慢性阻塞性肺疾病患者HRCT定量指标与肺功能的相关性研究
2023-11-06张凤方著袁艺周芸慧张国晋蒲红刘欢
张凤 方著 袁艺 周芸慧 张国晋 蒲红 刘欢
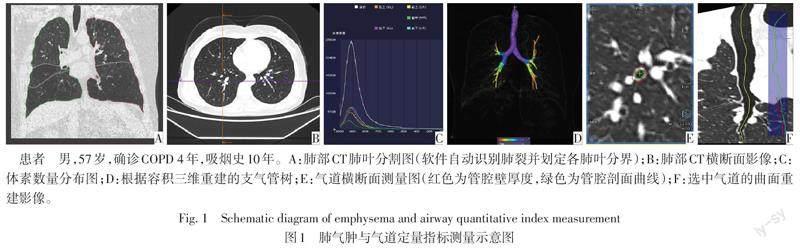
摘要:目的 利用高分辨率CT(HRCT)定量指標分析慢性阻塞性肺疾病(COPD)患者的肺气肿及气道重塑改变,并探讨上述指标与肺功能的相关性。方法 收集COPD患者(COPD组)109例及正常组33例,COPD组患者根据COPD全球倡议指南,分为GOLDⅠ级组(29例)、GOLDⅡ级组(38例)、GOLDⅢ+Ⅳ级组(42例)。患者均行HRCT和肺功能检查;利用后处理软件自动测量各肺叶HRCT肺气肿定量指标:肺气肿指数(EI)、各肺叶平均肺密度(MLD);气道定量指标:支气管壁面积(WA)、支气管腔面积(LA)、管壁面积占支气管断面总面积的百分比(WA%)。比较上述参数在正常组和COPD不同严重程度亚组之间的差异。应用Spearman法分析上述CT定量指标与肺功能指标的相关性。结果 与正常组比较,GOLDⅠ级组双肺上叶EI升高,GOLDⅡ级组各肺叶EI均升高,双肺上叶MLD及左肺下叶MLD降低,GOLDⅢ+Ⅳ级组各肺叶EI均升高,MLD降低(P<0.05)。与正常组比较,GOLDⅠ级组5级支气管WA,4~6级支气管WA%升高,5、6级支气管LA降低;与GOLDⅠ级组比较,GOLDⅡ级组仅6级支气管WA、WA%升高,LA降低;与GOLDⅡ级组比较,GOLDⅢ+Ⅳ级组仅5级支气管WA%降低(P<0.05)。各肺叶EI与第1秒用力呼气容积实测值与预测值百分比(FEV1%)呈负相关,MLD与FEV1%呈正相关(P<0.01),5、6级支气管WA%与FEV1%呈负相关(P<0.05)。结论 HRCT肺气肿定量指标、气道定量指标与肺功能具有一定相关性,且随着COPD分级不同而变化,可对COPD评估提供参考价值。
关键词:肺疾病,慢性阻塞性;肺气肿;体层摄影术,X线计算机;肺功能;肺密度
中图分类号:R445.3 文献标志码:A DOI:10.11958/20221162
The correlation research of quantitative index of HRCT and lung function in patients with chronic obstructive pulmonary disease
ZHANG Feng FANG Zhu YUAN Yi ZHOU Yunhui ZHANG Guojin PU Hong LIU Huan
1 North Sichuan Medical College, Chengdu 610072, China; 2 Department of Radiology, Sichuan Academy of Medical Sciences Sichuan Provincial People's Hospital, Affiliated Hospital of University of Electronic Science and
Technology of China; 3 GE Healthcare
Corresponding Author E-mail: ph196797@163.com
Abstract: Objective To analyze quantitative indexes of emphysema and airway remodeling in patients with chronic obstructive pulmonary disease (COPD) by using high resolution CT (HRCT), and to explore the correlation between the above indexes and pulmonary function. Methods A total of 109 COPD patients (the COPD group) and 33 normal controls (the control group) were