Aggregation Morphology of Perylene Bisimide Acceptors and the Role on Exciton Processes and Electron Transport in Organic Solar Cells
2023-10-10ZHANGYuCHENJiehuanZHOUJiadongLIULinlinXIEZengqi
ZHANG Yu, CHEN Jiehuan, ZHOU Jiadong, LIU Linlin, XIE Zengqi
Aggregation Morphology of Perylene Bisimide Acceptors and the Role on Exciton Processes and Electron Transport in Organic Solar Cells
ZHANGYu, CHENJiehuan, ZHOUJiadong, LIULinlin, XIEZengqi*
(,,,510640,)
Head-to-tail bonded perylene bisimide(PBI) dyads with different branched alkyl chains substituted at the terminal imide position show various stacking modes, which results in different effects on the excitonic processes and electron transportation. The dyad bearing branched alkyl chains with the branching sites close to the imide positions forms homogeneously amorphous state,while with branching sites being away from the imide positions the PBI core tend to stack with multiple modes. There are fewer energy trapping sites in the homogeneously amorphous state, but in the multiple stacking system the strong-interactions give more trapping sites. Our study demonstrates that the aggregation state of PBI-based acceptors plays an important role in the performances of organic solar cells(OSCs). Multiple stacking needs to be diminished to avoid the constrained exciton dissociation and retarded charge transport in the active layer.
Perylene bisimide; Amorphous state; Coexistence of multiple stacking; Organic solar cell
1 Introduction
In the past few decades, perylene bisimide(PBI) derivatives have received extensive attention in the field of organic electronics due to their outstanding physicochemical properties such as high absorption coefficient and excellent photo-thermal stability[1—4]. The planar central core of PBIs enables strong intermolecular-interaction, thereby showing excellent semi-conductivity. Considering their remarkable electron transporting properties, PBIs are widely applied as electron acceptors and cathode interfacial materials in organic solar cells(OSCs)[5—11]. Although PBIs possess many excellent properties, the large planar structures tend to form large aggregate domains, destroying proper phase separation with donors in the bulk heterojunction(BHJ) and limiting the charge transporting performance[12,13]. Many research groups have focused on the excessive aggregation behavior of PBIs and proposed numerous strategies to reduce the self-aggregation of PBIs and optimize phase separation in the BHJ, such as synthesizing oligomers and introducing sterically hindered groups[14—18]. However, till now the understanding of aggregation modes of PBIs in thin films is still superficial and it needs in-depth and detailed studies.
In-conjugated system, depending on the strength of intermolecular electron coupling, different aggregates(H-aggregates and J-aggregates) possess distinct excitonic energy, leading to “wavy” potential energy surfaces[19—21]. For example, H-aggregates with face-to-face parallel stacking have higher excitonic energy, while J-aggregates with slipped stacking have lower excitonic energy[22]. In this case, the sites with lower excitonic energy(local minimum) play the role of energy traps, constraining the migration of excitons or charge carriers in solid films. Besides, as described in our previous work,both H-aggregates and J-aggregates coexisted simultaneously in the crystal of a perylene bisimide dyad and the energy transfer from H-type stacking moieties to J-type stacking moieties occurred easily due to the higher excitonic energy of H-type stacking[23]. J-type stacking moieties form the lowest-lying energy valleys capture excitons and facilitate highly emissive processes.
Here, we reveal that the homogeneous stacking of PBI-based acceptors was conducive to the improvement of OSCs performance whereas the multiple stacking modes of PBI-based acceptors restrained the exciton dissociation and charge carrier transport properties, based on the analysis of the aggregation modes of a serial of PBI dyads named dPBI(1-1), dPBI(1-2), and dPBI(2-2) and the device performance of PBI-based OSCs. By comparing the crystallinity, film morphology, and photophysical process, enhanced emission properties were found in dPBI(1-2) and dPBI(2-2) with multiple stacking characteristics on account of more trapping sites. The OSCs based on homogeneously aggregated dPBI(1-1) showed satisfactory device performances with decent carrier transport properties. On the contrary, the dPBI(1-2) and dPBI(2-2)-based OSCs exhibited inferior power conversion efficiencies(PCE), especially short circuit current density. Furthermore, the lower exciton dissociation efficiency and the enhancement of recombination in dPBI(1-2) and dPBI(2-2)-based OSCs suggested that the wide excitonic energy distribution derived from multiple stacking characteristic has a negative impact on carrier dynamics.
2 Experimental
2.1 Materials and Methods
All reagents and active layer donors were purchased from commercial sources and used as received without further purification. PM6 were purchased from Solarmer Materials Inc. ITO glass substrates(<15 Ω/□) were purchased from South China Science and Technology Co., Ltd..
