Effect of Substitution Positions of Alkyl Chains in Small Molecular Donor Bridged Units on the Performance of Photovoltaic Devices
2023-10-10GUOZiqiJIAOCancanWUSiminMENGLingxianSUNYannaKEXinWANXiangjianCHENYongsheng
GUO Ziqi, JIAO Cancan, WU Simin, MENG Lingxian, SUN Yanna, KE Xin, WAN Xiangjian*, CHEN Yongsheng*
Effect of Substitution Positions of Alkyl Chains in Small Molecular Donor Bridged Units on the Performance of Photovoltaic Devices
GUOZiqi1, JIAOCancan1, WUSimin1, MENGLingxian2*, SUNYanna1, KEXin1, WANXiangjian1*, CHENYongsheng1*
(,,,,300071,;,,450001,)
Two isomeric small molecule donors, C2-C-F and C2-M-F, with only the different substitution positions of the alkyl chains on the intermediate bridged trithiophene units, were designed and synthesized. It was found that the alkyl chain substitution position has little effect on their absorptions and energy levels, but has a significant impact on the morphology of the active layers when blended with the acceptor BTP-4F-12. Better morphology was obtained for the active layer based on small molecule donor C2-C-F, and an efficiency of 12.84% was achieved for the C2-C-F-based photovoltaic device. This result indicates that the morphology of the active layer can be finely regulated through the alkyl substitution positions, providing a useful avenue for the design of efficient small molecule donors.
All-small-molecule organic solar cell; Alkyl chain; Small molecule donor
Organic solar cells(OSCs) have attracted significant attention due to their excellent properties such as low cost, light weight, flexibility, etc.[1—3]. Recently, with the emergence of Y6 and so on[4,5], power conversion efficiencies(PCEs) over 19% have been achieved for the polymer-based devices with active layer mate- rials designing and devices engineering[6—10]. However, polymer-based OSCs generally suffer from the batch-to-batch issue of polymers and thus the variation of device performance[11—13]. In contrast, small molecules exhibit unique advantages of definite chemical structures, less batch-to-batch variation and better device performance repeatability[14—16]. However, the PCEs of all-small-molecule organic solar cells(ASM-OSCs) still lag behind those of polymer-based OSCs[17—20]. Compared with polymer-based OSCs, the morphology of ASM-OSCs is difficult to regulate since small molecules are hard to form pre-aggregation in the solution and most of efficient small molecule donors and acceptors have the similar acceptor-donor-acceptor(A-D-A) architecture.
Among various methods for regulating morphology, alkyl chains engineering, such as the length of side chains, straight or branched chains and halogenation on the alkyl chains, have proved to be an effective strategy. The fine-tuning of alkyl chain could adjust the molecular planarity and solubility, optimize the aggregation and crystallinity. For example, Wei’s group[21]achieved a PCE of 14.78% by adjusting the branch points of side chain to control the crystallinity and miscibility of ZR2-C3. Hou’s group[22]increased the length of end-group alkyl chains to investigate the molecular orientation and intermolecular aggregation behavior. After optimizing, the devices based on DRTB-T-C4 obtained the highest PCE, which is mainly attributed to the better short-circuit current density(sc) and fill factor(FF). Ge’s group[23]reported two donors by fluorinating the donor DCAO3TBDTT to further optimize the blend morphology. After fluorinating, the devices based on BTEC-2F obtained a PCE of 13.34%, which is mainly attributed to the enhanced phase separation and compact molecular stacking of active layers.
In our previous works, we have designed a series of acceptor-donor-acceptor(A-D-A) type small-molecule donors based on benzodithiophene(BDT) and studied the impact of alkyl chains at central core and end groups on device performance[24,25]. Based on these, in this work, two A-D-A type small-molecule donors, C2-C-F and C2-M-F, were synthesized and blended with the acceptor BTP-4F-12[26]to investigate the influence on photovoltaic properties caused by changing the alkyl chain substitution position at-bridges. It was found that the alkyl chain substitution position has little effect on their absorptions and energy levels, but has a significant impact on the morphology of the active layers. The blend film with C2-C-F∶BTP-4F-12 possessed denser packing and stronger crystallinity. After optimizing, the device based on C2-C-F achieved a PCE of 12.84% with an FF of 62.32% and ascof 23.68 mA/cm2, higherthan the devices based on C2-M-F with a PCE of 11.84% with an FF of 59.51% andscof 21.57 mA/cm2.
