High Frequency Induction and Transformation of Somatic Embryos From Mature Seed-derived Leaf in Soybean*
2023-08-14SUNMingJieHOUHengZHAOChunMeiXUERenGao
SUN Ming-Jie, HOU Heng, ZHAO Chun-Mei, XUE Ren-Gao*
(1)College of Life Sciences, Key Lab of Plant Biotechnology in Universities of Shandong Province, Qingdao Agricultural University,Qingdao 266109, China;2)Shandong Baolai-Leelai Bio-Industrial Group, Taian 271000, China)
Abstract Objective Although many improvements in soybean transformation have been made, the efficiency of transformation is still low. Thus, a more useful and efficient soybean transformation system required to be established. The objective of this study is to develop high frequency induction and transformation of somatic embryos (SEs) using mature seed-derived leaf (MSDL) of soybean as target explants. Methods The leaves detached from 7-d-old germinated seedlings were divided into 1.2 cm×1.2 cm sections. These sections were used to induce somatic embryogenesis on the embryo induction medium (EIM) and to infect with Agrobacterium EHA105 strain harboring the pCAMBIA1301 for transformation. Results The MSDL including primary leaf and secondary leaf was able to produce SEs. Most of SEs occurred along the incision of leaves. This system was shown to be applicable to different genotypes. The highest induction frequency of SEs was 95.0% and the average transformation efficiency based on β-glucuronidase (GUS) assay reached to 75.4%. The transgenic events were further confirmed by Southern blot. Conclusion High frequency induction and transformation of somatic embryos using soybean MSDL as target tissue were developed in this study. This system can be applied to the development of genome editing technology in soybean.
Key words embryogenesis, mature seed-derived leaf (MSDL), somatic embryos (SEs), soybean, transformation
Soybean (Glycine max(L.) Merrill) is an important crop as a source of both protein and oil, so large efforts have been made to develop both efficient regeneration and transformation system for genetic improvement in traits of soybean. Although many improvements in both particle bombardment-mediated transformation andAgrobacterium-mediated transformation of soybean have been made, the transformation efficiency is still low that cannot serve functional genomics research in soybean[1]. The main reason for low transformation efficiency in soybean is the limitation in a high efficient regeneration system that can serve for transformation. Thus, a more useful and efficient regeneration system that can be used for soybean transformation required to be established[2].
Soybean regeneration was achieved through both organogenesis and somatic embryogenesis. Soybean regeneration through organogenesis was successfully obtained using both immature seed-derived tissue such as shoot meristems[3], immature cotyledons[4]and mature seed-derived tissue such as cotyledonary node[5-6], leaf[7], embryonic tip[8], and cotyledonary nodal callus[9-10]. Although regenerationviaorganogenesis has been successfully used as a target system for soybean transformation, somatic embryogenesis is still preferred over organogenesis as transformants derived through embryogenesis are of single cell origin[11]. Somatic embryogenesis was also successfully established by using the explants such as embryonic axes[12], embryonic calli[13], epicotyls and primary leaves[14], immature cotyledons[15-23],immature embryos[24]and embryonic shoot tip[11].However, to date all the explants used to induce somatic embryos in soybean were derived from immature tissues, it has not been reported that somatic embryogenesis was established by using mature seedderived tissues as explants.
We report here high frequency induction and transformation of soybean somatic embryos from mature seed-derived leaf (MSDL). This system has three obvious advantages over the established immature tissue embryogenesis system: (1) the leaf explants are easy to get from germinating soybean mature seeds that are not restricted by seasons; (2)most of somatic embryos occurred along the incision of leaves, so the prepared leaf explants could be directly used as target tissues for genetic transformation; (3) the explants are easy to prepare.The purpose of this study was to develop a new method for somatic embryogenesis and transformation using MSDL (primary leaf and/or secondary leaf) as target tissue in soybean.
