紫杉醇对实验性自身免疫性脑脊髓炎(EAE)小鼠细胞焦亡的影响及其机制的研究
2023-11-06潘婷李作孝晚丽
潘婷 李作孝 晚丽
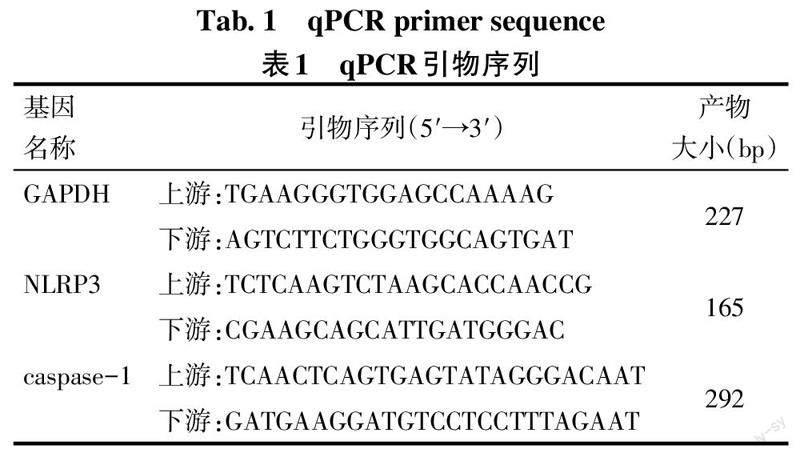
摘要:目的 探讨紫杉醇(PTX)通过抑制NLRP3/caspase-1通路减轻实验性自身免疫性脑脊髓炎(EAE)小鼠细胞焦亡的影响机制。方法 将50只雌性C57BL/6小鼠按照随机数字表法分为正常对照组、EAE模型组及PTX低、中、高剂量组,每组10只。除正常对照组外,其余组建立EAE模型。PTX低、中、高剂量组每日分别腹腔注射PTX 1、2、4 mg/(kg·d),正常对照组和EAE模型组腹腔注射等量生理盐水,连续14 d。从免疫当日开始,每日定时观察并记录小鼠体质量变化、精神、活动情况及进行神经功能障碍评分;对各组脊髓组织进行尼氏染色和LFB染色观察病理改变;荧光定量PCR(qPCR)检测脊髓组织NOD样受体热蛋白结构域相关蛋白3(NLRP3)及胱天蛋白酶(caspase)-1 mRNA表达水平。酶联免疫吸附试验(ELISA)检测外周血白细胞介素(IL)-18和IL-1β含量。结果 正常对照组小鼠无明显症状,EAE模型组和PTX低、中、高剂量组体质量下降、活动减少、反应迟钝。与EAE模型组相比,PTX各剂量组发病潜伏期延长,高峰期延迟,神经功能障碍评分降低(P<0.05),脊髓组织尼氏小体数量增多且相对规则,脱髓鞘、空泡改变减轻,外周血IL-18、IL-1β含量降低,NLRP3、caspase-1 mRNA表达水平降低(P<0.05);PTX剂量越高,发病潜伏期和高峰期延迟越明显;脊髓组织尼氏小体数量增多,排列及形态更规则,脱髓鞘及空泡改变越轻,外周血IL-1β、IL-18含量越低,caspase-1 mRNA表達水平越低(P<0.05)。结论 PTX可能通过抑制细胞焦亡,减轻EAE小鼠炎症反应,其机制可能与抑制NLRP3/caspase-1通路有关。
关键词:多发性硬化;脑脊髓炎,自身免疫性,实验性;细胞焦亡;紫杉醇;NLRP3/caspase-1通路
中图分类号:R744.51文献标志码:ADOI:10.11958/20221146
Study on the effect and mechanism of paclitaxel on pyroptosis in mice with experimental autoimmune encephalomyelitis
PAN Ting, LI Zuoxiao WAN Li
Department of Neurology, the Affiliated Hospital of Southwest Medical University, Luzhou 646000, China
△Corresponding Author E-mail: lzx3235@sina.com
Abstract: Objective To investigate the effect of taxol (PTX) on pyroptosis of experimental autoimmune encephalomyelitis (EAE) mice by inhibiting NOD-like receptor thermoprotein domain-associated protein 3 (NLRP3)/caspase-1 pathway. Methods Fifty female C57BL/6 mice were randomly divided into the normal control group, the EAE model group, the PTX low-dose, medium-dose and high-dose groups according to the random number table