1 Experimental
All the reactions and manipulations were carried out under argon atmosphere with the use of standard schlenk techniques. All starting materials were purchased from commercial suppliers and used without further purification unless indicated otherwise. The experimental details were provided in the Supporting Information, including the synthetic route and structural characterization of the molecules, fabrication of the devices, the measurements of cyclic voltammogram(CV), the UV-Visible spectroscopy, photoluminescence spectroscopy, the space-charge-limited current(SCLC) mobility, atomic force microscope(AFM) and grazing incidence wide angle X-ray scattering(GIWAXS).
2 Results and Discussion
The materials used to fabricate the ASM-OSCs were depicted in Fig.1(A). The synthetic routes of C2-C-F and C2-M-F are showed in the Supporting Information of this paper and the characterization data are also provided. The energy level of two donors were measured by means of cyclic voltammetry[27]. Fig.1(B) showed the corresponding energy level diagram of two donors. The HOMO and LUMO energy levels were calculated by onset oxidation(Ox) and reduction(Red) potentials from the equationHOMO/LUMO=-e[Ox/Red+(4.8 -Fc/Fc+)], whereFc/Fc+is the redox potential of Fc/Fc+. According to the equation and as shown in Fig.S1(see the Supporting Information of this paper), the HOMO/LUMO energy levels of C2-C-F and C2-M-F were calculated as ‒5.29/‒3.62 eV and ‒5.33/‒3.67 eV, corresponding to the band gap of 1.67 and 1.66 eV, respectively. Density functional theory(DFT) was performed to calculate the energy levels and optimal geometries of two donors, as shown in Fig.S2(see the Supporting Information of this paper), and the DFT-calculated HOMO and LUMO energy level of C2-C-F and C2-M-F are ‒5.06/‒2.87 and ‒5.03/‒2.85 eV. As shown in Fig.S3(see the Supporting Information of this paper), compared with the C2-M-F, the optimal geometries of C2-C-F shows better planarity with smaller dihedral angles of 3.13°, 9.98°, 7.12° and 3.19° between the bridged trithiophene units, which were favorable for the ordered packing and better carrier transport.