UV-Visible absorption spetra(UV-Vis) absorption spectra and photoluminescence(PL) spectra were recorded on Shimadzu UV-3600 spectrometer and Shimadzu RF-5301 spectrometer, respectively. Conventional quartz substrates(1.5 cm×1.5 cm) were used. Cyclic voltammetry(CV) measurements were performed on a CHI600D electrochemical workstation(CHI Instruments, Shanghai Chenhua Instrument Corp., China) with the platinum electrode as the counter electrode, the platinum carbon electrode as the working electrode, Ag/Ag+as the reference electrode and Fc/Fc+as external standard. CV measurements was carried out in dichloromethane(DCM) containing tetrabutylammonium hexafluorophosphate(TBAPF6) as electrolyte at a scan rate of 0.1 V/s. Power X-ray diffraction spectroscopy(PXRD) data were recorded on a Rigaku model RINT Ultima III diffractometer by depositing powder on glass substrate, from 2=5°—55° with 0.02° increment(Rigaku Corporation, Japan). The Grazing incidence wide-angle X-ray scattering(GIWAXS) measurement was performed by Xenocs 2.0 system. Thewas 0.134144 nm and the incident angle was 0.2°. Atomic force microscopy(AFM)measurement was carried out using a Digital Instrumental DI Multimode Nano-scope IIIa in tapping mode. The scanning electron microscopy(SEM) images were collected by field emission scanning electron microscope(Regulus 8100, Hitachi, Japan). The temperature-dependent steady fluorescence spectra and fluorescence decay were recorded by homebuilt optical paths. The heat-up process of dPBIs films was carried out from 80 K to 300 K in vacuum. The data was collected every 10 K and kept at constant temperature for 5 min before recording. The incident excitation was femtosecond laser pulses at a central wavelength of 500 nm with pulse duration of 80 fs and a repetition rate of 80 MHz(Spectra Physics, Mai Tai & Inspire HF 100). The film was placed at the sample holder inside the vacuum nitrogen cryostat(OptistatDN, Oxford). The static exchange gas was controlled to obtain the stable temperature from 80 K to 300 K by Mercury iTC temperature controller. The emission signal was split into two exit ports: one port was integrated with CCD(Princeton Instruments, Pixis 100B) for steady spectra measurement; the other one was directed into a monochromator(Princeton Instruments, Acton 2300i), and mounted with a photon counting detector combined with Time-correlated single photon counting(TCSPC) system(HydraHarp 400) for transient fluorescence measurements.
Mobilities were measured through space charge limited current(SCLC) method by fabricating single- carrier devices. Electron mobilities were measured by electron-only devices of ITO/ZnO/PM6∶dPBIs/PFN-Br/Ag. Hole mobilities were measured by hole-only devices of ITO/PEDOT∶PSS/PM6∶dPBIs/MoO3/Ag. The darkcharacteristics of the single-carrier devices was plotted as1/2.and the mobilities were calculated by Mott-Gurney equation of=(9/8)εεµ[(2)/(3)], whereis the current density(A/m2);0is the permittivity of vacuum(F/M);ris the relative permittivity of the active layer(usually 2—4 for organic semiconductor);is the mobility(m2·V-1·s-1);(V) is the effective voltage; andis the thickness of active layer(m). The effective voltage is obtained by=appl-bi, whereappl(V) is the applied voltage andbi(V) is the built-in voltage(regarded as 0 V).
2.2 Device Fabrication and Characterizations
The OSCs were fabricated with a conventional structure of ITO/PEDOT∶PSS/Active layer/PFN-Br/Ag. ITO glass substrates were cleaned by sonication in acetone,detergent, deionized water and isopropyl alcohol in sequence for 15 min each. Before fabrication, the ITO substrates were cleaned by UVO for 15 min. PEDOT∶PSS(4083) was purchased from commercial sources and filtered by 0.22 μm aqueous-phase filter. A 30 nm layer of PEDOT∶PSS was spin-coated onto the substrates and annealed at 150 ℃ for 15 min under ambient conditions. The thickness of the films was determined by the surface profile(Bruker). The substrates were then transferred into a nitrogen glove box. PM6∶dPBIs blends(the mass ratio of donor/acceptor was 1∶1) were prepared in chlorobenzene(CB) with 1.5% 1,8-diiodooctane(DIO) and 1.5% 1-chloronaphthalene (CN) at a total concentration of 20 mg/mL. The solutions were stirred at 50 ℃ overnight for complete dissolution. The active layer was prepared by spin coating the blend solutions at 3000 r/min for 40 s to form a 90 nm thin film and then annealed at 130 ℃ for 20 min. For PM6∶dPBI(2-2) blend solution, the total concentration was 6 mg/mL. The solution was stirred at 75 ℃ overnight. The substrates were maintained at 75 ℃ during spin-coating at a rate of 600 r/min, and the coated films were subsequently annealed at 130 ℃ for 20 min. 5 nm PFN-Br cathode interfacial layers were prepared by spin-coating. The solution of PFN-Br is 0.5 mg/mL in MeOH. Top silver electrode of 100 nm thickness was thermally evaporated at a pressure of about 10-4Pa with a shadow mask to define the active area of device(5.00 mm2).
The photovoltaic performance of devices was determined fromcurve measurements using a Keithley 2400 source measure unit under 1 sun, AM 1.5G spectrum from a solar simulator(SAN-EI; 1000 W/m). The solar simulator illumination intensity was determined using a monocrystal silicon reference cell calibrated by the National Renewable Energy Laboratory(NREL) and Konica Minolta Inc.(follows JIS C8904-2).
3 Results and Discussion
3.1 Synthesis of dPBIs
The head-to-tail bonded PBI dyads were synthesized according to our previous reported method[23]. By changing different substituted alkyl chains at the terminal imide positions, symmetrical dPBI(1-1) and dPBI(2-2) and asymmetrical dPBI(1-2) were obtained as shown in Fig.1(A). The detailed synthetic route is illustrated in the Scheme S1(see the Electronic Supplementray Material of this paper). These PBI dyads have similar molecular structures but show different stacking modes in solid states. The structure of dPBI(1-1) is similar to the structure of previously reported electron acceptor[24,25], and the only difference is the length of alkyl chains at the terminal imide position. The alkyl chain R1with the branching site at the first carbon atom close to the imide position greatly reduces the tendency of PBI aggregation and eventually manifested as structural disorder in the thin film. The structural design of dPBI(1-2) originated from the peculiar stacking modes of dPBI(2-2) previously reported by our research group, and PBI units in the crystal of dPBI(2-2) exhibited multiple stacking modes with columnar rotary-stacking(H-type stacking) and discrete dimeric slip-stacking(J-type stacking), as shown in Fig.1(B). However, due to the strong-stacking in aggregates, the solu- bility of dPBI(2-2) is extremely low in common organic solvents(2 mg/mL in CB at room temperature) and thus unfavorable for film or device fabrication. Through structural modification, the solubility of dPBI(1-2) with the substituted group R1is superior to that of dPBI(2-2), which make it applicable for convenient solution processing.