method, with 10 mice in each group. EAE model was established in other groups except the normal control group. PTX low-dose, medium-dose and high-dose groups were intraperitoneally injected with 1, 2 and 4 mg/kg PTX everyday, respectively, and normal control group and EAE model group were intraperitoneally injected with the same amount of normal saline for 14 consecutive days. From the day of immunization, changes of body weight, mental state, activity state and neurological dysfunction score of mice were observed and recorded regularly every day. Nissl staining and LFB staining were used to observe pathological changes. The mRNA expression levels of NLRP3 and caspase-1 in crushed tissues were detected by quantitative fluorescence PCR (qPCR). Levels of interleukin (IL) -18 and IL-1β in peripheral blood were detected by enzyme-linked immunosorbent assay (ELISA). Results There were no obvious symptoms in mice of the normal control group. Mice in the EAE model group and the PTX low-, medium-, and high-dose groups showed decreased body weight, decreased activity and sluggish response. Compared with the EAE model group, the latency and peak period were delayed in the PTX dose groups, the neurological dysfunction score at peak period was decreased (P<0.05). The number of Nyssome in spinal cord tissue was increased and relatively regular, demyelination and vacuolar changes were alleviated, and the mRNA expression levels of NLRP3 and caspase-1 were decreased. The higher the PTX dose, the more obvious the latency and peak delay. The number of Nyssome in spinal cord increased, the arrangement and morphology were more regular, the demyelination