1 Materials and methods
1.1 Preparation of MSDL explants
1.1.1Germination of mature seeds
Seeds of soybean varieties such as Jungery,Kennong18, Hefeng25, Hefeng39, K06-82, and Jilinxiaoli1, were surface-sterilized for 24 h using chlorine gas produced by mixing 4 ml of 12 mol/L HCl and 100 ml commercial bleach in a tightly sealed desiccator. Sterilized seeds were transferred to MS[25]medium for germination. The genotype Jungery was tested in all the experiments in the present study besides genotype investigation.
1.1.2Preparation of primary leaf explants
Primary leaves were detached from 1, 3, 5, 7, 10,14-d germinated seedlings. The detached primary leaves were divided vertically against the vein and separated into two sections. Subsequently, we found that SEs occurred along the incision and the main veins. So in the rest of our tests, bigger leaves were divided longitudinally through the midvein, and divided into 1.2×1.2 cm2sections with removing edge and the sections were placed into embryo induction medium (EIM) under fluorescent illumination (5-10 μmol/L photons/m2/s) in 16 h/8 h light/ dark. The EIM includes MS salts, B5 vitamins[26], 2 mg/L 2-(Nmorpholino) ethanesulfonic acid (MES), sucrose(3%), different concentrations (1, 2, 3, 5, 10, 20 mg/L)of 2,4-dichlorophenoxyacetic acid (2,4-D), 0.8% agar(Sigma), pH5.8. We also evaluated the effect of leaf size on the induction of somatic embryos. The size of primary leaves obtained from 5, 7, 10, 14-d germinated seedlings was measured. Five ranges of leaf size were designed: 2.0-4.0 mm,4.1-6.0 mm,6.1-10.0 mm,10.1-14.0 mm, 14.1-17.0 mm. Smaller or larger leaves were discarded. All the explants were placed into EIM supplemented with 10 mg/L 2,4-D and 8 g/L agar.
1.1.3Preparation of other tissue explants
Besides primary leaf, mature cotyledon, petiole,hypocotyl from 7-d germinated seedlings and secondary leaves from 14-d germinated seedlings were also tested. The hypocotyls were cut into 2-3 mm long sections. The cotyledons were cut into 1 mm slices. Petioles were detached from leaves. All explants were cultured on EIM for 4 weeks.
1.2 Induction and maturation of somatic embryos
1.2.1Induction of somatic embryos
The explants were placed into 100 ml Erlenmeyer flasks or 60 mm (diameter)×100 mm (height) glass tubes containing EIM and incubated under the conditions of cool white fluorescent light (5-10 μmol/L photons/m2/s) and a light/dark (16 h/8 h) photoperiod at 26℃.
1.2.2Maturation of somatic embryos
One month later, the induced somatic embryos were matured in FN Lite liquid medium[27]consisting of FN Lite macro salts, MS micro salts, and B5 vitamins supplemented with 1 g/L asparagine, 2 mg/L 2, 4-D, 1% sucrose and adjusted to pH 5.8 and maintained on a rotary shaker at 130 r/min. 2 d latter,leaves without any embryos were discarded and the embryos were transferred to the same fresh liquid medium. The number of the separated embryos was then counted. The embryo germination experiments are in progress. All the experiments were carried out with three replications.
1.3 Vector and Agrobacterium preparation
The vector pCAMBIA1301 was used in this experiment. The T-DNA region of the vector contains geneshygromycin phosphotransferase II(hpt II) andgus(β-glucuronidase) with a catalase intron. The pCAMBIA1301 was introduced into theAgrobacteriumstrain EHA105. TheAgrobacteriumcells harboring pCAMBIA1301 were inoculated into 100 ml liquid YEP containing 50 mg/L kanamycin and 50 mg/L rifampicin and cultured untilA600=1-1.5 at 28°C. TheAgrobacteriumcells were collected and resuspended in infection medium (MS salts, B5 vitamins, 10 mg/L 2,4-D, 2 mg/L MES, 3% sucrose,200 µmol/L acetosyringone, 0.02% Silwet L-77,pH5.4). TheAgrobacteriumcell density was adjusted toA600=0.5 for infection of MSDL explants.