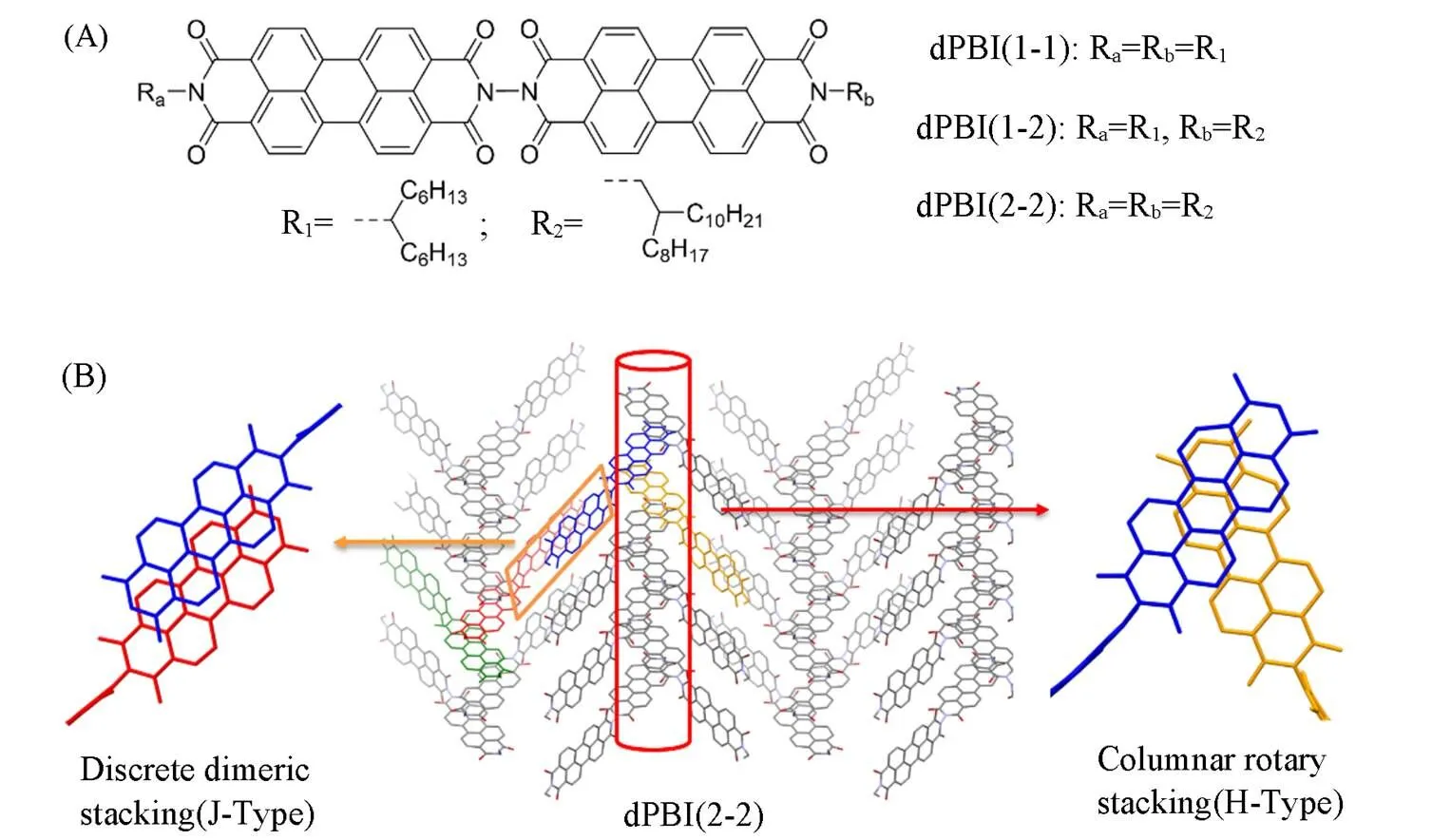
Fig.1 Molecular structures of dPBIs(A) and molecular stacking in the dPBI(2⁃2) crystal(B)
3.2 Electrochemical and Optical Properties of dPBIs
The lowest unoccupied molecular orbital(LUMO) and the highest occupied molecular orbital(HOMO) energy levels of dPBIs were measured by CV(Fig.S3, see the Electronic Supplementary Material of this paper). The LUMO/HOMO energy levels of dPBI(1-1), dPBI(1-2), and dPBI(2-2) estimated from the onset redox potentials are -3.87/-6.00, -3.87/-6.00, and -3.88/-6.03 eV, respectively(Table S1, see the Electronic Supplementary Material of this paper). The energy levels and the band gaps of dPBIs show no significant difference since modification of the imide substituents having little impact on the electronic and optical properties[26]. Fig.2(A) and (B) present the steady-state absorption and emission spectra of dPBIs as thin films measured by UV-Vis absorption and fluorescence spectroscopies, respectively. The 0-0 absorption peak of dPBI(2-2) located at 561 nm was 17 nm bathochromic-shifted compared with the peak of dPBI(1-1) at 544 nm, which is attributed to the formation of strong J-type stacking moieties in dPBI(2-2). The 0-0 peak of dPBI(1-2) at 548 nm exhibited a 4 nm bathochromic shift relative to that of dPBI(1-1) and a 13 nm hypsochromic shift relative to that of dPBI(2-2). The 0-1 absorption peak of dPBI(2-2) at 508 nm with an absorbance close to the 0-0 peak was attributed to the formation of H-aggregates, suggesting intensive H-type stacking in the film. For comparison, the ratio of the 0-1/0-0 absorbance of dPBI(1-2) was slightly higher than that of dPBI(1-1), indicating the enhanced H-type stacking in dPBI(1-2) film. The emission spectra showed a similar pattern. The emission peak of dPBI(1-2) at 629 nm is bathochromic-shifted by 5 nm compared to that of dPBI(1-1)(624 nm) and hypsochromic-shifted by 5 nm compared to that of dPBI(2-2)(634 nm). Considering the molecular structure of dPBI(1-2) is the combination of each half of dPBI(1-1) and dPBI(2-2), it can be reasonably inferred from the shifts of the spectra that the intermolecular packing structure of dPBI(1-2) may be partially crystalline with slightly multiple stacking characteristic.