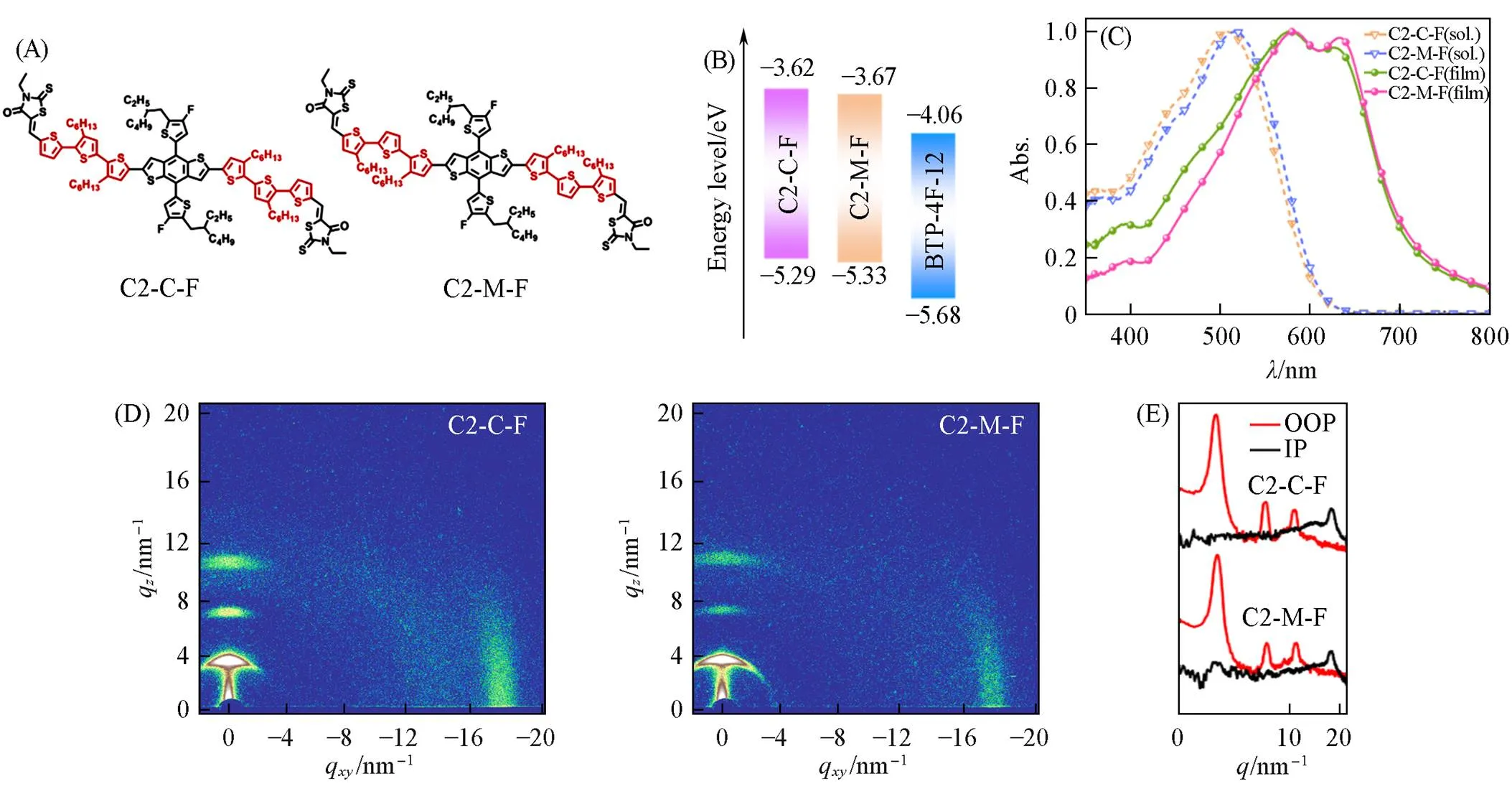
Fig.1 Chemical structures(A), energy level diagrams(B), normalized UV⁃Vis absorption spectra(C) and 2D GIWAXS of neat film(D) of C2⁃C⁃F, C2⁃M⁃F and 1D plots of the neat films in the in⁃plane and out⁃of⁃plane direction(F)
Fig.1(C) depicted the normalized absorption spectra of two donors in chloroform solution and neat films. Both C2-C-F and C2-M-F showed good absorption in short wavelength range(450—690 nm). Compared with the absorption in solution, the maximum absorption peaks of C2-C-F and C2-M-F in neat film showed obvious redshifts to 582 and 578 nm, respectively. Besides, the shoulder peaks were located at 633 and 627 nm, which indicates strong intermolecular interaction and aggregation in these two films[28,29]. The absorption onsets of the C2-C-F and C2-M-F were 719 and 727 nm, and the corresponding optical bandgaps were 1.73 and 1.71 eV, respectively[Table S1(see the Supporting Information of this paper)].
In order to investigate the difference of molecular packing information between two donors, the GIWAXS measurements were employed. The two-dimensional(2D) diffraction images of two donors’ neat films and the corresponding out-of-plane(OOP) and in-plane(IP) curves were depicted in Fig.1(D) and (E). As can be seen, both neat films showed strong-stacking diffraction peaks(010) in OOP direction and multiple lamellar packing diffraction(100, 200 and 300) in IP direction, implying a high degree of molecular ordering and a predominant edge-on orientation. In addition, the film of C2-C-F showed the more compact-stacking at 17.8 nm‒1(=0.353 nm) than the film of C2-M-F at 17.6 nm‒1(=0.357 nm), indicating that the C2-C-F had a closer-stacking, which may be ascribed to its better planarity and was beneficial to accelerate the hole transport behaviour.