and vacuolation changes were lighter, the mRNA expression level of caspase-1 and the contents of IL-1β and IL-18 in peripheral blood were lower (P<0.05). Conclusion PTX may reduce the damage of pyroptosis in EAE mice by inhibiting NLRP3/caspase-1 pathway related inflammatory factors.
Key words: multiple sclerosis; encephalomyelitis, autoimmune, experimental; pyroptosis; paclitaxel; NLRP3/caspase-1 pathway
多发性硬化(multiple sclerosis,MS)是一种自身免疫性疾病,主要以神经系统脱髓鞘和神经退行性变为特征[1]。目前MS发病趋年轻化且易复发,治疗手段有限[2-4]。实验性自身免疫性脑脊髓炎(experimental autoimmune encephalomyelims,EAE)可破坏血脑屏障并使其通透性增高,病理改变与MS相似,可作为研究MS常用的动物模型[5]。细胞焦亡是细胞在胱天蛋白酶(caspase)家族调控下介导的炎症反应[6]。当细胞受到刺激时,细胞内的受体识别相应的配体,促使NOD样受体热蛋白结构域相关蛋白3(NLRP3)、凋亡相关斑点样蛋白(ASC)及caspase-1组成NLRP3炎性小体,炎性小体剪切活化caspase-1前体,caspase-1活化后,使gasdermin D(GSDMD)结合细胞膜形成裂孔,同时活化白细胞介素(IL)-18、IL-1β,促进细胞焦亡[7]。研究表明,MS炎症反应与NLRP3炎性小体信号通路及细胞焦亡关系密切[8]。紫杉醇(paclitaxel,PTX)广泛用于肿瘤疾病治疗[9],也可调节炎症反应[10],但其对EAE小鼠细胞焦亡炎症影响的研究较少。本研究旨在观察PTX对EAE模型小鼠的影响,探讨其作用机制是否与NLRP3/caspase-1信号通路介导的细胞焦亡途径有关。
1 材料与方法
1.1 实验动物 SPF级C57BL/6健康雌性小鼠50只,6~8周龄,体质量20~25 g,购于武汉恒意赛生物科技有限公司。动物生产许可证号:SCXK(鄂)2020-0018,由西南医科大学忠山动物实验室统一饲养。环境通风清洁、室温26 ℃左右,维持湿度50%左右,12 h明/暗循环,进食及饮水自由。实验操作符合西南医科大学实验动物福利伦理(20210601-4)。
1.2 主要试剂及仪器 PTX购自武汉阿拉丁生物有限公司,MOG35-55多肽购自国泰生物,灭活结核分枝杆菌购自BD公司,完全弗氏佐剂(CFA)购自Sigma,RNA提取试剂盒、逆转录及PCR试剂盒、IL-18和IL-1β酶联免疫吸附试验(ELISA)检测试剂盒均购自ELK Biotechnology;普通光学显微镜(OLYMPUS公司,型号CX21)、台式离心机(上海安亭科学仪器厂)、荧光定量PCR仪(Life technologies,型号StepOneTM Real-Time PCR System)、冷冻离心机(湖南湘仪实验室仪器开发有限公司)等。
1.3 方法
1.3.1 实验动物分组及EAE小鼠模型建立 按照随机数字表法将50只小鼠分为正常对照组、EAE模型组及PTX低、中、高剂量组,每组10只。MOG35-55多肽用生理盐水稀释成5 g/L,再与相同体积的CFA和一定量灭活结核分支杆菌混合,形成油包水乳剂,配制成4 g/L的结核杆菌H37Ra溶液,于除正常对照组外的其他各组小鼠于脊柱两侧选取4点予以0.1 mL皮下注射完成造模,正常对照组注射等量生理盐水。