collected. According to the COPD Global Initiative guidelines, COPD patients were divided into the GOLD class Ⅰ group (n=29), the GOLD classⅡ group (n=38) and the GOLD class Ⅲ+Ⅳgroup (n=42). All patients underwent HRCT examination and pulmonary function examination. The post-processing software was used to automatically measure HRCT quantitative emphysema indexes of each lung lobe, including emphysema index (EI), mean lung density (MLD) of each lobe and airway quantitative indexes [bronchial wall area (WA), bronchial lumen area (LA) and the percentage of the wall area in the total bronchial section area (WA%)]. The above parameters were compared between the normal group and the different severity COPD subgroups. Spearman method was used to analyze the correlation between the above CT quantitative indexes and pulmonary function. Results Compared with the normal group, EI in upper lobe of both lungs was increased in the GOLD class Ⅰ group, EI in all lobes was increased in the GOLD class Ⅱ group, MLD values in upper lobe of both lungs and lower lobe of left lung were decreased, EI in all lobes was increased in the GOLD class Ⅲ+Ⅳ group, and MLD was decreased (P<0.05). Compared with the normal group, the WA of grade 5 bronchus and WA% of grade 4-6 bronchus were increased in the GOLD class Ⅰ group, and LA of grade 5 and 6 bronchus decreased. Compared with the GOLD class Ⅰ group, only WA and WA% of grade 6 bronchi were increased in the GOLD class Ⅱ group, and LA was decreased. Compared with the GOLD class Ⅱ group, WA% of grade 5 bronchial was decreased in the GOLD class Ⅲ+Ⅳ group (P<0.05). The measured and predicted percentages of forced expiratory volume in the first second (FEV1%) were negatively correlated with the EI of each lung lobe, and the MLD was