1.4 Transformation of MSDL explants
MSDL explants were prepaired from 7-d germinated seedlings and infected withAgrobacteriumEHA105cells harboring the pCAMBIA1301 in the infection medium in Petri dishes for 15-30 min at room temperature. During this infection, the Petri dishes were shaken every 2-3 min to allow good contact of the explants with theAgrobacteriumcells. After drying the leaves on sterilized filter paper, the infected explants were transferred to cocultivation medium (MS salts, B5 vitamins, 10 mg/L 2,4-D, 2 mg/L MES, 3% sucrose,200 µmol/L acetosyringone, 0.8% agar (Sigma),pH5.4). The cocultivation was maintained for 3 d at 25°C in the dark. After co-cultivation, the explants were washed in liquid EIM containing 100 mg/L Timentin to remove excessA. tumefacienson the explants. The explants were then transferred to recovery medium (EIM containing 250 mg/L Timentin) to stimulate embryo induction for the first 7 d under fluorescent illumination (5-10 mol/L photons/m2/s) with 16 h light/8 h dark at an average of 26°C. Then the explants were transferred to the EIM containing 8 mg/L hygromycin for 20-30 d to induce embryogenesis as well as select for resistant embryos.The leaves with SEs and SEs isolated from leaves were used for GUS assay and molecular identification.
1.5 Histochemical GUS assay
Histochemical GUS staining of leaves and SEs was carried out as described by Jeffersonet al.[28]. The resistant leaves with SEs and SEs isolated from leaves were stained in GUS assay buffer (50 mmol/L phosphate buffer at pH7.0, 10 mmol/L EDTA,1 mmol/L 5-bromo-4-chloro-3-indolyl-β-glucuronic acid (X-Gluc), 5 mmol/L K3Fe(CN)6, 5 mmol/L K4Fe(CN)6, and 1.0% Triton X-100) and incubated overnight at 37°C. Subsequently, the chlorophyll was removed using several applications of 95% ethanol at 37°C, and kept in 95% ethanol at 4°C before being photographed.
1.6 Southern blot analysis
10 μg of genomic DNA was digested withHindIII and separated on 0.8% agarose gel and transferred onto a nylon Hybond-N+membrane(Roche, Germany). The PCR products of thehpt IIwere used as probes by labelling with digoxigenin(DIG) and hybridized to the digested genomic DNA on the membrane. All steps of the probe labelling,membrane transfer and hybridization were performed according to the instructions of the manufacturer(Roche, Germany).
2 Results and discussion
2.1 Effect of leaf age and 2,4-D concentration on the SEs induction
Both leaf age (germination day) and 2, 4-D concentration had a significant effect on induction of soybean SEs. When the MSDL was cultured on SEs induction medium, the leaf tissue continued to grow and expand. During the 3-4 weeks on this medium,globular SEs developed from the leaves (Figure 1b).After suspension culture of SEs in FN Lite liquid medium, some globular embryos (Figure 1d)developed into cotyledonary embryos (Figure 1e). The MSDL from 1 to 5-d germinated seedlings produced SEs at low 2,4-D concentrations, the percent induction of SEs reached highest at 2 mg/L 2,4-D and then decreased as 2,4-D concentration increased from 2 to 10 mg/L (Table 1). While the induction frequency of the SEs from 7 to 14-d MSDL increased as 2,4-D concentration increased from 2 to 10 mg/L (Table 1).These results suggest an interaction between leaf age and 2, 4-D concentration for SEs induction. A synergistic effect of combination of L-cysteine and dithiothreitol (DTT) on soybean transformation efficiency was reported by Olhoftet al.[1]. The transformation efficiency was significantly higher when L-cysteine and DTT were combined than either L-cysteine or DTT alone. Franklinet al.[4]also reported that the regeneration efficiency in soybean was significantly higher in the medium supplemented with both benzylaminopurine (BAP) and thidiazuron(TDZ) than in the medium supplemented with one cytokine alone. Similarly, the frequency of soybean shoot induction, shoot elongation and transformation remarkably enhanced in medium supplemented with both L-asparagine and L-glutamine compared with medium supplemented with either L-asparagine or L-glutamine alone[29]. In this study, the highest frequency (94.1%) of the SEs induction was observed when 7-d MSDL cultured on induction medium containing 10 mg/L 2,4-D.
