李小食心虫GfunOBP2的原核表达及气味配体结合特性
2023-07-09年和粉张钰析李伯辽陈秀琳罗坤李广伟
年和粉,张钰析,李伯辽,陈秀琳,罗坤,李广伟
李小食心虫GfunOBP2的原核表达及气味配体结合特性
年和粉1,2,张钰析2,李伯辽1,2,陈秀琳1,2,罗坤1,2,李广伟1,2
1陕西省红枣重点实验室(延安大学),陕西延安 716000;2延安大学生命科学学院,陕西延安 716000
【目的】通过测定李小食心虫()Plus-C气味结合蛋白2(odorant binding protein,GfunOBP2)结合性信息素和苹果树挥发化合物的能力,分析GfunOBP2的嗅觉功能,为阐释李小食心虫定位寄主植物的嗅觉分子机理打下基础。【方法】利用RT-PCR扩增2的ORF序列,通过同源注释和比对氨基酸序列中半胱氨酸(Cys)的分布模式确定GfunOBP2属于Plus-C OBP亚家族;利用RT-qPCR检测2在李小食心虫3日龄成虫触角、头、胸、足、翅、腹部和性腺中的相对表达量;构建pET30a(+)/GfunOBP2原核表达载体,在大肠杆菌()BL21(DE3)细胞中表达GfunOBP2重组蛋白。利用荧光竞争结合试验测定重组GfunOBP2蛋白对5种性信息素和35种苹果树挥发化合物的结合能力;通过分子对接预测GfunOBP2与具有强结合能力的气味配体的相互作用力及关键氨基酸残基。【结果】克隆获得2(GenBank登录号:OQ054799.1)的全长序列,共编码183个氨基酸,氨基酸序列中有12个保守的Cys,其分布模式表明2属于Plus-C OBP。2主要在成虫触角中表达,在雄虫触角中的相对表达量显著高于雌虫触角(<0.05)。重组GfunOBP2蛋白对反-2-己烯-1-醇、苯甲醇、1-庚醇、1-癸醇、己醛、庚醛、乙酸-顺-3-己烯酯、2-甲基丁酸叶醇酯、-罗勒烯、-石竹烯、-蒎烯和柠檬烯具有强结合能力,抑制常数Ki均小于5.0 μmol·L-1。分子对接结果显示氢键、Donor-Donor相互作用和烷基相互作用是GfunOBP2结合反-2-己烯-1-醇、1-庚醇和1-癸醇的主要弱相互作用力,氢键和碳氢键是GfunOBP2结合乙酸-顺-3-己烯酯和2-甲基丁酸叶醇酯的主要弱相互作用力,烷基相互作用是GfunOBP2结合-罗勒烯和-石竹烯的唯一弱作用力。疏水性氨基酸Ile、Pro、Phe、Ala、Leu和Val在GfunOBP2结合气味配体中起着重要作用。【结论】2主要在李小食心虫成虫触角中表达,重组GfunOBP2蛋白对被测的35种苹果树挥发化合物中的12种具有强结合能力、对10种化合物具有中等结合能力,表明其在识别寄主植物挥发化合物的过程中起着重要作用。研究结果为证实Plus-C OBP参与李小食心虫外周嗅觉通讯提供了理论依据。
李小食心虫;化学感受;气味结合蛋白;寄主植物挥发物;分子对接
0 引言
【研究意义】李小食心虫()是鳞翅目卷蛾科的一种重要果树害虫,主要以幼虫蛀果危害,常造成幼果大量脱落,严重影响水果的产量和品质[1-2]。李小食心虫的寄主有李、郁李、杏、樱桃、桃等,其中以李受害最重[3-4]。近年来,随着我国北方苹果种植面积的不断扩大,李小食心虫在新疆、陕西、甘肃、吉林等地苹果园的种群数量逐年增加,危害日趋严重,在部分苹果种植区,李小食心虫已成为果园食心虫的优势种类[5-12]。目前,利用性诱剂诱捕和迷向丝迷向是无公害防治该虫的主要措施[13-14]。由于李小食心虫雄蛾具有多次交配的习性,在性诱剂或者迷向剂防治的果园,雌虫仍可获得较高的交配率,仅依靠诱捕和迷向防治不能达到理想的效果。深入开展李小食心虫定位寄主植物的嗅觉感受机制研究,筛选能够显著增效性信息素的寄主植物挥发性化合物,可为以嗅觉通讯系统为靶标设置“陷阱”开发绿色、高效的李小食心虫行为调控技术提供新思路。【前人研究进展】昆虫,特别是鳞翅目昆虫,雄虫主要依靠雌虫释放的性信息素寻觅配偶完成交配,雌虫借助寄主植物释放的挥发化合物定位最适合子代幼虫生长的产卵寄主植物[15-18]。性信息素和寄主植物挥发化合物分子通过昆虫触角感器表皮上的微孔进入其内,气味结合蛋白(odorant binding protein,OBP)能够选择性地识别和结合气味分子,并携带疏水性的气味分子穿过亲水性的感器淋巴液屏障[19-20]。当OBP/气味分子复合物移动至接近位于嗅觉感受器神经元(olfactory receptor neuron,ORN)树突膜上的气味受体(odorant receptor,OR)时,由于感器淋巴液酸性逐渐增强导致OBP构象发生变化从而释放气味分子,气味分子被OR识别后激活了OR/Orco(气味共受体,odorant receptor co-receptor)异源四聚体组成的门控离子通道,将气味分子的化学信号转变为电生理信号[21-24]。OBP作为昆虫嗅觉通讯系统中重要的运输蛋白参与了外周嗅觉识别的初始过程。OBP是一类分子量小、水溶性强、在触角感器中高浓度(可达10 mmol·L-1)存在的球状蛋白[25-26]。OBP通常编码140—180个氨基酸,典型OBP具有6个保守的半胱氨酸(cysteine,Cys)残基、1个疏水性的结合口袋以及3对二硫键[27]。