Fig.2 Optical properties of dPBIs as thin films
(A) Normalized UV-Vis absorption spectra of dPBIs films;(B) photoluminescence spectra of dPBIs films.
3.3 Crystalline and Morphological Properties of dPBIs
GIWAXS measurement which can clarify the molecular arrangement was used to obtain more insights into the molecular packing of dPBIs(Fig.3). From the uniform diffraction rings in the two-dimensional(2D) patterns shown in Fig.3(A) and (B), it can be seen that dPBI(1-1) and dPBI(1-2) exhibited isotropic orientations in films. Compared to dPBI(1-1), dPBI(1-2) exhibited slightly stronger crystallinity, as evidenced by higher diffraction intensity and larger lamellar coherence length, which is 3.72 nm for dPBI (1-1) and 8.60 nm for dPBI(1-2). In contrast, a distinctly sharp 2D diffraction pattern was observed in dPBI(2-2) film, and it was surprising to find that the diffraction pattern of the film closely matched that of the lamellar single crystals, as shown in Fig.3(C) and (D). The remarkable agreement between the diffraction patterns of the film and the single crystals suggests that during the spin-coating process of film formation, dPBI(2-2) was capable of growing crystals that closely resemble its single-crystal structure, which will be of great help in analyzing the relationship between stacking behavior, excited state processes, and carrier dynamics in the device. One-dimensional(1D) linecuts shown in Fig.3(E) were extracted along the in-plane(IP) and out-of-plane(OOP) directions from the 2D patterns. The-distances of dPBI(1-1) and dPBI(1-2) were calculated to be 0.378 nm from the (010) peaks at 16.6 nm-1. For dPBI(2-2), the (010) peak was located at 14.2 nm-1, corresponding to a-distance of 0.442 nm.

Fig.3 2D GIWAXS patterns and 1D linecuts of dPBIs
2D GIWAXS patterns of dPBIs neat films(A)—(C) and dPBI(2⁃2) lamellar crystals(D). (E) The corresponding 1D linecuts along the IP(dash lines) and OOP(solid lines) directions.
PXRD analysis was carried out to investigate the stacking structure and the degree of crystallinity. According to the XRD patterns of dPBIs(Fig.S4, see the Electronic Supplementary Material of this paper), dPBI(1-1) exhibited amorphous nature, while dPBI(2-2) pronounced strong crystallization signal. The bulge near 23° in the dPBI(1-2) pattern suggested relatively strong-stacking compared with the signal of dPBI(1-1)[27].
AFM and SEM were implemented to investigate the surface morphology of dPBIs films(Fig.S5 and Fig.S6, see the Electronic Supplementary Material of this paper). The surfaces of both dPBI(1-1) and dPBI(1-2) films exhibited a high level of smoothness, with no significant difference observed in the average surface roughness(a=1.26 nm and 1.17 nm, respectively). In contrast, the dPBI(2-2) film displayed highly rough surfaces(a=19.9 nm), indicating strong aggregation properties. Notably, AFM and SEM images reveal the formation of the crystals resembling “trilobite fossils” patterns on the film surface, further emphasizing the highly crystalline nature of the dPBI(2-2) film.
3.4 Photophysical Properties and Excited-state Dynamics of dPBIs
Time-resolved fluorescence measurements of dPBIs films at room temperature were carried out, as shown in Fig.4(A), to obtain the fluorescence lifetimes of dPBIs through the exponential fitting of the emission decays. The fluorescence lifetimes of dPBI(1-1), dPBI(1-2) and dPBI(2-2) were 2.90, 3.93 and 4.69 ns, respectively. Longer fluorescence lifetime might be attributed to the significant suppression of non-radiative processes which contain internal conversion or energy transfer[28]. The distinction between the radiative processes of dPBIs was further confirmed by the photoluminescence quantum yield(PLQY) measurements (Table 1), in which dPBI(1-2) and dPBI(2-2) films possessed higher quantum yield than dPBI(1-1) film.