1.3.2 药物干预 实验药物剂量参照体表面积等效剂量比换算,PTX低、中、高剂量组小鼠于免疫当日开始分别给药PTX 1、2、4 mg/(kg·d),正常对照组和EAE模型组同时给予等量生理盐水,均采用腹腔注射给药,连续14 d。
1.3.3 发病情况观察 从免疫当日开始,每日定时观察并记录小鼠体质量变化、精神、活动情况;各组小鼠按Kono评分标准进行神经功能障碍评分:0分,无相关临床症状;1分,尾部力量下降;2分,双侧后肢无力,可自主翻身;3分,双侧后肢瘫痪,不可自主翻身;4分,四肢瘫痪;5分,濒死或者死亡;症状介于两者则±0.5分。小鼠自造模开始至出现症状时判定为发病潜伏期,神经功能障碍评分连续3日不增加,四肢瘫痪甚至死亡判定为发病高峰期。
1.3.4 取材及标本处理 发病小鼠于发病高峰期眼球取血后处死、取材,未发病小鼠及正常对照组小鼠于造模后28 d眼球取血后处死、取材。(1)血清标本:采用眼球取血,离心收集血清。(2)病理取材:小鼠于麻醉后开胸,充分暴露心脏,注入生理盐水充分冲洗,予以40 g/L多聚甲醛固定,随后断头处理,取出脊髓组织,放置于固定液中浸泡1 d,固定标本采用常规石蜡包埋,以脊髓腰膨大处取样连续切片,切片厚度约4 μm,每组选取其中3张用于尼氏染色,另选3张用于LFB染色。
1.3.5 脊髓组织病理学观察 尼氏染色:将石蜡切片脱蜡至水、组化笔圈画组织、纯水洗脱、尼氏染色液染色、纯水洗脱、晾干后中性树胶封片、尼氏小體计数。LFB染色:石蜡切片脱蜡至水、组化笔圈画组织、纯水洗脱、LFB染色液染色、乙醇分化纯水洗脱、脱水封片。
1.3.6 荧光定量PCR(qPCR)检测小鼠NLRP3、caspase-1 mRNA表达 提取各组脊髓组织总RNA,根据逆转录试剂盒合成cDNA,由武汉金开瑞生物工程有限公司设计并合成NLRP3、caspase-1引物,见表1。根据扩增条件在PCR仪上进行基因片段扩增。PCR反应体系:2×PCR Master Mix 5.0 μL,引物工作液(2.5 μmol/L)上下游各0.5 μL,cDNA模板1.0 μL,ddH2O 2.0 μL,Rox 1.0 μL。反应条件:95 ℃预变性1 min;95 ℃变性15 s,58 ℃退火20 s,72 ℃延伸45 s,循环重复40次;测得Ct值后,采用2-ΔΔCt法计算相对表达量。
1.3.7 ELISA检测IL-18和IL-1β含量 按照ELISA试剂盒说明书检测血清中IL-18和IL-1β的表达量。
1.4 統计学方法 采用SPSS 26.0软件进行数据分析,计量资料以均数±标准差(x±s)表示,多组间比较采用单因素方差分析,组间多重比较采用LSD-t检验,P<0.05为差异有统计学意义。
2 结果
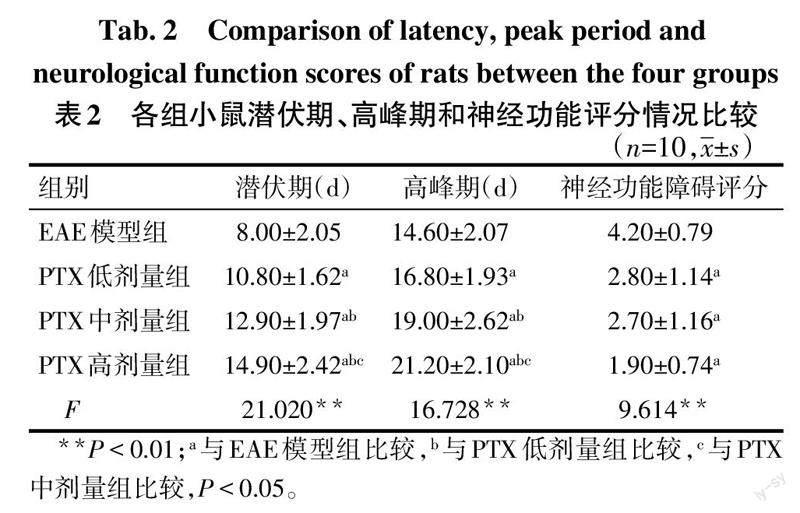
2.1 PTX对各组小鼠行为学和神经功能的影响 正常对照组小鼠无明显症状。EAE模型组和PTX低、中、高剂量组体质量下降、活动减少、反应迟钝,以尾部力量降低为先发症状。与EAE模型组比较,PTX各剂量组小鼠发病潜伏期延长,高峰期延迟,神经功能障碍评分下降(P<0.05);PTX低、中、高剂量组发病潜伏期逐渐延长,高峰期逐渐延迟(P<0.05),神经功能障碍评分差异无统计学意义,见表2。
2.2 PTX对脊髓组织病理学影响 尼氏染色结果显示,正常对照组小鼠脊神经元分布及形态未见明显异常;EAE模型组小鼠病程高峰期脊髓神经细胞中炎性细胞增多,尼氏小体数量减少、形态及排列不规则;PTX各剂量组尼氏小体数量较EAE模型组增多且相对规则,随着PTX剂量升高,尼氏小体数量增多,排列及形态更规则,见图1。LFB染色结果显示,正常对照组未见明显脱髓鞘及空泡改变,EAE模型组和PTX各剂量组存在不同程度脊髓组织脱髓鞘和空泡样改变,且PTX剂量越高,脱髓鞘及空泡改变越少,见图2。