positively correlated with FEV1% (P<0.01). The WA% of 5-6 bronchi was negatively correlated with FEV1% (P<0.05). Conclusion HRCT emphysema quantitative indexes and airway quantitative indexes have certain correlation with pulmonary function. It varies with different COPD grades, which can provide reference value for COPD evaluation.
Key words: pulmonary disease, chronic obstructive; pulmonary emphysema; tomography, X-ray computed; pulmonary function; lung density
慢性阻塞性肺疾病(COPD)是全球第4大致死疾病[1-2],其特征为进行性发展的气流受限从而导致肺功能障碍和相关症状。COPD早期起病隐匿,只有在肺实质破坏30%以上时才会出现临床症状或肺功能检查异常。肺气肿和小气道重塑是其主要病理表现,其中小气道重塑是COPD发展过程中空气潴留和气道阻力增加的核心机制[3-5]。早期准确识别小气道重塑,防止可逆性炎症变为不可逆性肺气肿是临床诊断和治疗的关键[6]。胸部高分辨率CT(HRCT)的肺气肿定量参数能间接反映肺通气功能;气道定量参数可直接测量支气管管壁及管腔厚度的变化,间接反映小气道重塑。上述参数可定量评估肺实质损伤程度,与肺功能具有一定相关性,相较于肺功能检查,其能在早期准确识别COPD病理改变,有利于临床精准化诊断、治疗及预后评价[7-9]。但目前有关不同程度COPD肺气肿和小气道重塑改变的定量及定位评估研究较少。本研究旨在探讨HRCT肺气肿定量参数和气道定量参数在不同分级COPD中的应用价值。
1 对象与方法
1.1 研究对象 选取2021年3月—2022年2月四川省人民医院收治的COPD患者为COPD组。纳入标准:(1)COPD诊断标准依据COPD全球倡议(GOLD)2022诊疗建议,除外其他疾病,肺功能检查吸入支气管扩张剂后第1秒用力呼气容积占用力肺活量的百分比(FEV1/FVC)<70%。(2)肺功能检查与HRCT扫描间隔时间≤2周。排除标准:(1)具有胸部手术史。(2)其他呼吸系统疾病史,如肺癌、哮喘、支气管扩张、肺结核、严重的肺部感染、胸腔积液等。共纳入109例,其中男89例,女20例,年龄36~84岁,平均(66.9±8.6)岁。根据GOLD严重程度分级,将患者分为不同亚组:第1秒用力呼气容积实测值与预测值百分比(FEV1%)≥80%为轻度(GOLDⅠ级组,29例),50%≤FEV1%<80%为中度(GOLDⅡ级组,38例),30%≤FEV1%<50%为重度(GOLDⅢ级组,31例),FEV1%<30%为极重度(GOLDⅣ级组,11例),因极重度组患者较少,将重度、极重度患者合并为GOLDⅢ+Ⅳ级组(42例)。选取同期肺功能检查正常,无呼吸系统相关病史的其他患者为正常组(33例),其中男22例,女11例,年龄31~84岁,平均(63.8±8.7)岁。正常组与COPD组性别(χ2=3.333)、年龄(t=1.769)差异无统计学意义(均P>0.05)。
1.2 CT扫描 采用德国西门子SOMATOM Force第3代双源CT进行扫描,检查前对患者进行呼吸训练,取仰卧位,头先进,嘱患者深吸气末屏气,扫描范围为肺尖至肺底。扫描参数:管电压120 kV,管电流自动调节,分别经肺算法、软组织算法重建,重建矩阵512×512,探测器准直96×0.6,重建层厚1.00 mm,层间距1.00 mm。