Temperature-dependent steady photoluminescence(TDPL) measurements were applied to study the stacking structure and exciton dynamics at different temperatures. As shown in Fig.4(B)—(D), fluorescence intensities of dPBI films significantly increased at low temperatures due to the localization of excitons and the slowdown of non-radiative decays. The fluorescence integral intensity of dPBI(1-1) was only doubled with the temperature decreasing from 300 K to 80 K, while the integral intensities of dPBI(1-2) and dPBI(2-2) were observably increased sevenfold as the temperature decreased. Significant variation in the fluorescence intensities of the dPBI(1-2) and dPBI(2-2) films indicated that the excitons were highly localized at low temperatures, which could be attributed to large amount of trapping sites caused by multiple stacking. The fluorescence peaks exhibited bathochromic shifts as the temperature decreased, which could be attributed to the increase in stacking density and chromophoric interaction at low temperature[29]. Meanwhile, the normalized fluorescence spectra shown in Fig.S7(see the Electronic Supplementary Material of this paper) revealed significant changes in the relative fluorescence intensities of the peaks at different positions in each dPBI film, which could be understood as a vibrationally induced shift known as Herzberg-Teller effect[23]. For instance,in the case of dPBI(2-2), the notable increase in intensity of the middle main peak at low temperatures is a result of energy transfer from the high-energy emission of the H aggregates.

Fig.4 Fluorescence decays and temperature⁃dependent steady photoluminescence spectra of dPBIs
(A) Fluorescence decays(ex=470 nm,em=630 nm) of dPBIs as thin films;(B)—(D) temperature-dependent steady photoluminescence spectra of dPBI(1-1) film(B), dPBI(1-2) film(C) and crystalline dPBI(2-2)(D).

Table 1 Photophysical properties of dPBIs
. Measured in thin films under ambient condition;. the excitation wavelength for solutions(5×10-5mol/L) wasex, sol=535 nm;. the excitation wavelength for films wasex, film=550 nm.
To further confirm that the exciton dynamics at various temperatures is connected with the multiple stacking characteristics of dPBIs, time-correlated single photon counting(TCSPC) method was used to investigate the dependency of the fluorescence lifetime on the temperature(Fig.5). As the temperature decreased, the fluorescence lifetime of dPBI(1-1) slightly increased from 0.9 ns(300 K) to 5.7 ns(80 K). Nevertheless, the fluorescence lifetimes of dPBI(1-2) and dPBI(2-2) significantly increased from 1.8 ns and 4.5 ns at 300 K to larger than 10 ns below 190 K, indicating the formation of excimer-like state with long lived emission[30]. According to literature, as the temperature decreases, the non-radiative transition rate decreases rapidly because of the suppressed intramolecular vibration[31]. Consequently, the longer lifetimes of dPBI(1-2) and dPBI(2-2) at low temperatures suggested that the presence of energy valleys originated from multiple stacking characteristic enhanced localized exciton emission.

Fig.5 Temperature⁃dependent photoluminescence decays of dPBIs
(A)—(C) Temperature-dependent photoluminescence decay curves of dPBI(1-1), dPBI(1-2), and dPBI(2-2) using TCSPC module, respectively;(D) fitted photoluminescence lifetimes of dPBIs under different temperatures.
Steady-state photoluminescence bulk quenching measurement was employed to obtain the exciton diffusion length[32]. In this technique, photoluminescence spectra of dPBIs films doped with various concentrations of PC71BM as fluorescence quencher were detected(Fig.S1, see the Electronic Supplementary Material of this paper) and used to calculate the relative PL quenching efficiencies(QE) which are strongly related to the diffusion coefficients. Since the measurement accuracy depends to a large extent on the uniformity of the thin film, dPBI(1-1) and dPBI(1-2) which are easy to form smooth films were selected. Details of experiments and simulations were presented in the Supporting Information. The fluorescence quenching efficiency increased with the addition of the volume fraction of PC71BM, according to which diffusion coefficients were fitted with the assistance of Monte Carlo simulation. The exciton diffusion lengths of dPBI(1-1) and dPBI (1-2) were calculated as 8.79 nm and 10.25 nm, respectively, which are consistent with typical PBI derivatives[1]. The difference in exciton diffusion lengths is mainly due to the difference in fluorescence lifetimes and therefore the short diffusion length of dPBI(1-1) is originated from excess non-radiative recombination processes in the thin film. However, such non-radiative recombination processes may contribute to the generation of photocurrent under sustained photoexcitation of the photovoltaic device, since excitons can easily interact with carriers, potentially detrapping the localized carriers and resulting in increase in photocurrent[33,34]. On the contrary, radiative sites originated from the multiple stacking characteristic in dPBI(1-2) are likely to capture excitons to generate fluorescence rather than photocurrent.
3.5 Charge Transport Properties of dPBIs
To assess the disparity in charge transport properties between the dPBIs, the carrier mobilities were measured using SCLC method. Electron-only device of ITO/ZnO/active layer/PFN-Br/Ag and hole-only device of ITO/PEDOT∶PSS/active layer/MoO3/Ag were employed for this measurement. Fig.S8 and Table S2(see the Electronic Supplementary Material of this paper) presents the results obtained from the measurements. The electron and hole mobilities(eandh) of dPBI(1-1) were determined to be 3.6×10-4cm2·V-1·s-1and 1.0× 10-4cm2·V-1·s-1, respectively. For dPBI(1-2), carrier mobilities exhibited a slightly decreased tendency with aeof 3.4×10-4cm2·V-1·s-1and ahof 9.5×10-5cm2·V-1·s-1. The reduction in carrier mobilities was further pronounced in dPBI(2-2), with aeof 2.5×10-4cm2·V-1·s-1andhof 5.3×10-5cm2·V-1·s-1, indicating a decline in the charge transport properties of the multi-stacked dPBIs.