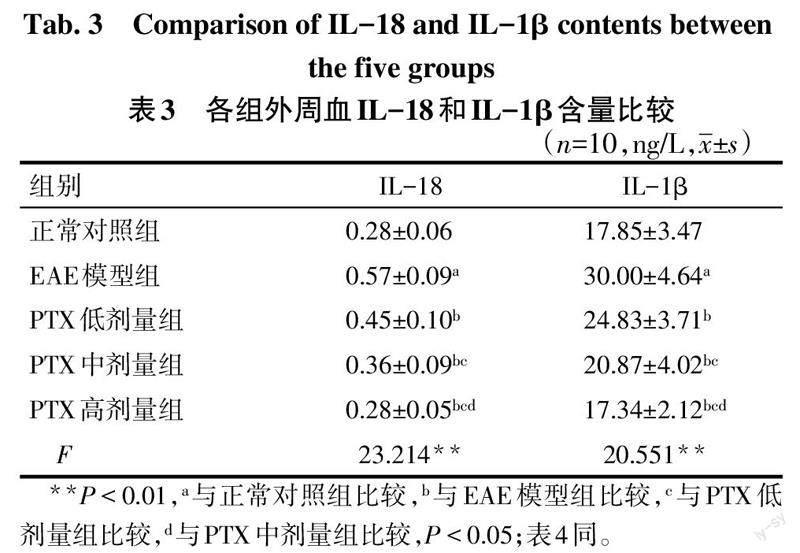
2.3 各组小鼠外周血IL-1β、IL-18含量变化 与正常对照组相比,EAE模型组小鼠外周血IL-1β、IL-18含量增加(P<0.05);与EAE模型组相比,PTX各剂量组小鼠外周血IL-1β、IL-18含量降低,且PTX剂量越高,小鼠外周血IL-1β、IL-18含量越低(P<0.05),见表3。
2.4 各组小鼠脊髓组织NLRP3、caspase-1 mRNA表达变化 与正常对照组相比,EAE模型组小鼠脊髓组织NLRP3、caspase-1 mRNA水平升高(P<0.05);与EAE模型组相比,PTX各剂量组NLRP3、caspase-1 mRNA表达水平降低(P<0.05);与PTX低剂量组比较,PTX中剂量组caspase-1 mRNA水平降低,PTX高剂量组NLRP3、caspase-1 mRNA水平降低(P<0.05);与PTX中剂量组相比,PTX高剂量组caspase-1 mRNA水平降低(P<0.05)。见表4。
3 讨论
MS发病主要损害中枢神经系统,是青年人群非创伤性神经功能障碍的主要原因之一。现阶段MS尚缺乏有效药物防治,治疗费用高昂,且不可完全逆转疾病发展。PTX是一种从红豆杉植物中提取的萜类次生代谢产物,PTX及其衍生物具有免疫调节、抗炎等功能,临床应用于多种肿瘤,如卵巢癌、乳腺癌、非小细胞肺癌等疾病[11]。研究表明其也可能是阿尔茨海默病[12]、风湿性关节炎[13]的潜在治疗药物。PTX的免疫调节机制可能与抑制有丝分裂有关,PTX可作用于微管系统,影响微管聚合,抑制微管降解,使细胞分裂停滞于G2/M期,诱导多种癌细胞的凋亡或焦亡。有研究表明,PTX通过影响微管蛋白及其二聚体的动态平衡来诱导肿瘤细胞的程序性死亡[14];其作为一种微管稳定药物,通过诱导α-微管蛋白乙酰化,增强NLRP3炎症小体的激活,诱导细胞发生凋亡或焦亡[10,15]。已有研究提出,抗肿瘤药物(如阿伦托珠单抗、奥瑞组单抗等)可用于MS治疗,降低MS复发率,延缓残疾进展[16-18]。本研究探究PTX是否减轻MS脊髓炎症反应,发现PTX能够有效减轻EAE小鼠的临床症状,降低神经功能障碍评分,减轻EAE小鼠中枢神经系统炎症、脱髓鞘及空泡改变,PTX剂量越高,发病潜伏期越长、高峰期推迟越明显,且中枢神经系统炎症、脱髓鞘及空泡改变越轻。
正常情况下,炎性因子发挥清除、修复、保护等功能,对机体产生积极影响[19]。MS持续自身免疫性炎症可导致细胞焦亡启动,炎性细胞过度激活,各种细胞因子透过血脑屏障,参与局部中枢神经系统炎症调节[20-21]。在EAE小鼠中,细胞焦亡诱发炎症,促进T细胞分化为Th1和Th17细胞,加重炎症及脱髓鞘表现[22]。研究发现,细胞焦亡与MS及EAE密切相关[8,22],NLRP3炎性小体的激活在细胞焦亡经典途径中发挥重要作用[23]。caspase-1抑制剂VX-765可抑制其激活,减轻EAE小鼠炎症反应[24]。本研究发现,与正常对照组相比,EAE小鼠脊髓组织NLRP3 mRNA、caspase-1 mRNA表达明显升高,外周血IL-1β、IL-18含量显著增加。说明EAE小鼠的发病机制与NLRP3/caspase-1通路介导的细胞焦亡密切相关。PTX干预后降低了EAE小鼠脊髓组织NLRP3 mRNA、caspase-1 mRNA表达水平及外周血IL-1β、IL-18含量,且剂量越高变化越明显,说明PTX可能通过抑制NLRP3/caspase-1通路减少细胞焦亡,减轻对EAE小鼠的损伤。
综上所述,PTX可能通过抑制细胞焦亡减轻EAE小鼠炎症反应,其机制可能与抑制NLRP3/caspase-1通路有关。PTX对EAE小鼠发挥保护作用的机制是否与其他通路相关有待进一步研究。PTX用于MS的治疗尚无明确报道,本实验可能为MS的治疗提供新的方向。
参考文献