1.3 CT图像后处理及参数测量 将获得的图像数据传输至后处理工作站(syngo.via VB20),使用后处理软件Pulmo 3D进行参数测量,由2名分别具有4年和5年阅片经验的放射科医师进行独立测量,并评估测量一致性,各参数影像测量见图1。肺气肿定量参数测量:在Pulmo 3D软件的肺实质分析模块中,设定图像窗宽以300 HU为上限,-1 024 HU为下限,软件自动识别肺裂并进行肺叶分割,当自动识别不准确时手动干预。软件自动获得各肺叶体素数量分布图,由此计算各肺叶肺气肿指数(EI):CT值<-950 HU区域占该肺叶容积的百分比;各肺叶平均肺密度(MLD):该肺叶的平均像素密度。
气道定量参数测量:利用Pulmo3D软件进行多平面重组技术重建支气管树,使支气管走行垂直于测量方向,软件自动对选中的支气管沿中心线进行曲面重建,测量者在曲面重建图上选择测量的位置。为全面了解气道情况,每个肺叶选取1支气管为研究对象,共选取6支气管,分别为右肺上叶尖段、右肺中叶外侧段、右肺下叶外基底段、左肺上叶尖后段、左肺上叶上舌段及左肺下叶后基底段。以段支气管为4级支气管,选择距离两端支气管开口约同等距离处为测量点,分别测量4~6级支气管支气管壁面积(WA)、支气管腔面积(LA)、管壁面積占支气管断面总面积的百分比[WA%,WA/(WA+LA)]。
1.4 肺功能测定 采用德国耶格公司MasterSereen PET型肺功能仪,受检者取坐位,在定量吸入支气管扩张剂后测量FEV1%和FEV1/FVC。
1.5 统计学方法 采用SPSS 26.0软件进行数据分析。符合正态分布的计量资料采用x±s表示,2组间比较采用独立样本t检验,多组间比较采用单因素方差分析,组间多重比较采用LSD-t检验。非正态分布的计量资料采用M(P25,P75)表示,多组间比较采用Kruskal-Wallis H检验,组间多重比较采用Nemenyi检验。计数资料以例表示,组间比较采用χ2检验。观察者间一致性检验采用组内相关系数(ICC)分析:ICC=0表示不可信,ICC<0.4表示信度较差,ICC为0.40~0.75表示信度一般,ICC>0.75表示信度良好,ICC=1表示完全可信。相关性分析采用Spearman法。P<0.05为差异有统计学意义。
2 结果
2.1 2名医生对肺气肿、气道定量指标独立测量结果的一致性分析 肺气肿定量指标一致性良好:左肺上叶、左肺下叶、右肺上叶、右肺中叶、右肺下叶EI的ICC值分别为0.874、0.872、0.869、0.870和0.868,MLD的ICC值分别0.897、0.897、0.883、0.864和0.871(P<0.01)。气道定量指标一致性一般:4~6级支气管WA ICC值分别为0.739、0.721、0.715,LA ICC值分别为0.743、0.727、0.726(P<0.01)。
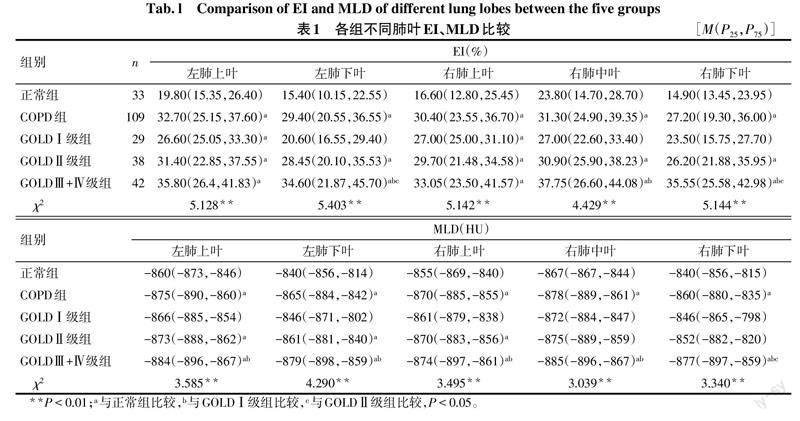
2.2 肺气肿定量参数分析 与正常组比较,GOLDⅠ级组双肺上叶EI升高;GOLDⅡ级组各肺叶EI均升高,双肺上叶MLD及左肺下叶MLD降低;GOLDⅢ+Ⅳ级组各肺叶EI均升高,MLD降低(P<0.05),见表1。
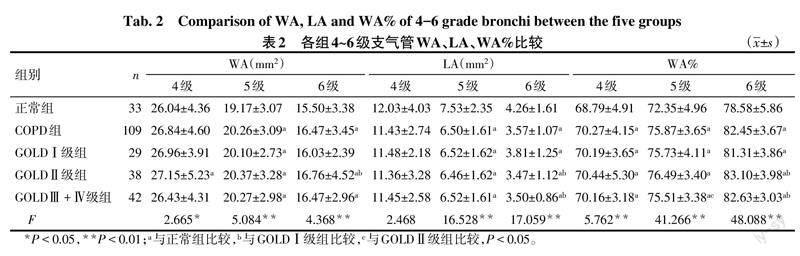
2.3 气道定量参数分析 与正常组比较,GOLDⅠ级组5级支气管WA和4~6级支气管WA%升高,5、6级支气管LA降低;与GOLDⅠ级组比较,GOLDⅡ级组仅6级支气管WA、WA%升高,LA降低;与GOLDⅡ级组比较,GOLDⅢ+Ⅳ级组仅5级支气管WA%降低(P<0.05),见表2。
2.4 COPD患者肺气肿定量参数与肺功能相关性分析 各肺叶EI与FEV1%均呈负相关,MLD与FEV1%均呈正相关(P<0.01),见表3。
2.5 COPD患者气道定量参数与肺功能的相关性分析 4~6级支气管WA、LA与FEV1%均无相关性,5、6级支气管WA%与FEV1%呈负相关(P<0.05),见表4。
3 讨论