3.6 Performance of OSC Based on PM6∶dPBIs
To confirm the effect of exciton and carrier dynamics on device performance, conventional bulk heterojunction OSCs were fabricated with the structure of ITO/PEDOT∶PSS/active layer/PFN-Br/Ag, where PM6 was used as donor(Scheme S2, see the Electronic Supplementary Material of this paper) and dPBIs were used as acceptor[35]. The absorption and emission spectra of PM6∶dPBIs blend films are shown in Fig.S2(see the Electronic Supplementary Material of this paper). Fig.6 shows thecurves of the OSCs based on PM6∶dPBIs under 1 sun illumination. The devices based on dPBI(1-1) exhibited superior PCE compared to the dPBI(1-2) and dPBI(2-2)-based devices. As summarized in Table 2, the OSCs based on PM6∶dPBI (1-1) achieved a prominent PCE of 6.48%, with open circuit voltage(OC) of 0.93 V, short circuit current density(SC) of 10.13 mA/cm2, and fill factor(FF) of 68.79%. PM6∶dPBI(1-2)-based OSCs offered a slightly inferior PCE of 6.03%, which was primarily attributed to a lowerSCof 9.57 mA/cm2. The PM6∶dPBI(2-2)-based devices demonstrated even worse performance, with an unsatisfactorySCof 4.36 mA/cm2and a mediocre FF of 61.51% contributing to the significantly low PCE of 2.47%.

Table 2 Summary of photovoltaic performance of PM6∶dPBIs-based OSCs under the illumination of AM 1.5G, 100 mW/cm2

Fig.6 J⁃V curves of the OSCs under 1 sun illumination(100 mW/cm2) of PM6∶dPBI⁃based OSCs
The charge transporting properties of the blend films were further characterized by SCLC method, as s ummarized in Fig.S8 and Table S2. The hole mobilities of the blend films exhibited only subtle difference, as the migration of holes is primarily governed by the donor PM6 in these blends. In contrast, there was a more pronounced difference in electron mobility among the three blend films. Theeof PM6∶dPBI(1-1), PM6∶dPBI(1-2), and PM6∶dPBI(2-2) were measured to be 5.1×10-4, 4.5×10-4and 1.4×10-4cm2·V-1·s-1, respectively. These variations ine, as well as thee/hratio, may significantly contribute to the observed differences inSCamong the devices.
In order to gain a deeper insight into the relationship between film morphology and device performance, the molecular packing and orientation of the blend films were characterized using GIWAXS measurement (Fig.7). All three blend films pronounced distinct(100) diffraction signals at approximately 3 nm-1, corresponding to a lamellar coherence length of 2.1 nm. Notably, PM6∶dPBI(1-2) exhibited an additional lamellar diffraction peak at 2 nm-1, corresponding to a larger lamellar coherence length of 3.1 nm compared to PM6∶dPBI(1-1). Furthermore, the 2D diffraction pattern of PM6∶dPBI(2-2) fitted well with that of the dPBI(2-2) neat film[Fig.7(A)—(C)], indicating that the presence of multi-stacking behavior in the blend film. The (010) peaks along the OOP direction of PM6∶dPBI(1-1), PM6∶dPBI(1-2), and PM6∶dPBI(2-2) were located at 17.2, 17.4, and 17.0 nm-1, respectively(Fig.8), corresponding to-distances of 0.365, 0.361 and 0.369 nm, respectively. The AFM and SEM images of the blend films(Fig.S5 and Fig.S6) suggested smooth surface morphology of PM6∶dPBI (1-1) and PM6∶dPBI(1-2), whereas the surface of PM6∶dPBI(2-2) appeared comparatively rough, which could potentially have a negative impact on charge transport and interfacial contact within the device.

Fig.7 2D GIWAXS patterns of PM6∶dPBIs blend films

Fig.8 1D linecuts of PM6∶dPBIs blend films
The corresponding 1D linecuts were extracted along the IP(dash lines) and OOP(solid lines) directions.
To examine the exciton dissociation and carriers transporting properties of PM6∶dPBIs-based OSCs, several measurements correlated to carrier dynamics were carried out. To investigate the exciton dissociation and charge collection properties of the OSCs, the photocurrent density(ph) as a function of the effective bias (eff) was recorded as shown in Fig.9(A), whereph=L-DwithLandDbeing the current densities under illumination and in the dark(Fig.S9, see the Electronic Supplementary Material of this paper), respectively, andeff=0-awith0andabeing the zero-photocurrent voltage and the applied voltage, respectively. The photocurrent density is assumed to be saturated(sat) at higheff(2 V), in which case all excitons can be dissociated into free charge carriers and collected by electrodes[36]. By normalizingphwithsat(Fig.S10, see the Electronic Supplementary Material of this paper), exciton dissociation efficiencies(diss) and charge collection efficiencies(coll)were evaluated from the ratio ofph/satunder short circuit condition and maximum power output condition, respectively. PM6∶dPBI(1-1)-based OSCs exhibited distinguisheddissandcollvalues of 95.6% and 77.7%, respectively, compared to that of PM6∶dPBI(1-2)-based OSCs(94.9% and 76.6%, respectively) and PM6∶dPBI(2-2)-based OSCs(92.1% and 67.1%, respectively), demonstrating the optimized exciton dissociation and charge collection could explain the outstandingSCin PM6∶dPBI(1-1)- based OSCs.