[1] RODR?GUEZ MUR?A S,FAREZ M F,QUINTANA F J. The immune response in multiple sclerosis[J]. Annu Rev Pathol,2022,17:121-139. doi:10.1146/annurev-pathol-052920-040318.
[2] GHOLAMZAD M,EBTEKAR M,ARDESTANI M S,et al. A comprehensive review on the treatment approaches of multiple sclerosis:Currently and in the future[J]. Inflamm Res,2019,68(1):25-38. doi:10.1007/s00011-018-1185-0.
[3] AFSHAR B,KHALIFEHZADEH-ESFAHANI Z,SEYFIZADEH N,et al. The role of immune regulatory molecules in multiple sclerosis[J]. J Neuroimmunol,2019,337:577061. doi:10.1016/j.jneuroim.2019.577061.
[4] YAMOUT B I,ALROUGHANI R. Multiple sclerosis[J]. Semin Neurol,2018,38(2):212-225. doi:10.1055/s-0038-1649502.
[5] SMITH P. Animal models of multiple sclerosis[J]. Curr Protoc,2021,1(6):e185. doi:10.1002/cpz1.185.
[6] SHARMA B R,KANNEGANTI T D. NLRP3 inflammasome in cancer and metabolic diseases[J]. Nat Immunol,2021,22(5):550-559. doi:10.1038/s41590-021-00886-5.
[7] WANG L,HAUENSTEIN A V. The NLRP3 inflammasome:Mechanism of action,role in disease and therapies[J]. Mol Aspects Med,2020,76:100889. doi:10.1016/j.mam.2020.100889.
[8] MCKENZIE B A,FERNANDES J P,DOAN M,et al. Activation of the executioner caspases-3 and -7 promotes microglial pyroptosis in models of multiple sclerosis[J]. J Neuroinflammation,2020,17(1):253. doi:10.1186/s12974-020-01902-5.
[9] ALQAHTANI F Y,ALEANIZY F S,EL TAHIR E,et al. Paclitaxel[J]. Profiles Drug Subst Excip Relat Methodol,2019,44:205-238. doi:10.1016/bs.podrm.2018.11.001.
[10] LANGE B M,CONNER C F. Taxanes and taxoids of the genus Taxus-A comprehensive inventory of chemical diversity[J]. Phytochemistry,2021,190:112829. doi:10.1016/j.phytochem.2021.112829.