COPD是一种异质性疾病,不同患者的临床表现、影像学特征、疾病进展和预后存在较大差别,临床治疗决策亦不相同[10]。目前常规使用肺功能检查对COPD进行诊断,但其临床价值有限,无法对肺气肿和小气道病变定量分析,且部分重症患者往往配合较差。近年来,HRCT被广泛用于评估COPD。研究显示,HRCT可直观、定量地评估肺气肿的存在、模式和程度[11-13],有助于临床个性化治疗。COPD患者定量CT检查所显示的支气管壁厚度和肺气肿程度是肺功能检查时气流受限程度和COPD恶化的独立影响因素[14-16]。本研究对肺气肿定量指标分析,与正常组比较,GOLDⅠ级组仅双肺上叶EI升高;随着COPD分级增加,GOLDⅢ+Ⅳ级组各肺叶EI均升高,MLD均降低。可见在COPD早期肺气肿主要发生于双肺上叶,随着病情进展,肺气肿在全肺均表现明显。师美娟等[17]发现轻度COPD患者的肺气肿分布在双肺上叶较多,重度患者肺气肿在双肺下叶分布的比例明显增加,与本研究结果类似。推测其原因为:(1)重力作用下胸腔负压从肺尖到肺底逐渐减小,因此肺尖对气体交换的动力最小,早期空气潴留容易发生在双肺上叶。(2)肺血流受重力影响,从肺尖到肺底,肺循环血管开放程度逐渐增加,当受吸烟或空气污染等环境因素刺激时肺血管收缩、血管舒张因子释放,由于流体静力效应[18],肺血管重塑首先发生于双肺上叶,从而导致气道阻力增加,最终导致肺气肿发生。黄晓旗等[19]利用双气相定量CT测量吸烟合并COPD患者各肺叶肺气肿区域百分比(Emph%),发现COPD组Ⅰ、Ⅱ级主要以右肺中叶Emph%损伤程度最重,双肺上叶次之。而本研究中GOLDⅠ期右肺中叶EI无明显升高,虽然右肺中叶支气管窄长,早期易发生通气不足及感染,但可能残存少许正常肺组织,对右肺中叶平均肺密度有缓冲作用,肺气肿进展缓慢。本研究中CT定量肺气肿参数与肺功能指标FEV1%有较好的相关性,提示其可用于COPD患者肺气肿程度的评估,临床中可用于识别肺气肿较重的区域,以选择肺减容术时的靶组织。
在本研究中,在GOLDⅠ级组与GOLDⅡ级组间,6级支气管WA、WA%随着病情加重而逐渐增大,LA逐渐减小,与既往研究相似[20-22]。当长期慢性暴露于烟草烟雾或有毒颗粒环境时,机体会产生异常先天性和适应性免疫反应,表现为气道黏液产生增加、黏液纤毛清除缺陷、上皮屏障破坏以及形成淋巴滤泡的炎性免疫细胞浸润气道壁[23],导致气道腔内的炎性黏液渗出物积聚,长期发展导致气道壁增厚。Hogg等[24]研究表明,黏液阻塞气道管腔、气道壁内炎症程度以及气道壁增厚都与气流限制有关。但本研究中GOLDⅢ+Ⅳ级组各气道定量指标结果均较GOLDⅡ级组变化不明显,分析原因可能为部分中重度患者处于急性期,炎性反应较重,渗出明显增多,远端小气道尺寸急剧减小;而本研究的后处理软件Pulmo 3D对支气管远端极细小分支的检测效果一般,因6级支气管尺寸较小,测量时可能未能达到前述测量方法所示的准确位置,故可能对结果产生一定影响。另外,本研究结果显示5级支气管WA%与FEV1%的相关性高于6级支气管。Qin等[25]利用后处理软件ISP 9.0连续测量第3、5、9级支气管的气道参数,将其与肺功能进行相关性分析,结果显示气道分级越小,相关性越高,以9-LA/AA、9-WA/AA与FEV1%相关性最高,与本研究结果有差异。本研究由于技术限制,部分中重度患者处于慢阻肺气道炎症急性期,黏液过度分泌,管腔面积急剧减小[26],测量6级支气管参数时可能测量点位置选择不够准确,对结果产生一定影响。因此,肺功能检查需和影像学检查互補,更准确提示COPD进展情况。
本研究的局限性:由于COPD患者有时可伴有肺纤维化改变,肺纤维化在HRCT上表现为高衰减区,与肺气肿区相反,因此对于肺纤维化严重的患者,采用HRCT肺气肿定量指标评估可能会对结果产生一定影响,后续可利用定量软件识别肺纤维化区域进一步研究。
综上所述,HRCT肺气肿定量指标EI、MLD,气道定量指标WA%与肺功能具有相关性,可反映COPD的病理变化,且各指标结果随着病情严重程度不同而变化,对COPD诊断及评估提供参考价值,是肺功能检查的重要补充。但单一影像学因素对COPD的评估准确性不够,未来可纳入多重指标进一步研究其在COPD严重程度分级中的诊断效能。
参考文献
[1] BURNEY P G,PATEL J,NEWSON R,et al. Global and regional trends in COPD mortality,1990-2010[J]. Eur Respir J,2015,45(5):1239-1247. doi:10.1183/09031936.00142414.