在昆虫长期的进化过程中,OBP氨基酸序列中保守的Cys数量发生了缺失或增加,根据保守Cys数量,将OBP分为典型OBP、Minus-C OBP(具有4个Cys,第2和第5个Cys被其他氨基酸取代)和Plus-C OBP(具有8—12个Cys,在第4和第5个Cys之间存在1个Cys4a,第6个Cys后具有Cys6a和Cys6b)[28-30]。目前,通过敲除(knockout)、敲低(knockdown)昆虫OBP基因表达以及利用荧光竞争结合试验测定重组OBP结合配体的能力,证实典型OBP在昆虫识别性信息素或寄主植物挥发化合物中起着关键性的作用[31-34]。相比于典型OBP,Plus-C OBP在昆虫中数量少,对其功能研究相对不足,其在嗅觉通讯中的生理功能有待深入研究。【本研究切入点】本课题组前期成功构建了李小食心虫触角cDNA文库并进行了二代转录组测序,从中鉴定到23种OBP,其中22种OBP属于典型OBP,1种OBP属于Plus-C OBP(命名为GfunOBP2)[11],借助原核表达系统和荧光竞争结合试验对典型OBP亚家族中的3种普通气味结合蛋白(general odorant binding protein,GOBP)和4种信息素结合蛋白(pheromone binding protein,PBP)的嗅觉功能进行了研究,发现GfunGOBP能够结合性信息素和寄主植物挥发化合物,GfunPBP对性信息素具有强结合能力[11-12]。GfunOBP2属于Plus-C OBP,在序列长度、保守半胱氨酸分布模式、结合口袋形成等方面与典型OBP存在较大差异,GfunOBP2的嗅觉功能有待进一步明确。【拟解决的关键问题】明确GfunOBP2与性信息素和寄主挥发化合物的结合特性,为Plus-C OBP在李小食心虫外周嗅觉通讯中的生理功能研究提供理论依据,进一步为开发以寄主植物挥发化合物为增效剂的高效性诱剂打下基础。
1 材料与方法
试验于2022年在延安大学生命科学学院完成。
1.1 试虫来源及组织样品收集
所用试虫为李小食心虫的雌、雄成虫,其老熟幼虫采自于延安市宝塔区李渠镇的杏园,在杏成熟期将老熟幼虫从果实中移出置于潮湿的沙土中结茧化蛹。成虫羽化后分雌、雄置于一次性塑料杯中饲养并饲喂5%蜂蜜水补充营养。不同发育阶段的试虫均在人工气候箱中饲养,饲养条件:温度(25±1)℃,相对湿度60%±5%,光周期16L﹕8D。选择3日龄健康活泼的成虫分雌、雄分别收集触角(400个)、头(去除触角)(40个)、胸(20个)、足(100个)、翅(50对)、腹(去除性腺)(10个)、性腺(50个)作为测定2在成虫不同组织中相对表达量的样品。每个样品3次生物学重复。各样品收集后立即转至浸于液氮中的无酶离心管中,后保存于-80 ℃低温冰箱待用。
1.2 GfunOBP2基因克隆和序列结构分析
使用TransZol Up试剂(TransGen Biotech.,中国)参照说明书提取1.1节中所有样品的总RNA。以2 μg总RNA为底物模板、oligo(dT)18为引物,利用Thermo scientific RevertAid MM Kit试剂盒(ThermoFisher,美国)反转录合成cDNA第1链。以表1中GfunOBP2- F/R为引物、雄虫触角cDNA为模板、2×Taq Plus Master Mix II (Dye Plus)为聚合酶(Vazyme,中国)扩增2 ORF的全长序列。PCR目标产物使用SteadyPure DNA凝胶回收试剂盒(艾科瑞生物,中国)纯化回收后连接至TA/Blunt-Zero克隆载体(Vazyme,中国),然后转入大肠杆菌DH5感受态细胞,随机选取3个阳性克隆送测序公司测序验证。利用BLASTX比对非冗余蛋白数据库(non-redundant protein sequence database,Nr)对GfunOBP2进行同源注释,选取葡萄花翅小卷蛾()LbotOBP35、大豆食心虫()LglyOBP等17个鳞翅目昆虫Plus-C OBP的氨基酸序列分析GfunOBP2保守半胱氨酸残基的分布模式。利用在线程序SignalP-4.1(https://services.healthtech.dtu. dk/services/SignalP-4.1/)预测GfunOBP2的信号肽、ProtParam tool(https://web.expasy.org/protparam/)计算蛋白质的分子量和理论等电点pI。
1.3 GfunOBP2在成虫不同组织中的相对表达量检测
以1.2节合成的第1链cDNA为模板、李小食心虫和(GenBank登录号:JX258663.1)为双内参,利用实时荧光定量PCR系统(StepOnePlusTM,ABI,美国)检测2在李小食心虫3日龄雌、雄成虫触角、头(去除触角)、胸、足、翅、腹(去除性腺)和性腺中的相对表达量。首先,以李小食心虫雄虫触角cDNA为模板,用无酶水4倍梯度稀释至4-6,利用表1中基因表达量检测引物,优化目标基因和内参基因的扩增条件,使2、和的扩增效率在90%—105%且熔解曲线显示无非特异性的扩增。然后,以获得的最优RT-qPCR条件测定2在李小食心虫雌、雄成虫不同组织中的相对表达量。反应条件:94 ℃预变性30 s;94 ℃变性5 s,60 ℃(2和)/59 ℃()退火15 s,72 ℃延伸10 s,共40个循环。每个样品3次生物学重复,每个生物学样品3次技术重复。参照文献[35-36]方法计算2在不同组织中的相对表达量。