[11] LI L,JIANG M,QI L,et al. Pyroptosis,a new bridge to tumor immunity[J]. Cancer Sci,2021,112(10):3979-3994. doi:10.1111/cas.15059.
[12] 陳庆昌. 多功能纳米复合材料负载MDR-siRNA逆转肿瘤细胞多药耐药性及多金属氧酸盐抑制Aβ聚集的研究[D]. 广州:暨南大学,2015. CHEN Q C. Reverse multidrug resistance of tumor cells by MDR-siRNA loaded by multifunctional nanocomposite and inhibition of Aβ aggregation by polyoxometalate[D]. Guangzhou:Jinan University,2015.
[13] SHENG Z,ZENG J,HUANG W,et al. Comparison of therapeutic efficacy and mechanism of paclitaxel alone or in combination with methotrexate in a collagen-induced arthritis rat model[J]. Z Rheumatol,2022,81(2):164-173. doi:10.1007/s00393-020-00940-x.
[14] WANG X,LIU X,LI Y,et al. Sensitivity to antitubulin chemotherapeutics is potentiated by a photoactivable nanoliposome[J]. Biomaterials,2017,141:50-62. doi:10.1016/j.biomaterials.2017.06.034.
[15] ZENG Q Z,YANG F,LI C G,et al. Paclitaxel enhances the innate immunity by promoting NLRP3 inflammasome activation in macrophages[J]. Front Immunol,2019,10:72. doi:10.3389/fimmu.2019.00072.
[16] GRAF J,ALBRECHT P,GOEBELS N,et al. Ocrelizumab for treatment of multiple sclerosis[J]. Nervenarzt,2020,91(8):722-734. doi:10.1007/s00115-020-00937-6.
[17] VARYT? G,ARLAUSKIEN? A,RAMA?AUSKAIT? D. Pregnancy and multiple sclerosis:An update[J]. Curr Opin Obstet Gynecol,2021,33(5):378-383. doi:10.1097/GCO.0000000000000731.
[18] SYED Y Y. Alemtuzumab:A review in relapsing remitting multiple sclerosis[J]. Drugs,2021,81(1):157-168. doi:10.1007/s40265-020-01437-2.
[19] DISABATO D J,QUAN N, GODBOUT J P. Neuroinflammation:The devil is in the details[J]. J Neurochem,2016,139(Suppl 2):136-153. doi:10.1111/jnc.13607.
[20] ROTHHAMMER V,QUINTANA F J. Control of autoimmune CNS inflammation by astrocytes[J]. Semin Immunopathol,2015,37(6):625-638. doi:10.1007/s00281-015-0515-3.
[21] LIU B,GU Y,PEI S,et al. Interleukin-1 receptor associated kinase(IRAK)-M -mediated type 2 microglia polarization ameliorates the severity of experimental autoimmune encephalomyelitis(EAE)[J]. J Autoimmun,2019,102:77-88. doi:10.1016/j.jaut.2019.04.020.
[22] 娄雅琳. Zhx2激活巨噬细胞Nlrp3炎性体诱导焦亡参与EAE的作用及机制研究[D]. 济南:山东大学,2021. LOU Y L. Study on the role and mechanism of Zhx2-activated macrophage Nlrp3 inflammasome to induce pyroptosis in EAE[D]. Jinan:Shandong University,2021. doi:10.27272/d.cnki.gshdu.2021.004558.
[23] ZHEN Y,ZHANG H. NLRP3 Inflammasome and inflammatory bowel disease[J]. Front Immunol,2019,10:276. doi:10.3389/fimmu.2019.00276.
[24] MCKENZIE B A,MAMIK M K,SAITO L B,et al. Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis[J]. Proc Natl Acad Sci U S A,2018,115(26):E6065-E6074. doi:10.1073/pnas.1722041115.
(2022-07-21收稿 2022-08-15修回)
(本文編辑 李志芸)