The narrow bandgap material BTP-4F-12[Fig.2(A)] was selected as acceptor to blend with the donors for fabricating the ASM-OSCs. The devices with conventional structure[Fig.2(B)] of indium tin oxide(ITO)/poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate)(PEDOT∶PSS)/active layer/perylene diimide functionalized with amino N-oxide(PDINO)/Al were fabricated to investigate the effect of different position of side chains on photovoltaic properties. Current density-voltage(-) characteristic curves of the two ASM-OSCs were depicted in Fig.2(C) and the detailed photovoltaic performance data were listed in Table 1 for a clear comparison. The optimized device based on C2-C-F∶BTP-4F-12 obtained a PCE of 12.84% with aocof 0.871 V, ascof 23.68 mA/cm2and an FF of 62.32%, while the devices based on C2-M-F∶BTP-4F-12 attained a PCE of 11.41% with aocof 0.889 V, ascof 21.57 mA/cm2and an FF of 59.51%.

Fig.2 Chemical structure of BTP⁃4F⁃12(A), conventional device structure of ASM⁃OSCs(B), J⁃V curves of optimized ASM⁃OSCs based on C2⁃C⁃F and C2⁃M⁃F with TA treatment at 120 ℃ for 5 min under one sun illumination(AM 1.5 G 100 mW/cm2)(C) and EQE spectra of the corresponding devices(D)
The external quantum efficiency(EQE) curves of the optimal devices based on C2-C-F∶BTP-4F-12 and C2-M-F∶BTP-4F-12 are shown in Fig.2(D). The integral current densities obtained for devices based on C2-C-F and C2-M-F are 22.91 and 20.98 mA/cm2which consistent with thescvalue from the solar simulator(within 5% errors) and displayed in Table 1. As can be seen from Fig.2(D), the devices based on C2-C-F exhibited a stronger photo-response than that of the C2-M-F-based devices in 450—850 nm, which could be attributed to the stronger-interactions and improved morphology, indicating more effective exciton separation, higher charge transport and collection efficiency in C2-C-F-based device[30].

Table 1 Optimized photovoltaic data of C2-C-F and C2-M-F based devices under the illumination of AM 1.5G(100 mW·cm‒2)
* The PCE value was calculated from 15 devices.
In order to study the exciton dissociation and charge transfer behaviors in blend films, photoluminescence(PL)-quenching experiment was performed. As depicted in Fig.3(A) and (B), when exciting donors at 540 nm, the PL-quenching efficiencies of the optimized blend films of C2-C-F∶BTP-4F-12 and C2-M-F∶BTP-4F-12 were 99.56% and 92.41%, respectively. When exciting acceptor at 780 nm, the PL- quenching efficiencies of two blend films based on C2-C-F and C2-M-F were 90.68% and 88.66%, respectively. The higher PL quenching efficiency of the C2-C-F∶BTP-4F-12 blend film was consistent with the higherscand EQE response, which indicated more efficient exciton dissociation and photoinduced electron charge transfer between C2-C-F and BTP-4F-12[31,32].

Fig.3 PL spectra of neat films of two donors and blend films excited at 540 nm (A) and neat film of BTP⁃4F⁃12 and blend films exited at 780nm(B), Jphvs.Veff measurement of the devices(C), dependence of Voc(D) and Jsc(E) on light intensity(Plight) of two optimized ASM⁃OSCs
In order to investigate the exciton dissociation and charge extraction processes, the dependence of photocurrent density(ph)the effective voltage(eff) of these two devices were measured[Fig.3(C)][33].phwas defined asph=L-D, whereLandDrepresented the photocurrent density under illumination and dark conditions, respectively. The effective voltageeffwas defined aseff=0-bias, where0was the voltage at whichphwas zero andbiaswas the applied external voltage bias. The value of(E,T)[(E,T)=ph/sat, wheresatwas the saturation photocurrent density] represented the charge dissociation(diss) and collection probability. Under the short-circuit condition, thedissof devices based on C2-C-F∶BTP-4F-12 and C2-M-F∶BTP-4F-12 were 94.25% and 91.68%, respectively. Efficient exciton dissociation and charge extraction process contributes to the highscandin C2-C-F∶BTP-4F-12 device.