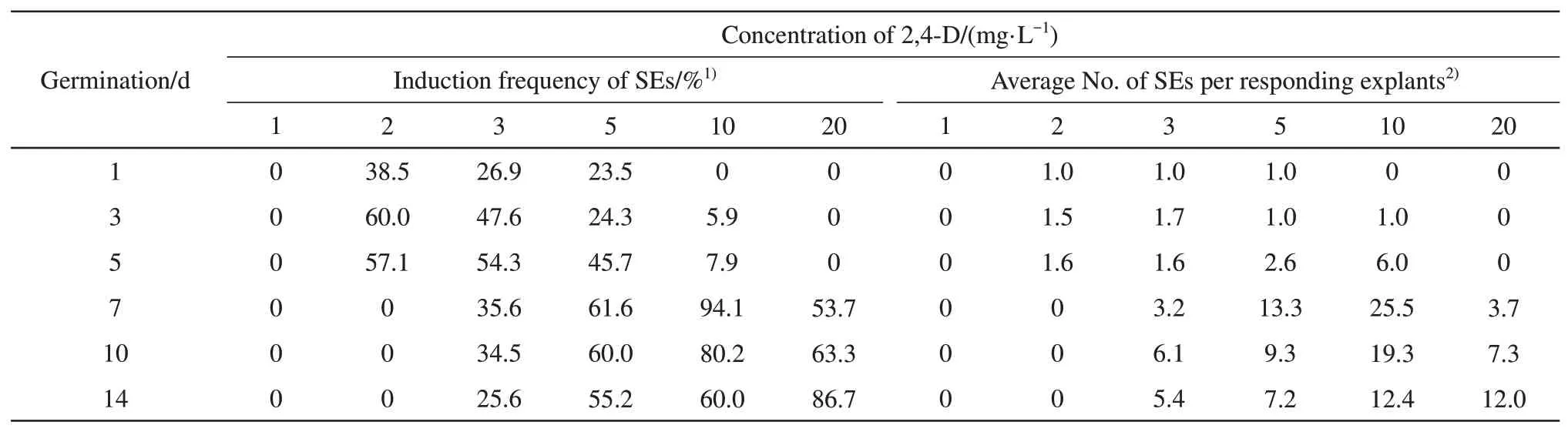
Table 1 Effect of leaf age and 2,4-D concentration on SEs induction
Highly significant effects for average number of SEs per responding explant were observed in different age MSDL. The younger MSDL produced a large amount of callus, but very small amount of SEs was induced from a responding callus. For 1 to 5-d MSDL, the average number of SEs per responding explant ranged from 1.0 to 6.0 (Table 1).The average number of SEs per responding explant was significantly greater for 7 to 14-d MSDL than for 1 to 5-d MSDL at the same concentration of 2, 4-D(Table 1). The highest production was 25.5 embryos per explant from 7-d MSDL under 10 mg/L 2,4-D(Table 1). Although SEs were also produced from the MSDL at 20 mg/L 2,4-D, the callus induced from MSDL became necrotic (Data not shown), indicating the concentration of 2,4-D is too high for MSDL.Rajasekaran and Pellow[14]reported similar result that high concentration of 2,4-D inhibited further growth of the isolated immature epicotyls and primary leaves.Previous reports indicated that somatic embryogenesis in soybean was obtained either with low[30-31]or high 2,4-D concentrations[16,32]. This could be attributed to the differences in the explants sensitivity to 2,4-D[20].
