的致病机制提供了新的理论基础。
参考文献
[1] IVES C W,SINKEY R,RAJAPREYAR I,et al. Preeclampsia-pathophysiology and clinical presentations:JACC state-of-the-art review[J]. J Am Coll Cardiol,2020,76(14):1690-1702. doi:10.1016/j.jacc.2020.08.014.
[2] ALFAIFI A A,HEYDER R S,BIELSKI E R,et al. Megalin-targeting liposomes for placental drug delivery[J]. J Control Release,2020,324:366-378. doi:10.1016/j.jconrel.2020.05.033.
[3] ZHOU Y,WANG J,WANG L,et al. Effect of compound danshen injection combined with magnesium sulfate on oxidative stress,TNF-alpha,NO,and therapeutic efficacy in severe preeclampsia[J]. Comput Intell Neurosci,2022,2022:9789066. doi:10.3390/biom10060953.
[4] SHIRVANI S O,SCHERR J,KAYVANPOUR E,et al. Marathon-induced cardiac strain as model for the evaluation of diagnostic microRNAs for acute myocardial infarction[J]. J Clin Med,2021,11(1):5. doi:10.3390/jcm11010005.
[5] KOLKOVA Z,HOLUBEKOVA V,GRENDAR M,et al. Association of circulating miRNA expression with preeclampsia,its onset,and severity[J]. Diagnostics (Basel),2021,11(3):476. doi:10.3390/diagnostics11030476.
[6] LUO X,PAN C,GUO X,et al. Methylation mediated silencing of miR-155 suppresses the development of preeclampsia in vitro and in vivo by targeting FOXO3[J]. Mediators Inflamm,2022,2022:4250621. doi:10.1155/2022/4250621.
[7] CHOI S,PARK M,KIM J,et al. TNF-alpha elicits phenotypic and functional alterations of vascular smooth muscle cells by miR-155-5p-dependent down-regulation of cGMP-dependent kinase 1[J]. J Biol Chem,2018,293(38):14812-14822. doi:10.1074/jbc.RA118.004220.
[8] 中華医学会妇产科学分会妊娠期高血压疾病学组. 妊娠期高血压疾病诊治指南(2020)[J]. 中华妇产科杂志,2020,55(4):227-238. Group of Hypertensive Disorders of Pregnancy,Obstetrics and Gynecology Branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of hypertensive disorders during pregnancy(2020)[J]. Chin J Obstetr Gynecol,2020,55(4):227-238. doi:10.3760/cma.j.cn112141-20200114-00039.
[9] LI H,OUYANG Y,SADOVSKY E,et al. Unique microRNA signals in plasma exosomes from pregnancies complicated by preeclampsia[J]. Hypertension,2020,75(3):762-771. doi:10.1161/HYPERTENSIONAHA.119.14081.
[10] ABDELZAHER W Y,MOSTAFA-HEDEAB G,BAHAA H A,et al. Leukotriene receptor antagonist,montelukast ameliorates L-NAME-induced pre-eclampsia in rats through suppressing the IL-6/Jak2/STAT3 signaling pathway[J]. Pharmaceuticals(Basel),2022,15(8):914. doi:10.3390/ph15080914.
[11] LIU X,WANG W,BAI Y,et al. Identification of a genome-wide serum microRNA expression profile as potential noninvasive biomarkers for chronic kidney disease using next-generation sequencing[J]. J Int Med Res,2020,48(12):1220769033. doi:10.1177/0300060520969481.
[12] CAI M,KOLLURU G K,AHMED A. Small molecule,big prospects:microRNA in pregnancy and its complications[J]. J Pregnancy,2017,201:6972732. doi:10.1155/2017/6972732.
[13] HEMMATZADEH M,SHOMALI N,YOUSEFZADEH Y,et al. MicroRNAs:small molecules with a large impact on pre-eclampsia[J]. J Cell Physiol,2020,235(4):3235-3248. doi:10.1002/jcp.29286.
[14] WU H Y,LIU K,ZHANG J L. LINC00240/miR-155 axis regulates function of trophoblasts and M2 macrophage polarization via modulating oxidative stress-induced pyroptosis in preeclampsia[J]. Mol Med,2022,28(1):119. doi:10.1186/s10020-022-00531-3.
[15] EVORA P,SOARES R,BASSETTO S,et al. After thirty years,we still cannot understand why methylene blue is not a reference to treat vasoplegic syndrome in cardiac surgery[J]. Braz J Cardiovasc Surg,2021,36(3):406-411. doi:10.21470/1678-9741-2021-0955.
[16] ITO H,MORISHITA R,NAGATA K I. Functions of rhotekin,an effector of rho GTPase,and its binding partners in mammals[J]. Int J Mol Sci,2018,19(7):2121. doi:10.3390/ijms19072121.
[17] RAMDANI G,SCHALL N,KALYANARAMAN H,et al. cGMP-dependent protein kinase-2 regulates bone mass and prevents diabetic bone loss[J]. J Endocrinol,2018,238(3):203-219. doi:10.1530/JOE-18-0286.
[18] BISWAS S,KOJONAZAROV B,HADZIC S,et al. IRAG1 deficient mice develop PKG1beta dependent pulmonary hypertension[J]. Cells,2020,9(10):2280. doi:10.3390/cells9102280.
[19] FERNANDO I P S,KIRINDAGE K G I S,JAYASINGHE A M K,et al. Hot water extract of Sasa borealis (hack.) makino & shibata abate hydrogen peroxide-induced oxidative stress and apoptosis in kidney epithelial cells[J]. Antioxidants(Basel),2022,11(5):1013. doi:10.3390/antiox11051013.
[20] ZHAO L,LI Y,YAO D,et al. Pharmacological basis for use of a novel compound in hyperuricemia:anti-hyperuricemic and anti-inflammatory effects[J]. Front Pharmacol,2021,12:772504. doi:10.3389/fphar.2021.772504.
[21] STAFF A C. The two-stage placental model of preeclampsia:an update[J]. J Reprod Immunol,2019:134-135. doi:10.1016/j.jri.2019.07.004.
[22] WANG Y,LI B,ZHAO Y. Inflammation in preeclampsia:genetic biomarkers, mechanisms,and therapeutic strategies[J]. Front Immunol,2022,13:883404. doi:10.3389/fimmu.2022.883404.
[23] SOCHA M W,MALINOWSKI B,PUK O,et al. The role of NF-κB in uterine spiral arteries remodeling,insight into the cornerstone of preeclampsia[J]. Int J Mol Sci,2021,22(2):704. doi:10.3390/ijms22020704.
[24] MANN M,MEHTA A,ZHAO J L,et al. An NF-κB-microRNA regulatory network tunes macrophage inflammatory responses[J]. Nat Commun,2017,8(1):851. doi:10.1038/s41467-017-00972-z.
(2022-11-18收稿 2023-03-02修回)
(本文編辑 李鹏)