表1 引物信息
下划线表示内切酶H I和d III的酶切位点The cutting sites of endonuclease ofH I andd III are underlined
1.4 重组GfunOBP2的原核表达及纯化
设计去除信号肽、正反向引物5′端分别带有H I和d III酶切位点的特异性引物用于原核表达重组GfunOBP2蛋白(表1)。以李小食心虫雄虫触角cDNA为模板、GfunOBP2-eF/eR为引物,利用2×Taq Plus Master Mix II(Dye Plus)聚合酶扩增目的片段。将PCR目的产物经胶纯化回收后连接至TA/Blunt-Zero克隆载体,提取TA/Blunt-Zero/GfunOBP2重组质粒后用H I和d III内切酶对其进行酶切,同时用以上两种内切酶对表达载体pET30a(+)进行酶切,然后利用T4 DNA连接酶(TaKaRa,大连)将酶切回收后的GfunOBP2 DNA片段连接至线性化的pET30a(+)载体上,先转DH5感受态细胞,提取阳性克隆的质粒后再转至BL21 (DE3)感受态细胞。将成功转化pET30a(+)/GfunOBP2质粒的大肠杆菌()BL21 (DE3)单克隆菌种扩大培养后,利用终浓度0.5 mmol·L-1异丙基--d-硫代半乳糖苷(isopropyl--d-thiogalactoside,IPTG)进行诱导表达,十二烷基硫酸钠聚丙烯酰胺凝胶电泳(SDS-PAGE)检测发现重组GfunOBP2蛋白以包涵体的形式存在,参照Calvello等[37-38]的方法对GfunOBP2的包涵体进行变性和复性处理。利用Ni-NTA His·Bind Resin(7sea Biotech.,中国)参照说明书对重组GfunOBP2蛋白进行纯化,利用肠激酶(New England Biolabs.,英国)切除重组蛋白的His-tag,使用BCA蛋白定量试剂盒(中晖赫彩,中国)测定重组GfunOBP2蛋白的浓度。
1.5 重组GfunOBP2蛋白结合气味配体的能力
以李小食心虫雌虫5种性信息素[39]和苹果树35种挥发化合物[40-41]的化学合成品作为待测配体,测定重组GfunOBP2蛋白结合气味配体的能力。性信息素乙酸-顺-8-十二碳烯酯、乙酸-反-8-十二碳烯酯和顺-8-十二碳烯醇(有效成分均>98%)购自北京中捷四方生物科技股份有限公司,乙酸-顺-8-十四碳烯酯和乙酸-顺-10-十四碳烯酯(有效成分均>90%)委托沈阳北欣景溢贸易有限公司合成。35种挥发化合物来自苹果树的花、叶片和果实,对应的化学合成品购自化学试剂公司。用色谱级甲醇将荧光探针N-苯基-1-萘胺(N-phenyl-1-naphthylamine,1-NPN)和气味配体配制成100 mmol·L-1的母液,然后稀释成1 mmol·L-1的工作液。将重组GfunOBP2蛋白用20 mmol·L-1Tris-HCl(pH 7.4)稀释至2 μmol·L-1的反应浓度。利用荧光分光光度计(F-2700,日本日立公司)在激发光337 nm、扫描发射波长370—550 nm条件下测定重组GfunOBP2蛋白结合气味化合物的能力。参照实验室前期测定重组OBP蛋白结合气味配体的方法,依次测定重组GfunOBP2蛋白与荧光探针1-NPN的抑制常数(K1-NPN)以及与不同气味配体的抑制常数(Ki)[42-43]。根据公式Ki=IC50/[l+(1-NPN)/Kl-NPN]计算GfunOBP2与气味配体的抑制常数Ki。IC50表示配体替代1-NPN使蛋白/1-NPN复合物的初始荧光强度值降低50%时的浓度;1-NPN表示未与蛋白结合的1-NPN浓度。为了更规范地描述GfunOBP2结合配体能力的强弱,参照Huang等[44]的方法,K<5、5≤K<10和K≥10 μmol·L-1分别表示蛋白对配体化合物具有强烈、中等和较弱的结合能力。
1.6 GfunOBP2同源建模及分子对接
以去除信号肽的GfunOBP2序列为查询序列,利用在线工具NCBI BLASTP(https://www.ncbi.nlm.nih. gov/)在蛋白质数据库(Protein Data Bank,PDB)比对与GfunOBP2序列一致性较高的模板序列,根据GfunOBP2与模板序列的覆盖度和相似性,选择以冈比亚按蚊()AgamOBP47(PDB: 3PM2)的晶体结构为模板利用SWISS-MODEL在线程序(https://swissmodel.expasy.org/)构建GfunOBP2的同源模型,GfunOBP2的3D结构,包括-螺旋、二硫键等的显示利用PyMOL(v 2.5.2)软件完成。由于在荧光竞争结合试验结果中发现GfunOBP2对反-2-己烯-1-醇、1-庚醇、1-癸醇、乙酸-顺-3-己烯酯、2-甲基丁酸叶醇酯、-罗勒烯和-石竹烯具有强结合活性,Ki值均≤4 μmol·L-1。故选择以上7种气味配体利用AutoDockTools-1.5.6软件通过分子对接预测GfunOBP2结合气味配体的弱相互作用力和关键氨基酸残基[45]。蛋白与配体对接的3D结构图和2D结构示意图分别用PyMOL(v 2.5.2)和Discovery Studio 2016(v 16.1)软件显示。