To further study the morphological characteristics of the active layer, the atomic force microscopy(AFM) and grazing-incidence wide-angle X-ray scattering(GIWAXS) measurements were carried out. The AFM images of C2-C-F and C2-M-F blended with BTP-4F-12 were showed in Fig.4(A) and (B). The roughness value decreased from 2.33 to 2.14 nm when the alkyl chain close to the end group(C2-M-F) moving to the middle thiophene(C2-C-F). The smaller roughness value indicated the smoother surface morphology of C2-C-F∶BTP-4F-12 film. As show in Fig.4(C)—(E), both blend films exhibited-stacking diffraction (010) peaks in OOP and a lamellar diffraction (100) peaks in IP direction, implying both blend films preferred face-on orientation. Furthermore, the blend film with C2-C-F has a smaller-stacking distance of 0.349 nm(-diffraction peak at 18.0 nm‒1) compared to 0.351 nm of blend film with C2-M-F, demonstra-ting that the C2-C-F-based blend film lead to stronger-stacking, which is beneficial for transport carriers and exciton extraction, supporting the higherJand FF values[38,39].

Fig.4 AFM images for the blend films based on optimal C2⁃C⁃F∶BTP⁃4F⁃12(A) and C2⁃M⁃F∶BTP⁃4F⁃12(B), 2D⁃GIWAXD diffraction images of C2⁃C⁃F∶BTP⁃4F⁃12(C) and C2⁃M⁃F∶BTP⁃4F⁃12(D) blend films and 1D plots of the blend films in the in⁃plane and out of plane direction(E)
3 Conclusions
In summary, two small-molecule donors C2-C-F and C2-M-F were synthesized to investigate the effect of alkyl chain substitution position at the-bridge on the molecular properties and photovoltaic performance. It was found that, the C2-C-F-based devices shows better planarity and closer-stacking, leading to more efficient charge transfer, less charge recombination and higher carrier mobility than C2-M-F-based device. This work demonstrates a facile strategy to fine-tune the molecular structure for achieving suitable morphology and high efficiency ASM-OCSs.
The supporting information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20230180.
[1] Zhou Z., Liu W., Zhou G., Zhang M., Qian D., Zhang J., Chen S., Xu S., Yang C., Gao F., Zhu H., Liu F., Zhu X.,.,2020,(4), 1906324
[2] Sun Y., Meng L., Wan X., Guo Z., Ke X., Sun Z., Zhao K., Zhang H., Li C., Chen Y.,.,2021,(16), 2010000
[3] Chen S., Wang Y., Zhang L., Zhao J., Chen Y., Zhu D., Yao H., Zhang G., Ma W., Friend R. H., Chow P. C. Y., Gao F., Yan H.,.,2018,(45), 1804215
[4] Nie Q., Tang A., Guo Q., Zhou E.,,2021,, 106174
[5] Guo Q., Guo Q., Geng Y., Tang A., Zhang M., Du M., Sun X., Zhou E.,,2021,(8), 3257—3280