2.2 Effect of leaf size on the SEs induction
Size of the MSDL is also very important in determining both induction frequency and the number of SEs. The optimum size range is 10.1-14.0 mm.The MSDL less than 4.0 mm in size produced only callus and those range from 4.1 mm to 6.0 mm in size produced callus and a small amount of SEs (Table 2).Similar results were reported by Wrightet al.[7]that the size of the explants was very important in obtaining shoot formation from immature primary leaves of soybean. It has been also reported that the optimum size of immature seed-derived cotyledons for somatic embryogenesis in soybean was 3-4 mm in length[15,33].
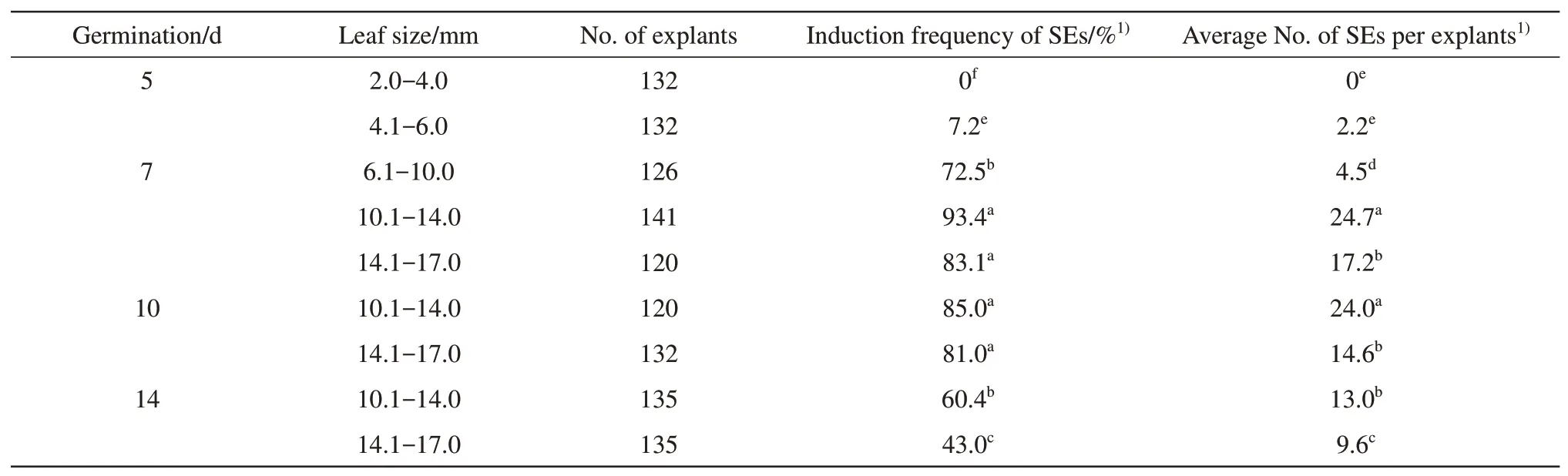
Table 2 Effect of leaf size on SEs induction
2.3 Effect of the culture vessel on the SEs induction
Culture vessel is crucial for induction of SEs in this study. MSDL cultured in different culture vessels gave different responses for induction of SEs. The MSDL cultured in petri dishes gave the poor responses with induction frequency of 1.3% whereas the explants cultured in flasks or glass tubes both with vent-containing closure produced high frequency of SEs induction (Table 3). The similar results were also reported by Wrightet al.[7]. Although the reasons for the difference in response are not exactly known, the difference in ventilation could be one of the most important factors because the induction frequency of SEs significantly reduced from 89.3% to 15.1% when the MSDL was cultured in the glass tubes with no vent closure under otherwise same culture conditions(Table 3).