1.7 数据分析
使用单因素方差分析、Tukey多重比较检验2在雌、雄虫不同组织中相对表达量的差异显著性,相同组织在不同性别中相对表达量的差异用独立样本检验进行检验。
2 结果
2.1 GfunOBP2的克隆和序列特征
2(GenBank登录号:OQ054799.1)ORF全长552 bp,编码183个氨基酸,N端有一个由18个氨基酸组成的信号肽。成熟的GfunOBP2蛋白其预测的分子量和等电点分别为20.41 kDa和4.73。将GfunOBP2与其他17种鳞翅目昆虫的Plus-C OBP的氨基酸序列进行比对,结果发现GfunOBP2具有12个保守的Cys位点,Cys分布符合Cys1-X12-Cys1a -Cys1b-X13-Cys1c-X19-Cys2-X4-Cys3-X43-Cys4-X11-Cys4a-X7-Cys5-X8-Cys6-X8-Cys6a-X9-Cys6b(X为除Cys以外的其他氨基酸)的模式。此外,Cys6残基后直接连接一个脯氨酸残基也是Plus-C OBP的重要特征。BLASTX比对发现GfunOBP2与葡萄花翅小卷蛾LbotOBP35的序列一致性最高(达79.78%),与大蜡螟()GmelGOBP66-like的序列一致性次之(达56.28%),与种内其他22个GfunOBP的序列一致性较低,介于1.6%—9.8%,表明GfunOBP2与近缘种间昆虫Plus-C OBP同源性更高,而与种内其他典型OBP的同源性低,分化程度高。
2.2 GfunOBP2的组织表达
RT-qPCR检测结果表明,2在雌、雄成虫触角、头、胸、足、翅、腹部和性腺中均有表达,但相对表达量存在显著差异(雌虫:=1 743.427,=6,14,<0.001;雄虫:=7 436.579,=6,14,<0.001)(图1)。2主要在触角中表达,相对表达量显著最高其他组织。2在触角、头、翅和性腺中的相对表达量存在性别差异,在雄虫触角(=6.318,=4,=0.003)、头(=9.674,=4,=0.001)和性腺(=-17.959,=4,<0.001)中的相对表达量极显著高于雌虫中的,雌虫翅中的相对表达量极显著高于雄虫中的(=47.399,=4,<0.001)。2在雌、雄虫胸(=0.382,=4,=0.722)、足(=0.650,=4,=0.551)和腹(=-2.406,=4,=0.074)中的相对表达量无显著差异。从2偏好表达于触角中的特点推测其可能参与成虫的嗅觉通讯过程。
2.3 GfunOBP2重组蛋白的原核表达和纯化
SDS-PAGE检测显示,重组GfunOBP2蛋白以包涵体的形式存在,经变性、复性以及利用Ni-NTA His·Bind Resin融合标签蛋白纯化树脂纯化蛋白后得到约24 kDa的蛋白条带,这与预测的含有His-tag的重组蛋白分子量大小(His-tag:5.45 kDa,去除信号肽的GfunOBP2:18.15 kDa)相一致。为了消除His-tag影响重组GfunOBP2蛋白对配体的结合能力,用肠激酶成功切除了His-tag(图2)。

图中数据为平均值±标准误(n=3)。柱上不同小写字母和大写字母分别表示雌虫和雄虫不同组织间的基因表达量差异显著(单因素方差分析,Tukey多重比较,P<0.05)。双星号和ns分别表示基因相对表达量在雌、雄虫相同组织中差异极显著(P<0.01)和不显著(P>0.05)(独立样本t检验)

M:蛋白质分子量标准 Protein molecular weight marker;1:未经IPTG诱导的GfunOBP2表达产物GfunOBP2 expression product without IPTG induction;2:IPTG诱导后的GfunOBP2表达产物GfunOBP2 expression product after IPTG induction;3:IPTG诱导GfunOBP2表达产物的上清Supernatant of GfunOBP2 expression product induced by IPTG;4:IPTG诱导GfunOBP2表达产物的包涵体Inclusion body of GfunOBP2 expression product induced by IPTG;5:纯化的GfunOBP2蛋白Purified GfunOBP2 protein;6:肠激酶切除His-tag后的GfunOBP2蛋白GfunOBP2 protein after excision of His-tag using enterokinase
2.4 重组GfunOBP2蛋白与气味配体的结合能力