[2] WANG C,XU J,YANG L,et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China(the China Pulmonary Health[CPH]study):A national cross-sectional study[J]. Lancet,2018,391(10131):1706-1717. doi:10.1016/S0140-6736(18)30841-9.
[3] 王雯婷,王晓华,贺蓓,等. 基于吸呼双相CT定量参数的慢性阻塞性肺疾病影像学表型研究[J]. 中华医学杂志,2021,101(28):2242-2245. WANG W T,WANG X X,HE B,et al. Imaging phenotypes of chronic obstructive pulmonary disease based on biphasic quantitative CT features[J]. Natl Med J China,2021,101(28):2242-2245. doi:10.3760/cma.j.cn112137-20201223-03443.
[4] OSTRIDGE K,WILLIAMS N P,KIM V,et al. Relationship of CT-quantified emphysema,small airways disease and bronchial wall dimensions with physiological,inflammatory and infective measures in COPD[J]. Respir Res,2018,19(1):31. doi:10.1186/s12931-018-0734-y.
[5] 郭佑民,金晨望,曹宪宪. 定量CT在慢性阻塞性肺疾病诊疗中的应用[J]. 中国医学影像技术,2020,36(3):332-334. GUO Y M,JIN C W,CAO X X. Application of quantitative CT in diagnosis and treatment of chronic obstructive pulmonary disease[J]. Chinese Journal of Medical Imaging Technology,2020,36(3):332-334. doi:10.13929/j.issn.1003-3289.2020.03.002.
[6] HOGG J C,PAR? P D,HACKETT T L. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease[J]. Physiol Rev,2017,97(2):529-552. doi:10.1152/physrev.00025.2015.
[7] 曹憲宪,金晨望,郭佑民. CT定量评估肺气肿的研究现状[J]. 临床放射学杂志,2019,38(9):1787-1790. CAO X X,JIN C W,GUO Y M. Research status of CT quantitative evaluation of emphysema[J]. Journal of Clinical Radiology,2019,38(9):1787-1790. doi:10.13437/j.cnki.jcr.2019.09.047.
[8] KONIETZKE P,WIELP?TZ M O,WAGNER W L,et al. Quantitative CT detects progression in COPD patients with severe emphysema in a 3-month interval[J]. Eur Radiol,2020,30(5):2502-2512. doi:10.1007/s00330-019-06577-y.
[9] 王强,罗勇,李君. 慢性阻塞性肺疾病患者胸部高分辨率计算机断层成像肺气肿定量指标、气道管壁定量指标与肺功能的相关性研究[J]. 上海医学,2020,43(12):734-739. WANG Q,LUO Y,LI J. Correlation between chest high-resolution computed tomography quantitative indicators of emphysema, measurements of airway wall and pulmonary function test results in patients with chronic obstructive pulmonary disease[J]. Shanghai Med J,2020,43(12):734-739. doi:10.19842/j.cnki.issn.0253-9934.2020.12.006.
[10] PARK J,HOBBS B D,CRAPO J D,et al. Subtyping COPD by using visual and quantitative CT imaging features[J]. Chest,2020,157(1):47-60. doi:10.1016/j.chest.2019.06.015.
[11] 马姣,石芳,崔涛,等. 慢性阻塞性肺疾病患者高分辨率CT下表型的临床特征分析[J]. 国际呼吸杂志,2019,39(2):86-90. MA J,SHI F,CUI T,et al. Clinical characteristics and prognosis of chronic obstructive pulmonary disease phenotype classified by high resolution computer tomography[J]. International Journal of Respiration,2019,39(2):86-90. doi:10.3760/cma.j.issn.1673-436X.2019.02.002.
[12] ZHANG L,JIANG B,WISSELINK H J,et al. COPD identification and grading based on deep learning of lung parenchyma and bronchial wall in chest CT images[J]. Br J Radiol,2022,95(1133):20210637. doi:10.1259/bjr.20210637.
[13] LYNCH D A,AUSTIN J H,HOGG J C,et al. CT-definable subtypes of chronic obstructive pulmonary disease:A statement of the fleischner society[J]. Radiology,2015,277(1):192-205. doi:10.1148/radiol.2015141579.