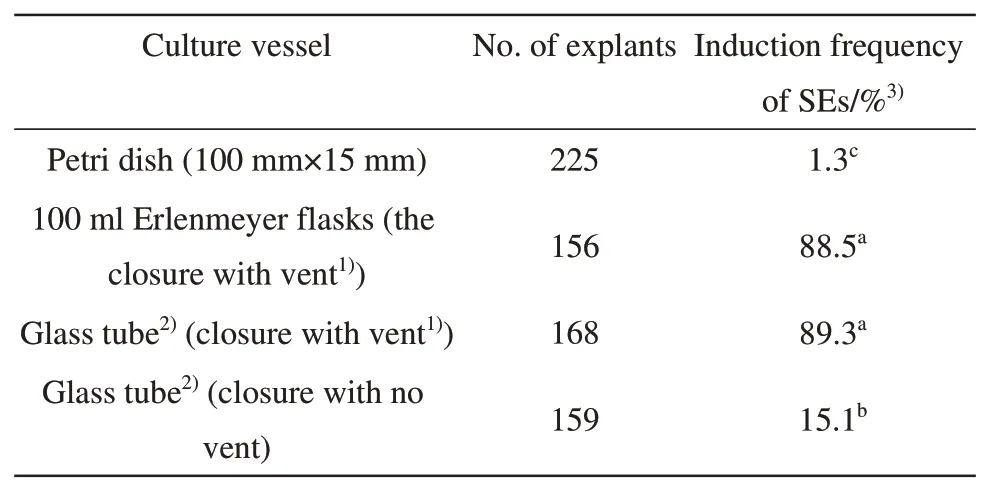
Table 3 Effect of the culture vessel on the SEs induction
2.4 Effect of explants on the SEs induction
In recent work, a wide range of mature and immature seed-derived explants of soybean cv. K10 were used to evaluate the responses for SEs induction.Unfortunately, all mature seed-derived explants(shoot, leaf, root, cotyledon and embryonic tip) did not induce SEs[11]. In the present study, we also evaluated the effect of mature seed-derived explants(primary leaf, secondary leaf, petiole, cotyledon and hypocotyl) of soybean cv. Junsery on the SEs induction. The explants such as petiole, cotyledon and hypocotyl did not produce SEs at any of the concentrations of 2,4-D tested, but both the primary leaf and secondary leaf were able to produce SEs(Table 4).
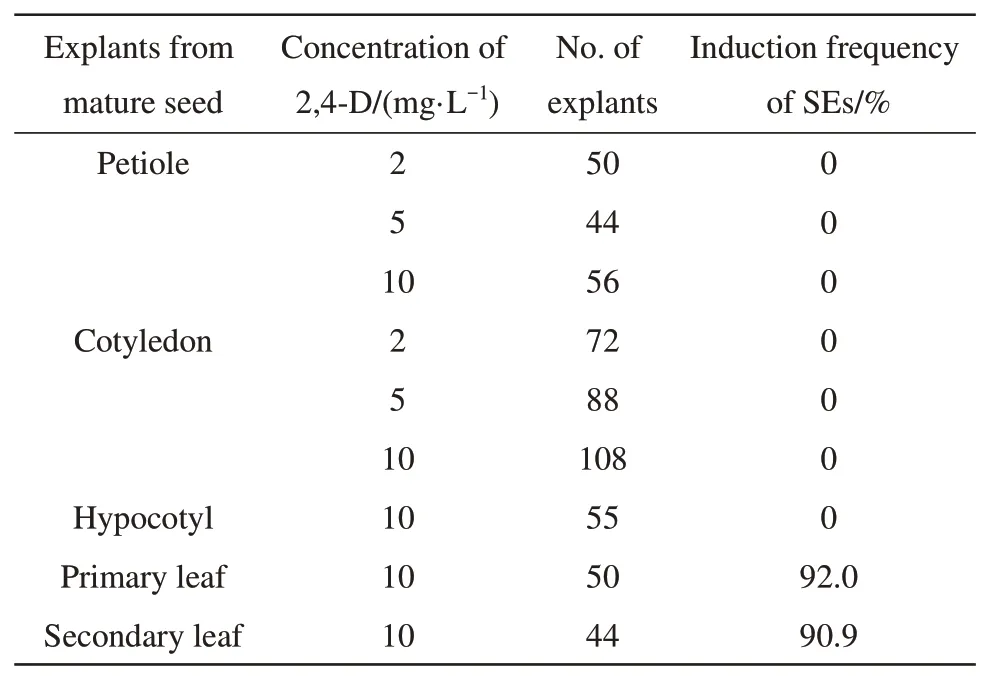
Table 4 Effect of explants on the SEs induction
2.5 Effect of genotype on the SEs induction
The six genotypes Kennong18, Hefeng25,Henfeng39, K06-82, Junsery and Jilinxiaoli1 were used to study the effects on SEs induction. Although the six genotypes gave the different responses for the SEs induction, high frequency (>80%) of SEs induction was observed in all genotypes tested(Table 5). MSDL of genotype Junsery gave the highest induction frequency (95.0%) of SEs and the greatest average number (28.5) of embryos per explant.