[6] Meng L., Liang H., Song G., Li M., Huang Y., Jiang C., Zhang K., Huang F., Yao Z., Li C., Wan X., Chen Y.,.,2023,(3), 808—815
[7] Chen T., Li S., Li Y., Chen Z., Wu H., Lin Y., Gao Y., Wang M., Ding G., Min J., Ma Z., Zhu H., Zuo L., Chen H.,.,2023,(21), 2300400
[8] Xu X.,Jing W., Meng H., Guo Y., Yu L., Li R., Peng Q.,.,2023, 2208997
[9] Zhu L., Zhang M., Xu J., Li C., Yan J., Zhou G., Zhong W., Hao T., Song J., Xue X., Zhou Z., Zeng R., Zhu H., Chen C. C., MacKenzie R. C. I., Zou Y., Nelson J., Zhang Y., Sun Y., Liu F.,.,2022,(6), 656—663
[10] Zheng Z., Wang J., Bi P., Ren J., Wang Y., Yang Y., Liu X., Zhang S., Hou J.,,2022,(1), 171—184
[11] Zhou R., Jiang Z., Yang C., Yu J., Feng J., Adil M. A., Deng D., Zou W., Zhang J., Lu K., Ma W., Gao F., Wei Z.,.,2019,(1), 5393
[12] Jiang M., Bai H., Zhi H., Yan L., Woo H. Y., Tong L., Wang J., Zhang F., An Q.,.,2021,(7), 3945—3953
[13] Huo Y., Gong X. T., Lau T. K., Xiao T., Yan C., Lu X., Lu G.,Zhan X., Zhang H. L.,.,2018,(23), 8661—8668
[14] Hu D., Yang Q., Chen H., Wobben F., Le Corre V. M., Singh R., Liu T., Ma R., Tang H., Koster L. J. A., Duan T., Yan H., Kan Z., Xiao Z., Lu S.,.,2020,(7), 2134—2141
[15] Bin H., Yao J., Yang Y., Angunawela I., Sun C., Gao L., Ye L., Qiu B., Xue L., Zhu C., Yang C., Zhang Z. G., Ade H., Li Y.,.,2018,(27), 1706361
[16] Li H., Wu Q., Zhou R., Shi Y., Yang C., Zhang Y., Zhang J., Zou W., Deng D., Lu K., Wei Z.,.,2018,(6), 1803175
[17] Meng L., Li M., Lu G., Shen Z., Wu S., Liang H., Li Z., Lu G., Yao Z., Li C., Wan X., Chen Y.,,2022,(21), 2201400
[18] Zhang L., Sun R., Zhang Z., Zhang J., Zhu Q., Ma W., Min J., Wei Z., Deng D.,.,2022,(50), 2207020
[19] Ma K., Feng W., Liang H., Chen H., Wang Y., Wan X., Yao Z., Li C., Kan B., Chen Y.,.,2023,(19), 2214926
[20] Sun Y., Nian L., Kan Y., Ren Y., Chen Z., Zhu L., Zhang M., Yin H., Xu H., Li J., Hao X., Liu F., Gao K., Li Y.,,2022,(12), 2835—2848
[21] Zhou R., Jiang Z., Shi Y., Wu Q., Yang C., Zhang J., Lu K., Wei Z.,.,2020,(51), 2005426
[22] Yang L., Zhang S., He C., Zhang J., Yang Y., Zhu J., Cui Y., Zhao W., Zhang H., Zhang Y., Wei Z., Hou J.,.,2018,(6), 2129—2134
[23] Ge J., Xie L., Peng R., Fanady B., Huang J., Song W., Yan T., Zhang W., Ge Z.,.,2020,(7), 2808—2815
[24] Zhou J., Zuo Y., Wan X., Long G., Zhang Q., Ni W., Liu Y., Li Z., He G., Li C., Kan B., Li M., Chen Y.,.,2013,(23), 8484—8487
[25] Kan B., Zhang Q., Li M., Wan X., Ni W., Long G., Wang Y., Yang X., Feng H., Chen Y.,.,2014,(44), 15529—15532
[26] Hong L., Yao H., Wu Z., Cui Y., Zhang T., Xu Y., Yu R., Liao Q., Gao B., Xian K., Woo H. Y., Ge Z., Hou J.,.,2019,(39), 1903441