由图3可以看出,重组GfunOBP2蛋白和1-NPN的结合存在饱和效应,利用Scatchard方程计算出GfunOBP2和1-NPN的抑制常数(K1-NPN)为(4.96±0.85)μmol·L-1。荧光竞争结合试验显示,在待测气味配体最大终浓度值为16 μmol·L-1时,40种气味配体中有31种配体能够成功替代1-NPN将GfunOBP2/1-NPN复合物的初始荧光强度值降低50%以上,表明GfunOBP2的结合谱较宽(表2)。在待测的5种李小食心虫雌虫性信息素中,GfunOBP2对乙酸-顺-8-十二碳烯酯、乙酸-反-8-十二碳烯酯、顺-8-十二碳烯醇和乙酸-顺-8-十四碳烯酯具有中等结合能力。在9种醇类配体中,GfunOBP2对反-2-己烯-1-醇、苯甲醇、1-庚醇和1-癸醇具有强结合能力,对1-辛烯-3-醇和异辛醇的结合能力较弱,对2-丁氧基乙醇和芳樟醇无明显的结合能力。在8种醛类配体中,GfunOBP2对己醛和庚醛表现出强结合能力,对十一醛和苯甲醛无明显的结合能力,对反-2-己烯醛、辛醛、壬醛和癸醛具有中等结合能力。在10种酯类配体中,GfunOBP2能够强烈结合乙酸-顺-3-己烯酯和2-甲基丁酸叶醇酯,对戊酸乙酯和肉豆蔻酸丁酯无明显结合能力,对其他6种物质表现出中等或者较弱的结合能力。在2种酮类挥发物中,GfunOBP2对6-甲基-5-庚烯-2-酮具有中等结合能力,对2-甲基-6-亚甲基-1,7-辛二烯-3-酮无明显结合能力。在6种萜烯类挥发物中,-罗勒烯和-石竹烯是所有待测物质中GfunOBP2最偏好结合的化合物。此外,GfunOBP2对-蒎烯和柠檬烯也表现出强结合能力。
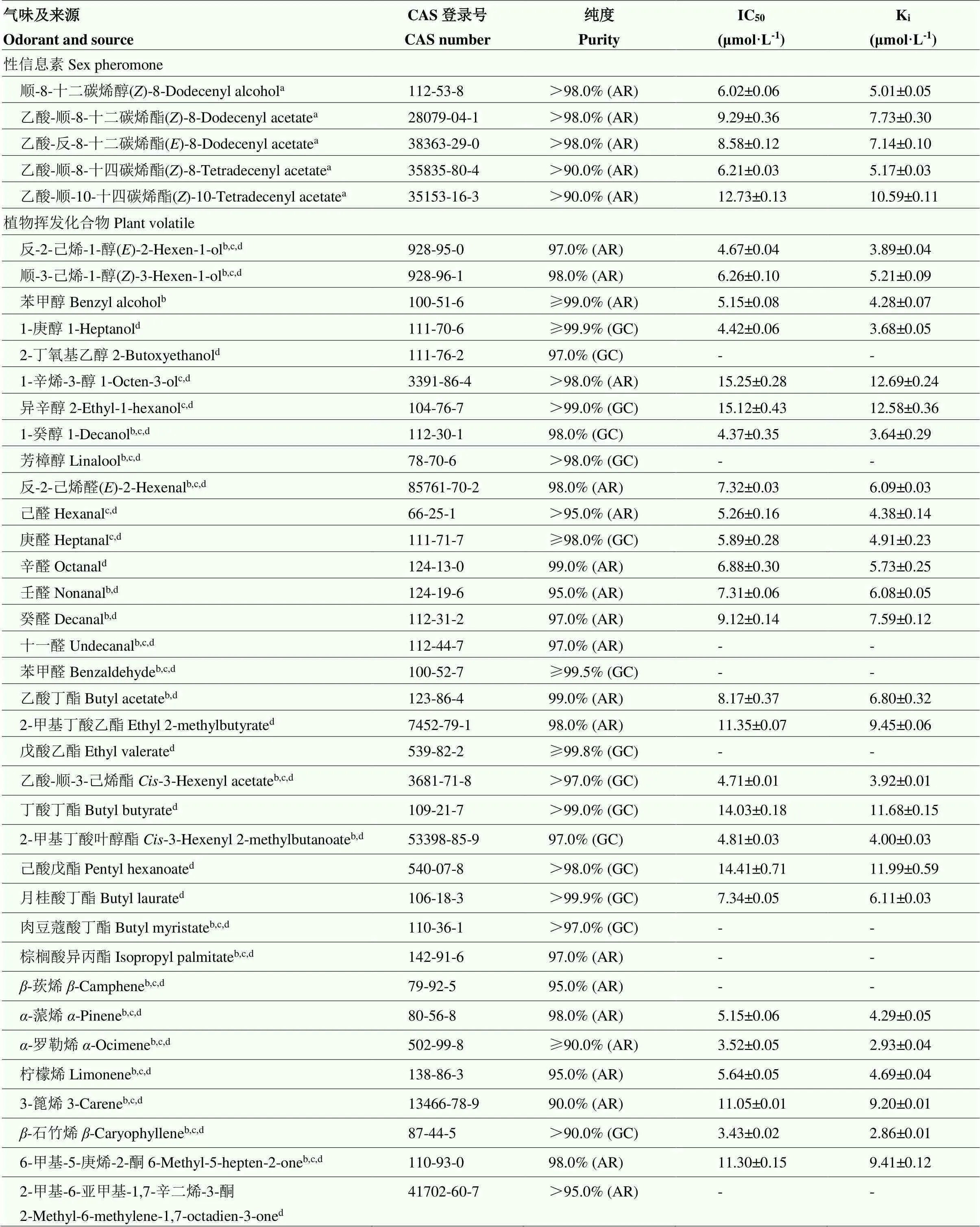
表2 重组GfunOBP2蛋白对性信息素和苹果树挥发化合物的结合能力
挥发化合物来源(根据文献报道)Origins of volatiles according to literature reports:a:李小食心虫雌成虫性信息素Sex pheromones of female adult of;b:苹果花Apple flowers;c:苹果叶片apple leaves;d:苹果果实apple fruits。“-”:无法计算出IC50和Ki值The values of IC50and Kican’t be calculated
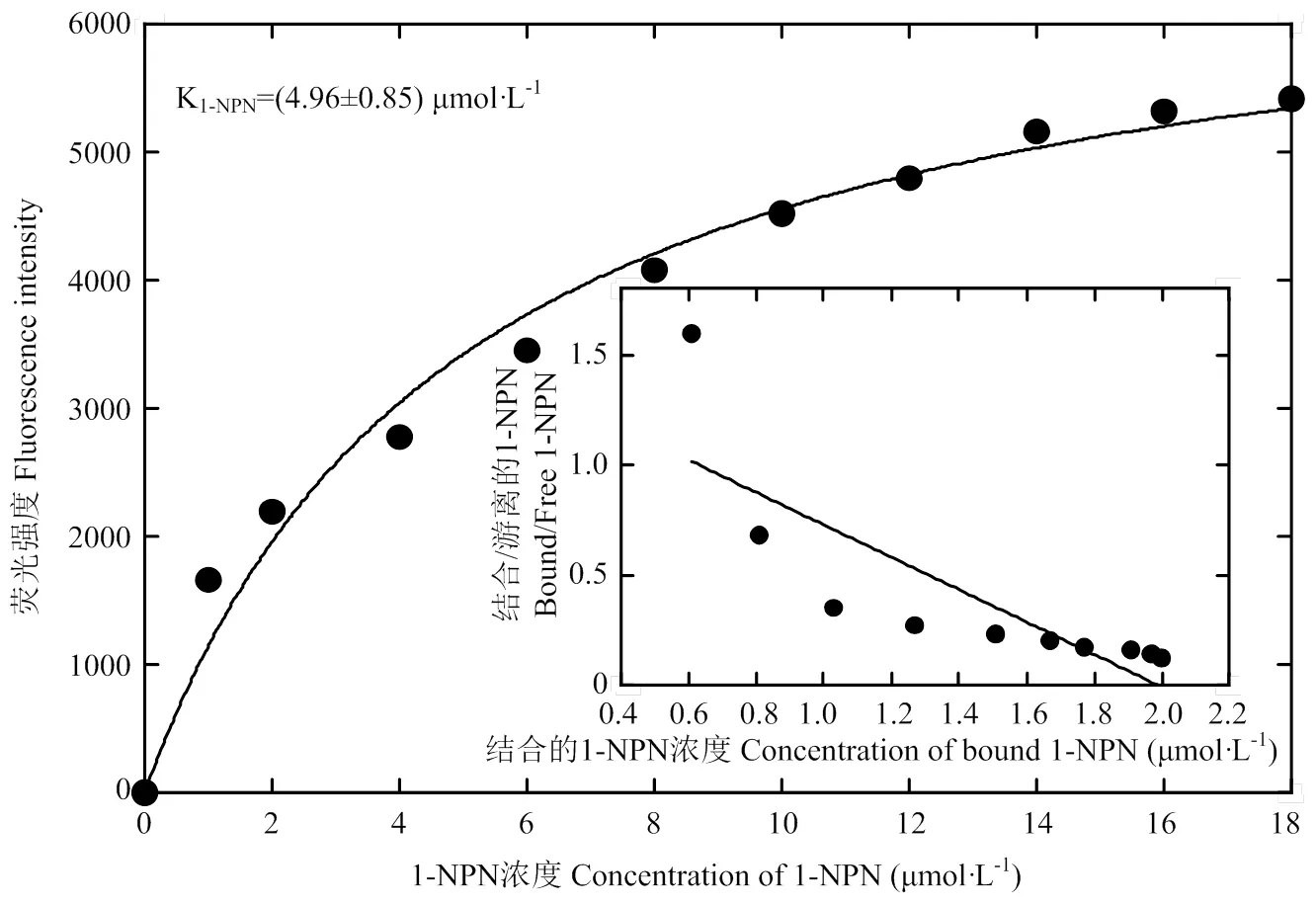
图3 重组蛋白GfunOBP2与1-NPN的结合曲线及线性化的斯卡查德图
2.5 GfunOBP2同源建模和分子对接
以冈比亚按蚊AgamOBP47(PDB: 3PM2)为结构模板构建了GfunOBP2的3D同源模型(图4-A),分析其结构发现GfunOBP2具有由8个-螺旋形成的球状结构,GfunOBP2的3D结构中Cys3-Cys159、Cys27-Cys149、Cys28-Cys140、Cys42-Cys67和Cys111-Cys131连接形成5个二硫键维持空间构型(图4-B)。由于反-2-己烯-1-醇、1-庚醇、1-癸醇、乙酸-顺-3-己烯酯、2-甲基丁酸叶醇酯、-罗勒烯和-石竹烯是荧光竞争结合试验中与GfunOBP2结合能力最强的前7种配体(Ki值≤4 μmol·L-1),通过分子对接计算和预测了GfunOBP2与以上7种配体的结合能(表3)以及相互作用的关键氨基酸残基(图5、图6),结果表明,蛋白结合口袋内部的疏水性氨基酸与气味分子形成弱相互作用力是GfunOBP2结合以上配体的重要原因,如GfunOBP2与反-2-己烯-1-醇和1-庚醇均可形成氢键、Donor-Donor相互作用和烷基相互作用(图5-A、5-B),与1-癸醇可形成氢键、σ-π键相互作用、π-孤对电子作用和烷基相互作用(图5-C),与乙酸-顺-3-己烯酯和2-甲基丁酸叶醇酯主要形成氢键和碳氢键(图5-D、5-E),与-罗勒烯和-石竹烯仅形成烷基相互作用(图5-F、5-G)。疏水性氨基酸如Ile、Pro、Phe、Ala、Leu、Val等是GfunOBP2结合气味配体的重要氨基酸残基(图6)。
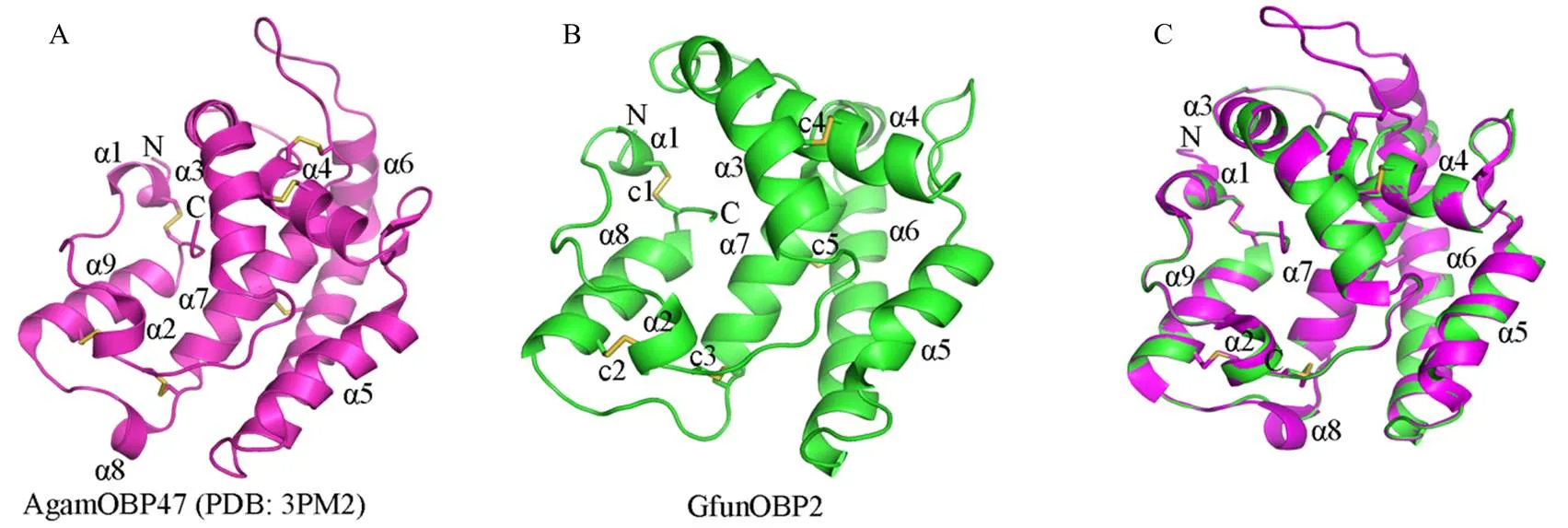
A:冈比亚按蚊AgamOBP47的晶体结构Crystal structure of AgamOBP47 from A. gambiae;B:预测的GfunOBP2 3D模型Predicted 3D model of GfunOBP2;C:AgamOBP47和GfunOBP2的3D结构比对3D structure aligned of AgamOBP47 and GfunOBP2。图中字母N和C分别表示OBP的N端和C端The letters N and C indicate the N- and C-terminal of OBPs, respectively
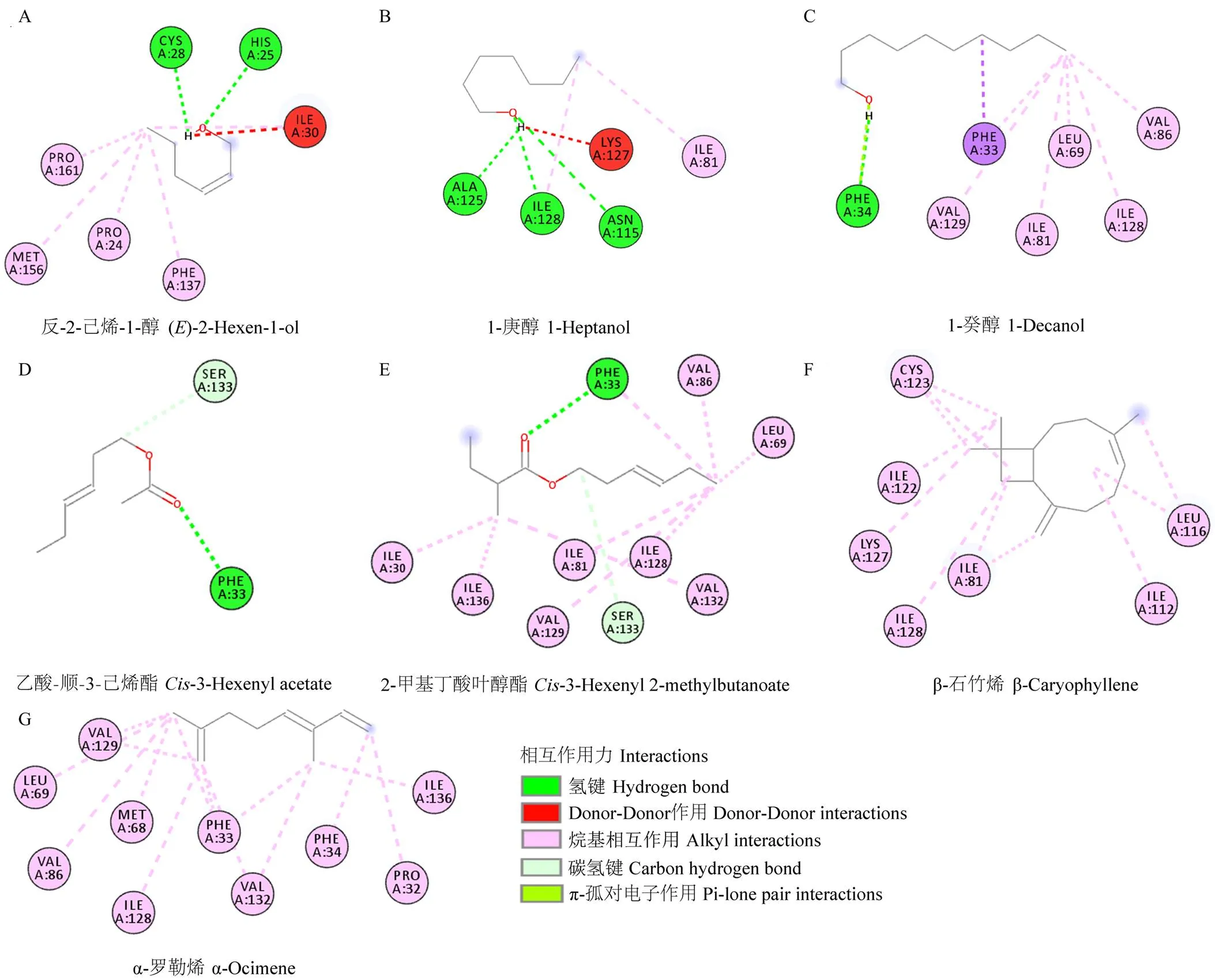
图5 GfunOBP2与部分具有强结合能力的气味配体的相互作用力及氨基酸残基(2D结构)
3 讨论
3.1 GfunOBP2的空间结构特征及其与气味配体结合和释放的关系