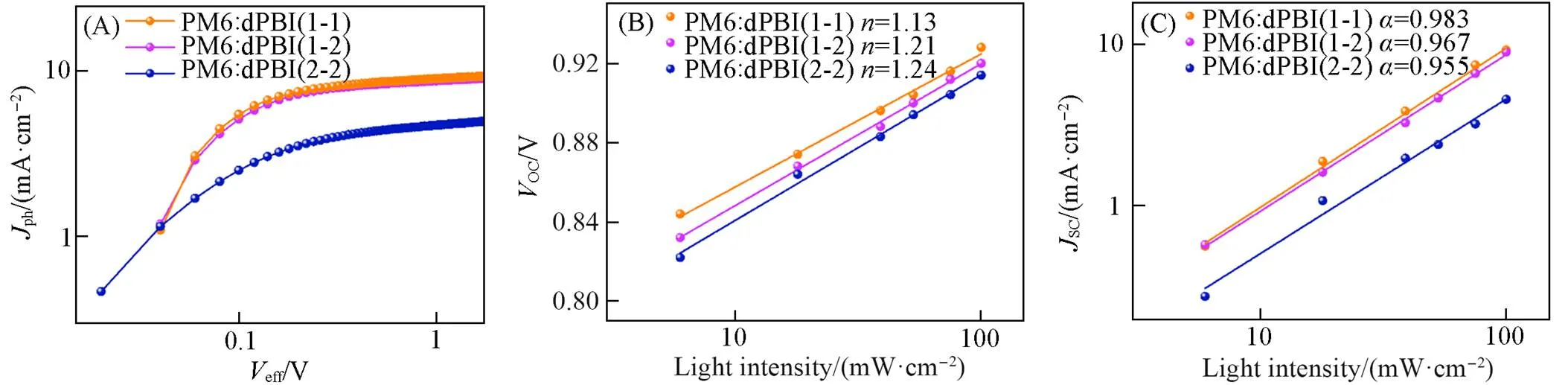
Fig.9 Photocurrent density(Jph) vs. effective bias(Veff) curves(A) and VOC and JSCvs. light intensity(C, D) of PM6∶dPBIs⁃based OSCs

The devices characterization revealed that PM6∶dPBI(1-2) and PM6∶dPBI(2-2)-based devices exhibited unsatisfactory exciton dissociation, charge transport, and charge collection. Compared to the significant reduction inSC, the loss of FF was relatively minor, indicating the efficient charge extraction. There are several factors contributing to the current loss. Firstly, the presence of multiple stacked structures acted as energy traps, limiting the dissociation of excitons and the generation of free charges. Secondly, the large difference in the electron mobility of dPBIs resulted in the imbalance of electron-hole mobility within the blend films, impeding efficient charge transfer. Additionally, the suboptimal surface morphology also restricted the charge transport at the interface. In contrast, homogeneous aggregation was advantageous for enhancing device performance due to the uniformly distributed excitonic energy. In this stacking mode, charge transfer can occur more efficiently, leading to improved PCE.
4 Conclusions
In summary, we demonstrated that the homogeneous aggregation in PBI-based acceptors plays an important role in improving the OSCs performance, while the multiple stacking modes significantly damaged the photovoltaic device performance, especiallySC. PBI dyads with different substituted alkyl chains at the terminal imide position, named dPBI(1-1), dPBI(1-2), and dPBI(2-2), were synthesized. The introduction of substituent2instead of1induced a transformation in the aggregation behavior of dPBIs from being amorphous to having multiple stacking modes(H-type and J-type stacking). More excitonic energy traps were formed in dPBI(1-2) and dPBI(2-2) due to the multiple stacking characteristics, thereby constraining excitons in J-aggregates and enhancing fluorescence emission. Accordingly, the OSCs based on dPBI(1-2) and dPBI(2-2) exhibited lower exciton dissociation and charge transport efficiencies as well as severe recombination, which explained the decline in device performance. In contrast, the OSCs based on homogeneously aggregated dPBI(1-1) possessed outstanding performance with efficient charge transport. This is the first time that the relationship between the stacking modes in acceptors and the OSCs performance has been demonstrated, providing a new perspective for further molecular design of electron acceptors.
The supporting information of this paper see http://www.cjcu.jlu.edu.cn/CN/10.7503/cjcu20230092.
[1] Nowak⁃Król A., Shoyama K., Stolte M., Würthner F.,,2018,(98), 13763—13772
[2] Zhan X., Facchetti A., Barlow S., Marks T. J., Ratner M. A., Wasielewski M. R., Marder S. R.,,2011,(2), 268—284
[3] Würthner F., Saha⁃Möller C. R., Fimmel B., Ogi S., Leowanawat P., Schmidt D.,,2016,(3), 962—1052
[4] Schaack C., Evans A. M., Ng F., Steigerwald M. L., Nuckolls C.,,2022,(1), 42—51
[5] Zhang M., Bai Y., Sun C., Xue L., Wang H., Zhang Z. G.,,2022,(3), 462—485
[6] Ding K., Shan T., Xu J., Li M., Wang Y., Zhang Y., Xie Z., Ma Z., Liu F., Zhong H.,,2020,(77), 11433—11436
[7] Deng M., Zhang G., Yu L., Xu X., Peng Q.,,2021,, 131910
[8] Wen X., Nowak⁃Król A., Nagler O., Kraus F., Zhu N., Zheng N., Müller M., Schmidt D., Xie Z., Würthner F.,,2019,(37), 13051—13055
[9] Yao J., Qiu B., Zhang Z. G., Xue L., Wang R., Zhang C., Chen S., Zhou Q., Sun C., Yang C., Xiao M., Meng L., Li Y.,,2020,(1), 2726
[10] Wen X., Zhang Y., Xie G., Rausch R., Tang N., Zheng N., Liu L., Würthner F., Xie Z.,,2022,(17), 2111706