[27] Kan B., Zhang J., Liu F., Wan X., Li C., Ke X., Wang Y., Feng H., Zhang Y., Long G., Friend R. H., Bakulin A. A., Chen Y.,.,2018,(3), 1704904
[28] Wang Y., Wang Y., Zhu L., Liu H., Fang J., Guo X., Liu F., Tang Z., Zhang M., Li Y.,.,2020,(5), 1309—1317
[29] Zhou R., Jiang Z., Shi Y., Wu Q., Yang C., Zhang J., Lu K., Wei Z.,.,2020,(51), 2005426
[30] Gao J., Ge J., Peng R., Liu C., Cao L., Zhang D., Fanady B., Hong L., Zhou E., Ge Z.,,2020,(15), 7405—7411
[31] Chen Y., Bai F., Peng Z., Zhu L., Zhang J., Zou X., Qin Y., Kim H. K., Yuan J., Ma L. K., Zhang J., Yu H., Chow P. C. Y., Huang F., Zou Y., Ade H., Liu F., Yan H.,.,2020,(3), 2003141
[32] Yan T., Ge J., Lei T., Zhang W., Song W., Fanady B., Zhang D., Chen S., Peng R., Ge Z.,,2019,(45), 25894—25899
[33] Wang Y., Zhang Y., Qiu N., Feng H., Gao H., Kan B., Ma Y., Li C., Wan X., Chen Y.,.,2018,(15), 1702870
[34] Ke X., Meng L., Wan X., Li M., Sun Y., Guo Z., Wu S., Zhang H., Li C., Chen Y.,,2020,(19), 9726—9732
[35] Ge J., Xie L., Peng R., Fanady B., Huang J., Song W., Yan T., Zhang W., Ge Z.,.,2020,(7), 2808—2815
[36] Jiao C., Guo Z., Sun B., Yi Y. Q. Q., Meng L., Wan X., Zhang M., Zhang H., Li C., Chen Y.,,2020,(18), 6293—6298
[37] Zhang Y., Feng H., Meng L., Wang Y., Chang M., Li S., Guo Z., Li C., Zheng N., Xie Z., Wan X., Chen Y.,.,2019,(45), 1902688
[38] Wu J., Fan Q., Xiong M., Wang Q., Chen K., Liu H., Gao M., Ye L., Guo X., Fang J., Guo Q., Su W., Ma Z., Tang Z., Wang E., Ade H., Zhang M.,,2021,, 105679
[39] Xu Y., Cui Y., Yao H., Zhang T., Zhang J., Ma L., Wang J., Wei Z., Hou J.,.,2021,(22), 2101090
小分子给体桥联单元烷基链取代位置对光伏器件性能的影响
郭子琦1,焦灿灿1,吴思敏1,孟令贤2,孙延娜1,柯鑫1,万相见1,陈永胜1
(1. 南开大学化学学院, 功能高分子材料教育部重点实验室, 天津 300071; 2. 郑州大学材料科学与工程学院, 郑州 450001)
设计合成了2个同分异构体小分子给体C2-C-F和C2-M-F, 二者仅中间桥联三噻吩单元上烷基链的取代位置不同. 研究结果表明, 烷基链取代位置对其吸光性能和能级影响较小, 但对与受体BTP-4F-12共混后的活性层形貌具有较大影响. 其中, 小分子给体C2-C-F与受体BTP-4F-12共混的薄膜获得了较好的形貌, 光伏器件效率达到12.84%. 研究结果表明, 可以通过烷基取代的位置来精细调控活性层的形貌, 为高效小分子给体的设计提供了有益的参考.
全小分子有机太阳能电池;烷基链;小分子给体
O649.5
A
10.7503/cjcu20230180
网络首发日期: 2023-05-29.
联系人简介:陈永胜, 男, 博士, 教授, 主要从事碳纳米材料、有机光电功能材料及其在能源转化与存储等方面的研究. E-mail: yschen99@nankai.edu.cn
万相见, 男, 博士, 教授, 主要从事有机光电功能材料设计、合成与器件制备方面的研究. E-mail: xjwan@nankai.edu.cn
孟令贤, 女, 博士, 讲师, 主要从事有机光电功能器件制备方面的研究. E-mail: lxmeng@zzu.edu.cn
2023-04-07
国家自然科学基金(批准号: 52025033, 21935007, 52203245)和中国博士后创新人才支持计划(批准号: BX20220274)资助.
Supported by the National Natural Science Foundation of China(Nos.52025033, 21935007 and 52203245) and the China Postdoctoral Innovative Talent Support Program(No.BX20220274).
(Ed.: L, H, W, K)