本研究克隆了一个在李小食心虫触角转录组测序中鉴定的候选Plus-C OBP基因,命名为2,通过比对GfunOBP2和其他鳞翅目昆虫Plus-C OBP的氨基酸序列发现,GfunOBP2具有Plus-C OBP亚家族蛋白的结构特征和保守的Cys残基分布模式。GfunOBP2与典型OBP、Minus-C OBP在序列组成上明显不同,如GfunOBP2的分子量更大(编码183个氨基酸),具有较长的N-末端且N-末端序列形成了2个小-螺旋;具有5对二硫键维持蛋白的空间构型,较典型OBP 3对二硫键维持的空间结构更加稳定[46]。典型OBP和Minus-C OBP具有6个-螺旋[47-48],而预测的GfunOBP2具有8个-螺旋,长N-末端形成的2个-螺旋1和2位于结合腔外,未参与结合腔的形成,根据鳞翅目昆虫OBP释放气味的分子机制[49-50],推测GfunOBP2在中性或者弱酸性的感觉器淋巴液中结合气味分子,当到达pH较低的ORN树突膜附近时,由于其构象发生变化,位于结合腔外的1和2螺旋进入结合腔中,促使GfunOBP2将结合的气味分子释放出来。典型OBP通常具有较长的C-末端,C-末端的肽链在中性感器淋巴液中成伸展构象,气味分子进入典型OBP结合腔形成复合物,当该复合物移动至ORN树突膜附近的酸性淋巴液时,典型OBP C-末端的肽链由伸展构象转变为-螺旋,进入结合腔促使蛋白将气味分子释放出来[51-53]。Tsitsanou等[54]对冈比亚按蚊Plus-C OBP AgamOBP48的晶体结构解析发现,AgamOBP48在溶剂中形成同源二聚体,蛋白以同源二聚体形成的疏水腔结合气味分子,与Plus-C OBP AgamOBP47形成的单体蛋白明显不同。尚未见Plus-C OBP在酸性环境中的晶体结构解析以及蛋白和气味分子复合物结构构象的报道,Plus-C OBP结合和释放气味化合物的分子机制有待深入研究。
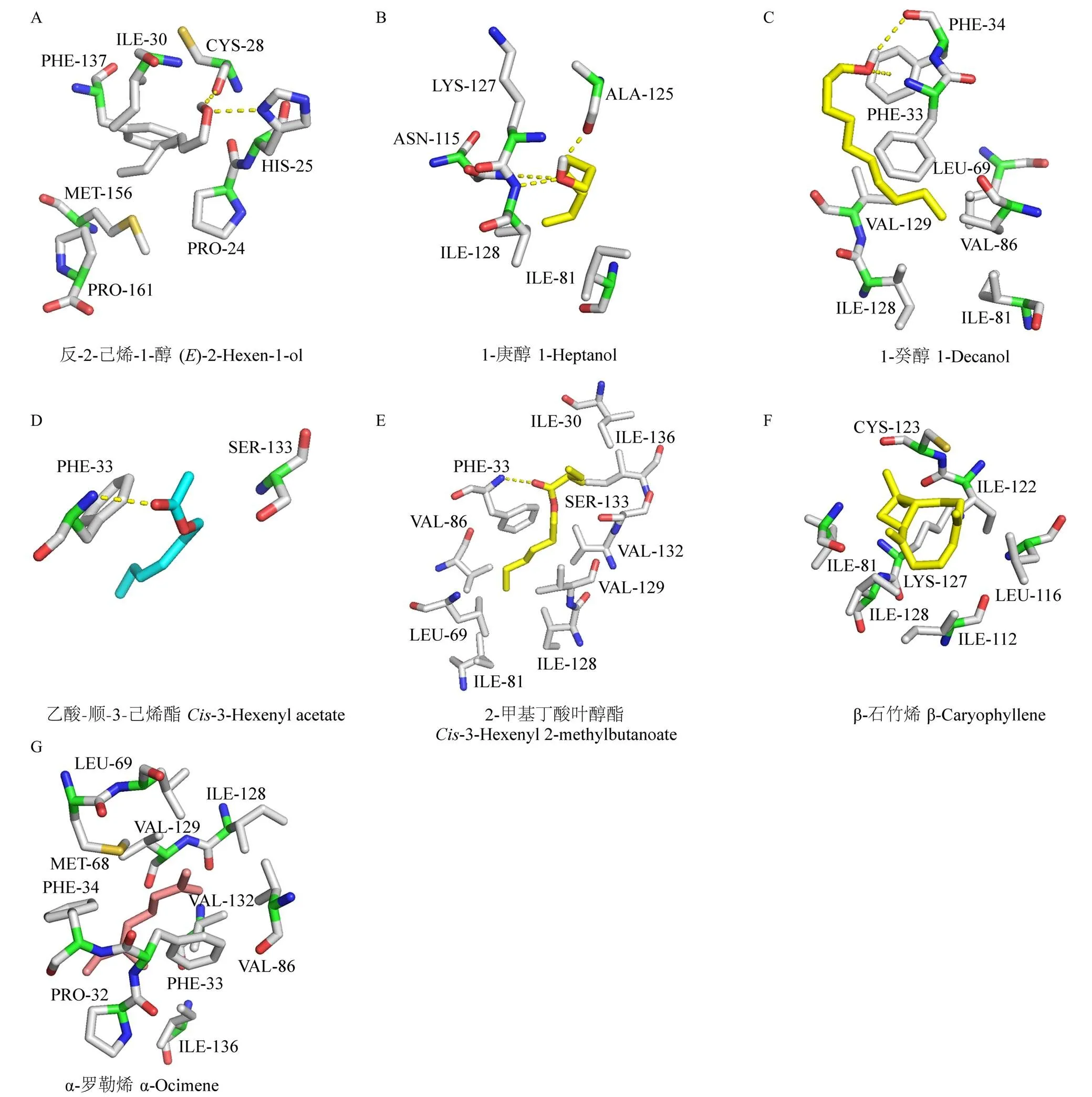
图6 GfunOBP2与部分具有强结合能力的气味配体的相互作用力及氨基酸残基(3D结构)
3.2 GfunOBP2是一种结合寄主植物挥发物的广谱型气味结合蛋白
荧光竞争结合试验发现GfunOBP2对李小食心虫雌虫性信息素和大部分苹果叶片、果实挥发化合物具有结合活性,表明GfunOBP2参与了该虫嗅觉通讯中对气味分子的选择性感受识别和运输。GfunOBP2对5种性信息素化合物表现出中等或较弱的结合能力,其结合性信息素的能力明显低于3种GfunPBP(GfunPBP1. 1/1.2/2)和3种GfunGOBP(GfunGOBP1/2/3)[11-12]。前人研究发现疆夜蛾()Plus-C OBP PsauOBP7对性信息素乙酸-顺-11-十六碳烯酯和乙酸-顺-9-十四碳烯酯没有结合活性[55]、柑橘木虱()Plus-C OBP DcitOBP2对性信息素月桂酸仅具有较弱的结合能力[56],表明Plus-C OBP不是转运性信息素的主要OBP。GfunOBP2对苹果树挥发化合物具有较宽的结合谱,对35种寄主挥发化合物中的26种具有结合活性,其中对反-2-己烯-1-醇、苯甲醇、1-庚醇、1-癸醇、己醛、庚醛、乙酸-顺-3-己烯酯、2-甲基丁酸叶醇酯、-蒎烯、-罗勒烯、柠檬烯和-石竹烯具有强结合活性,GfunOBP2对反-2-己烯-1-醇、己醛、庚醛、-罗勒烯和-石竹烯的结合能力强于GfunGOBP1、GfunGOBP2和GfunGOBP3[11],表明GfunOBP2能够选择性地识别寄主植物挥发化合物。在其他昆虫中也证实Plus-C OBP能够结合寄主挥发化合物或环境中的挥发性信息物质,如暗黑鳃金龟()Plus-C HparOBP14能够结合寄主植物挥发化合物6-甲基-5-庚烯-2-酮,有机肥挥发化合物3-甲基吲哚、对二甲苯、甲醇、甲醛、-蒎烯和香叶醇[57];柑橘木虱DcitOBP2能够结合寄主挥发化合物二甲基二硫醚和(+)-柠檬烯[56]。
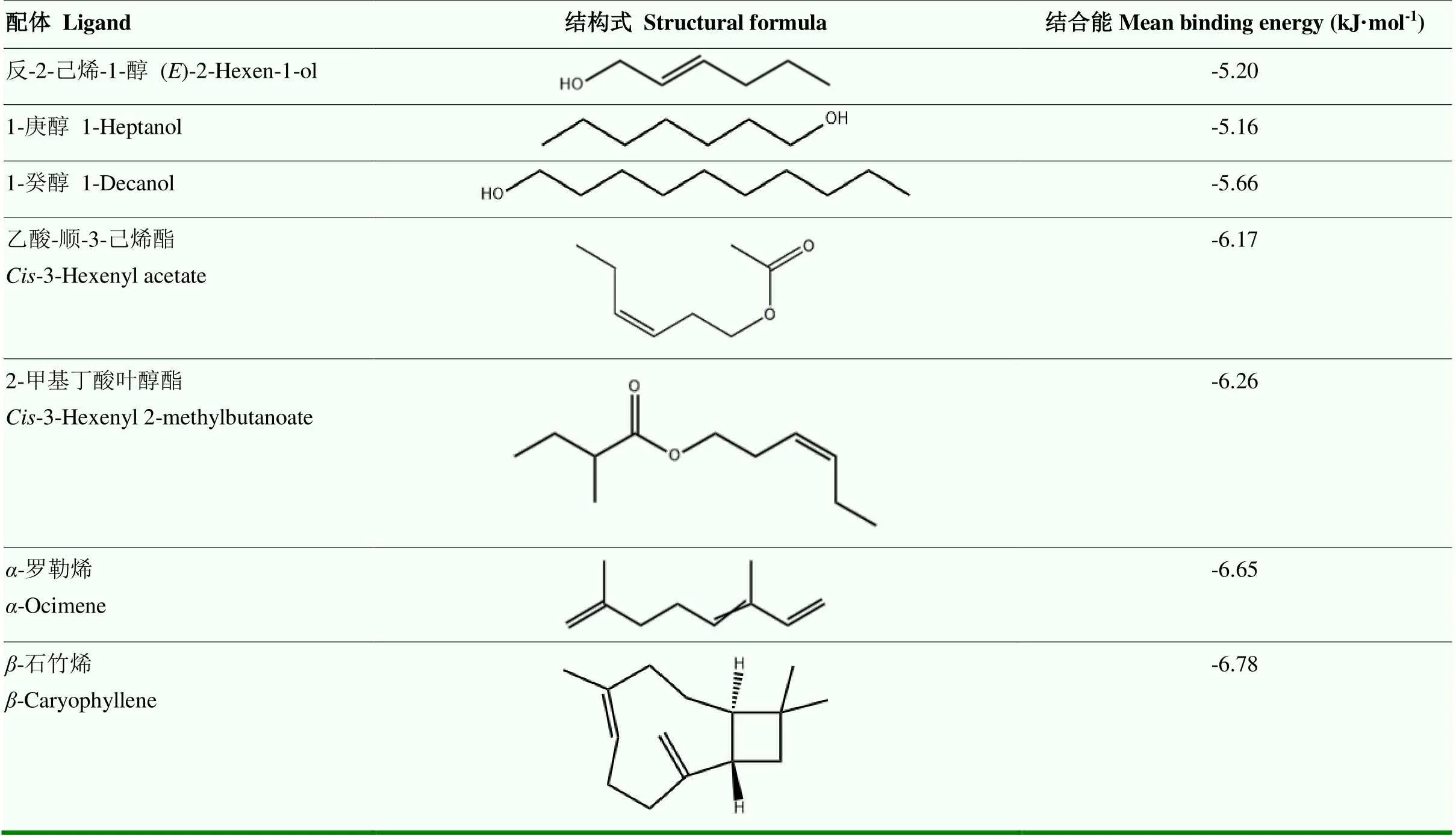
表3 GfunOBP2与气味配体的平均结合能
3.3 GfunOBP2结合不同类型气味配体的关键氨基酸残基及形成的弱相互作用力不同
为了更好地阐明GfunOBP2与不同类型挥发化合物的结合特性,本研究利用同源建模和分子对接技术分析了GfunOBP2与7种具有最强结合能力(Ki≤4.0 μmol·L-1)的气味配体的结合能和关键氨基酸残基,结果显示,GfunOBP2与7种气味配体的结合能均为负值,GfunOBP2与气味配体的结合能力与结合能具有相关性,结合能力越强,结合能越小(表3),这与已报道的松蛀螟()[58]、美国白蛾()[59]、异色瓢虫()[60]、多异瓢虫()[61]等昆虫的OBP相似,表明GfunOBP2与不同配体之间存在弱相互作用力。前人通过核磁共振解析OBP的晶体结构发现,OBP通常通过疏水性结合腔内的非极性或极性氨基酸残基与不同配体形成相互作用力[62-63]。GfunOBP2与3种醇类物质反-2-己烯-1-醇、1-庚醇和1-癸醇的结合均有氢键和烷基相互作用力的参与,不同的是GfunOBP2 Ile30的氨基与反-2-己烯-1-醇的羟基、Lys127的氨基与1-庚醇的羟基形成Donor-Donor相互作用力,而GfunOBP2 Phe33的-苯基与1-癸醇的C7碳原子形成了σ-π键相互作用、Phe30的-苯基与1-癸醇的羟基氧原子形成了π-孤对电子作用。GfunOBP2结合两种酯类物质乙酸-顺-3-己烯酯和2-甲基丁酸叶醇酯主要由氢键和碳氢键两种弱相互作用力维持,且与上述两种配体结合的关键氨基酸残基也相同,氢键均由Phe33的氨基氢原子与酯类物质的羰基氧原子形成、碳氢键均由Ser133的羟基氢原子与酯类配体的碳原子形成。烷基相互作用力是GfunOBP2结合两种萜烯类物质-罗勒烯和-石竹烯形成的唯一弱相互作用力,疏水性氨基酸如Val、Leu、Phe、Ile和Pro在形成烷基相互作用力中起着重要作用。本研究仅通过分子对接试验预测了GfunOBP2结合气味配体的关键氨基酸残基,今后将以上关键残基作为研究重点,借助定点突变[64-65]和荧光竞争结合试验验证关键残基突变后对GfunOBP2结合配体能力的影响。