[14] LYNCH D A,MOORE C M,WILSON C,et al. CT-based visual classification of emphysema:Association with mortality in the COPDGene study[J]. Radiology,2018,288(3):859-866. doi:10.1148/radiol.2018172294.
[15] SCHROEDER J D,MCKENZIE A S,ZACH J A,et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema,air trapping,and airways in subjects with and without chronic obstructive pulmonary disease[J]. AJR Am J Roentgenol,2013,201(3):W460-W470. doi:10.2214/AJR.12.10102.
[16] HAN M K,BARTHOLMAI B,LIU L X,et al. Clinical significance of radiologic characterizations in COPD[J]. COPD,2009,6(6):459-467. doi:10.3109/15412550903341513.
[17] 師美娟,沈聪,于楠,等. 基于CT定量探讨不同级别慢性阻塞性肺疾病患者肺气肿肺叶分布[J]. 西安交通大学学报(医学版),2019,40(2):182-186. SHI M J,SHEN C,YU N,et al. Emphysema lobar distribution in patients with different degrees of chronic obstructive pulmonary disease based on quantitative CT analysis[J]. Journal of Xi'an Jiaotong University(Medical Sciences),2019,40(2):182-186. doi:10.7652/jdyxb201902003.
[18] WRIGHT J L,LEVY R D,CHURG A. Pulmonary hypertension in chronic obstructive pulmonary disease:Current theories of pathogenesis and their implications for treatment[J]. Thorax,2005,60(7):605-609. doi:10.1136/thx.2005.042994.
[19] 黄晓旗,牛媛,雷禹,等. 基于CT双气相定量研究吸烟合并慢性阻塞性肺疾病患者的肺叶小气道病变及肺气肿损伤程度[J]. 中华放射学杂志,2022,56(5):536-541. HUANG X Q,NIU Y,LEI Y,et al. Quantitative study on the degree of small airway disease and emphysema injury in pulmonary lobes of patients with smoking combined with chronic obstructive pulmonary disease based on biphasic CT[J]. Chin J Radiol,2022,56(5):536-541. doi:10.3760/cma.j.cn112149-20210428-00418.
[20] XU F,VASILESCU D M,KINOSE D,et al. The molecular and cellular mechanisms associated with the destruction of terminal bronchioles in COPD[J]. Eur Respir J,2022,59(5):2101411. doi:10.1183/13993003.01411-2021.
[21] TAKAYANAGI S,KAWATA N,TADA Y,et al. Longitudinal changes in structural abnormalities using MDCT in COPD:Do the CT measurements of airway wall thickness and small pulmonary vessels change in parallel with emphysematous progression?[J]. Int J Chron Obstruct Pulmon Dis,2017,12:551-560. doi:10.2147/COPD.S121405.
[22] VASILESCU D M,MARTINEZ F J,MARCHETTI N,et al. Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease[J]. Am J Respir Crit Care Med,2019,200(5):575-581. doi:10.1164/rccm.201811-2083OC.
[23] POMPE E,MOORE C M,MOHAMED HOESEIN F,et al. Progression of emphysema and small airways disease in cigarette smokers[J]. Chronic Obstr Pulm Dis,2021,8(2):198-212. doi:10.15326/jcopdf.2020.0140.
[24] HOGG J C,CHU F,UTOKAPARCH S,et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease[J]. N Engl J Med,2004,350(26):2645-2653. doi:10.1056/NEJMoa032158.
[25] QIN S,YU X,MA Q,et al. Quantitative CT analysis of small airway remodeling in patients with chronic obstructive pulmonary disease by a new image post-processing system[J]. Int J Chron Obstruct Pulmon Dis,2021,16:535-544. doi:10.2147/COPD.S295320.
[26] ZHAO D,ZHOU Y,JIANG C,et al. Small airway disease:A different phenotype of early stage COPD associated with biomass smoke exposure[J]. Respirology,2018,23(2):198-205. doi:10.1111/resp.13176.
(2022-07-25收稿 2022-10-14修回)
(本文編辑 李志芸)