[11] Yao J., Ding S., Zhang R., Bai Y., Zhou Q., Meng L., Solano E., Steele J. A., Roeffaers M. B. J., Gao F., Zhang Z. G., Li Y.,,2022,(32), 2203690
[12] Rajaram S., Shivanna R., Kandappa S. K., Narayan K. S.,,2012,(17), 2405—2408
[13] Zheng J. M., Wen X. B., Zhou J. D., Zheng N., Xie Z. Q.,,2019,(8), 775—807(郑介明,文新博,周家东,郑楠,解增旗. 高分子学报,2019,(8), 775—807)
[14] Lin H., Chen S., Hu H., Zhang L., Ma T., Lai J. Y. L., Li Z., Qin A., Huang X., Tang B., Yan H.,,2016,(38), 8546—8551
[15] Zhang G., Feng J., Xu X., Ma W., Li Y., Peng Q.,,2019,(50), 1906587
[16] Russell J. C., Posey V. A., Gray J., May R., Reed D. A., Zhang H., Marbella L. E., Steigerwald M. L., Yang Y., Roy X., Nuckolls C., Peurifoy S. R.,,2021,(8), 1136—1141
[17] Pedersen V. B. R., Pedersen S. K., Jin Z., Kofod N., Laursen B. W., Baryshnikov G. V., Nuckolls C., Pittelkow M.,,2022,(48), e202212293
[18] Cao J., Yang S.,,2022,(12), 6966—6973
[19] Fink R. F., Seibt J., Engel V., Renz M., Kaupp M., Lochbrunner S., Zhao H. M., Pfister J., Würthner F., Engels B.,,2008,(39), 12858—12859
[20] Hestand N. J., Spano F. C.,,2018,(15), 7069—7163
[21] Bialas D., Kirchner E., Röhr M. I. S., Würthner F.,,2021,(12), 4500—4518
[22] Kasha M., Rawls H. R., El⁃Bayoumi M. A.,,1965,(3/4), 371—392
[23] Chen J., Tang N., Zhou J., Wang L., Jiang N., Zheng N., Liu L., Xie Z.,,2021,(13), 3373—3378
[24] Wu C. H., Chueh C. C., Xi Y. Y., Zhong H. L., Gao G. P., Wang Z. H., Pozzo L. D., Wen T. C., Jen A. K. Y.,,2015,(33), 5326—5332
[25] Ye L., Sun K., Jiang W., Zhang S., Zhao W., Yao H., Wang Z., Hou J.,,2015,(17), 9274—9280
[26] Nowak⁃Król A., Würthner F.,,2019,(8), 1272—1318
[27] Pradhan R., Dahiya H., Bag B. P., Keshtov M. L., Singhal R., Sharma G. D., Mishra A.,,2021,(3), 1563—1573
[28] Guo Q., Liu Y., Liu M., Zhang H., Qian X., Yang J., Wang J., Xue W., Zhao Q., Xu X., Ma W., Tang Z., Li Y., Bo Z.,,2020,, 2003164
[29] Matthew Menke S., Holmes R. J.,,2016,(16), 3437—3442
[30] Son M., Park K. H., Shao C., Würthner F., Kim D.,,2014,(20), 3601—3607
[31] Kamma I., Kommidi P., Reddy B. R.,,2008,(9), 096104
[32] Lin J. D. A., Mikhnenko O. V., Chen J., Masri Z., Ruseckas A., Mikhailovsky A., Raab R. P., Liu J., Blom P. W. M., Loi M. A., García⁃Cervera C. J., Samuel I. D. W., Nguyen T. Q.,,2014,(2), 280—285
[33] Wakayama N., Williams D. F.,,1971,(1), 45—47
[34] Wakayama N., Williams D. F.,,1972,(4), 1770—1779
[35] Zhang M., Guo X., Ma W., Ade H., Hou J.,,2015,(31), 4655—4660
[36] Mihailetchi V. D., Koster L. J. A., Hummelen J. C., Blom P. W. M.,,2004,(21), 19—22
[37] Schilinsky P., Waldauf C., Brabec C. J.,,2002,(20), 3885—3887
[38] Koster L. J. A., Mihailetchi V. D., Ramaker R., Blom P. W. M.,,2005,(12), 123509
苝酰亚胺受体的聚集形态对有机太阳电池中激子过程与电子传输的影响
张煜,陈介焕,周家东,刘琳琳,解增旗
(华南理工大学发光材料与器件国家重点实验室, 高分子光电材料及器件研究所, 广州 510640)
末端烷基链分叉位置对苝酰亚胺(PBI)线性二联体的堆积模式具有显著影响, 进而影响激子过程与载流子传输. 二联体两端烷基链分叉位点紧靠苝酰亚胺能够抑制分子长程有序堆积, 形成具有较少能量陷阱的无定形聚集态; 而两端烷基链分叉位点远离苝酰亚胺则导致多种聚集结构共存, 分子间强-相互作用位点成为能量陷阱. 结果表明, PBI受体的聚集方式对有机太阳电池的性能产生重要影响, 需要尽量减少活性层中的多种聚集结构共存以免引起激子解离受限以及载流子传输迟滞.
苝酰亚胺; 无定形态; 多种堆积共存; 有机太阳电池
O622
A
10.7503/cjcu20230092
2023-03-08
网络首发日期: 2023-07-11.
联系人简介:解增旗, 男, 博士, 教授, 主要从事有机电子领域的研究. E-mail: msxiez@scut.edu.cn
国家自然科学基金(批准号: 21975076, 52003089, 52103206)、广东省分子聚集发光重点实验室(批准号: 2019B030301003)和广州市科技计划项目(批准号: 202102020561, 202102020401)资助.
Supported by the National Natural Science Foundation of China(Nos.21975076, 52003089, 52103206), the Key Laboratory of Luminescence from Molecular Aggregates of Guangdong Province, China(No.2019B030301003) and the Science and Technology Projects in Guangzhou, China(Nos.202102020561, 202102020401).
(Ed.: V, K, S)