4 结论
2在李小食心虫成虫触角中高表达,重组GfunOBP2蛋白对苹果树叶片、果实的多种挥发化合物具有强结合活性,推测其可能在成虫寄主植物定位、雌虫产卵场所选择等嗅觉行为中发挥着重要作用。寄主挥发化合物反-2-己烯-1-醇、苯甲醇、1-庚醇、1-癸醇、己醛、庚醛、乙酸-顺-3-己烯酯、2-甲基丁酸叶醇酯、-蒎烯、-罗勒烯、柠檬烯和-石竹烯是GfunOBP2偏好结合的配体分子。
[1] 刘海荣, 赵百丽, 赵文琦, 张武杰, 杨晓华, 齐玉新, 顾广军, 刘畅. 黑龙江省果树食心虫区域分布及防治. 黑龙江农业科学, 2010(5): 65-68.
LIU H R, ZHAO B L, ZHAO W Q, ZHANG W J, YANG X H, QI Y X, GU G J, LIU C. Distribution and prevention of fruit trees borer in Heilongjiang Province. Heilongjiang Agricultural Sciences, 2010(5): 65-68. (in Chinese)
[2] STEFANOVA D, VASILEV P, KUTINKOVA H, ANDREEV R, PALAGACHEVA N, TITYANOV M. Possibility for control of plum fruit mothTr. by pheromone dispensers. Journal of Biopesticides, 2019, 12(2): 153-156.
[3] 吴维钧. 两种果树害虫——旋杖潜叶蛾及李小食心虫. 昆虫学报, 1961, 10(4/6): 395-400.
WU W J. A new record of two species of fruit tree pests from North China. Acta Entomologica Sinica, 1961, 10(4/6): 395-400. (in Chinese)
[4] RIZZO R, FARINA V, SAIANO F, LOMBARDO A, RAGUSA E, VERDE G L. Doinfestation rely on specific plum fruit features?. Insects, 2019, 10(12): 444.
[5] 蒋玉宝, 张素梅, 祁光增, 周科清, 程文波. 陇东苹果主要食心虫和卷叶蛾成虫发生动态监测. 甘肃农业科技, 2014(2): 30-32.
JIANG Y B, ZHANG S M, QI G Z, ZHOU K Q, CHENG W B. Dynamics monitoring of occurs of borers and leafrollers adult on apple in Longdong Areas. Gansu Agricultural Science and Technology, 2014(2): 30-32. (in Chinese)
[6] ZHENG Y, WU R X, DORN S, CHEN M H. Diversity of tortricid moths in apple orchards: evidence for a cryptic species of(Lepidoptera: Tortricidae) from China. Bulletin of Entomological Research, 2017, 107(2): 268-280.
[7] 杨磊, 孙惠敏, 林河州, 陈刘生, 王少山. 石河子垦区果树食心虫种类及其种群动态. 北方园艺, 2017(12): 132-135.
YANG L, SUN H M, LIN H Z, CHEN L S, WANG S S. Species and population dynamics of fruit borer in Shihezi Reclamation Zone. Northern Horticulture, 2017(12): 132-135. (in Chinese)
[8] 陈秀琳, 陈玉鑫, 包琳杰, 康乐, 孙勇, 李广伟. 延安地区苹果园食心虫种类及其种群消长动态调查. 植物保护, 2021, 47(2): 219-225.