2.6 High efficient transformation of MSDL
In order to verify whether the SEs system can be applied to soybean transformation, theAgrobacteriummediated soybean transformation was carried out using leaves of cv. Junsery as target tissue. Four weeks after culturing infected leaves, somatic embryos were induced from leaves on selective medium (Figure 1f).
The leaves with somatic embryos (Figure 1g)and somatic embryos (Figure 1h) were tested for GUS expression. The results showed that the average transformation efficiency was 75.4%, and reached a maximum of 80.6% (Table 6), indicating that leaves were excellent target tissues for soybean transformation. The results of Southern blot confirmed that the T-DNA was integrated into the genome, and the copy number ofhptIIranged from one to three (Figure 1i).
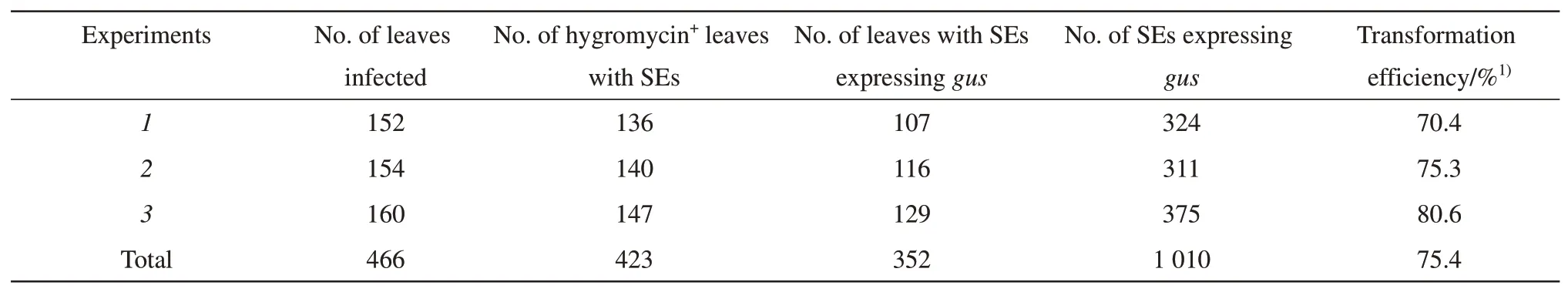
Table 6 Efficiency of MSDL transformation in soybean
Knowledge of the origin and timing of embryo induction could help to target the proper tissue at the proper time[2]. Earlier studies reported that soybean SEs originated from epidermal and subepidermal cells[2,34]. Because the target tissue used for transformation in this system was cells that lie below the surface, so proper wounding of the tissue was needed. In the present study, a majority of SEs was induced along the cut edges of the MSDL (Figure 1c).This origin of SEs induction could make our tissue culture system suitable forAgrobacterium-mediated transformation in soybean.
3 Conclusion
High frequency induction and transformation of somatic embryos using mature seed-derived leaf of soybean as target tissue were developed in this study.The highest induction frequency of SEs was 95.0%and the average transformation efficiency based on GUS assay reached up to 75.4%. This system can be applied to the development of soybean genome editing technology.