CHEN X L, CHEN Y X, BAO L J, KANG L, SUN Y, LI G W. Species and population dynamics of fruit-borer in apple orchards of Yan’an area. Plant Protection, 2021, 47(2): 219-225. (in Chinese)
[9] 李兴龙, 张馨月, 雷岳杰, 肖小容, 杨永棒, 张振兴, 孙惠敏, 王少山. 石河子垦区果树食心虫迷向技术. 新疆农业科学, 2020, 57(1): 173-180.
LI X L, ZHANG X Y, LEI Y J, XIAO X R, YANG Y B, ZHANG Z X, SUN H M, WANG S S. Study on the mating disruption control technology of fruit borer in Shihezi Reclamation Area. Xinjiang Agricultural Sciences, 2020, 57(1): 173-180. (in Chinese)
[10] 金岩. 吉林地区“123”苹果蛀果害虫发生规律及药剂防治. 北方园艺, 2013(14): 123-124.
JIN Y. Occurrence and chemical control of borer fruit pests of “123” apple. Northern Horticulture, 2013(14): 123-124. (in Chinese)
[11] LI L L, XU B Q, LI C Q, LI B L, CHEN X L, LI G W. Different binding affinities of three general odorant-binding proteins in(Treitscheke) (Lepidoptera: Tortricidae) to sex pheromones, host plant volatiles, and insecticides. Journal of Economic Entomology, 2022, 115(4): 1129-1145.
[12] LI L L, XU B Q, LI C Q, LI B L, LUO K, LI G W, CHEN X L. Functional disparity of four pheromone-binding proteins from the plum fruit mothTreitscheke in detection of sex pheromone components. International Journal of Biological Macromolecules, 2023, 225: 1267-1279.
[13] NASTAS T, RAILEANU N, CHEPTINARI V, ROSCA G. Methodological and technological methods for application of sexual pheromones againstTr.. Scientific Studies & Research, 2014, 23(2): 12-19.
[14] VERDE G L, GUARINO S, BARONE S, RIZZO R. Can mating disruption be a possible route to control plum fruit moth in mediterranean environments?. Insects, 2020, 11(9): 589.
[15] DU J, LI G W, XU X L, WU J X. Development and fecundity performance of oriental fruit moth (Lepidoptera: Tortricidae) reared on shoots and fruits of peach and pear in different seasons. Environmental Entomology, 2015, 44(6): 1522-1530.
[16] KARLSSON M F, PROFFIT M, BIRGERSSON G. Host-plant location by the Guatemalan potato mothis assisted by floral volatiles. Chemoecology, 2017, 27(5): 187-198.
[17] WOLFIN M S, CHILSON III R R, THRALL J, LIU Y X, VOLO S, CHA D H, LOEB G M, LINN JR C E. Proximate mechanisms of host plant location by a specialist phytophagous insect, the grape berry moth,. Journal of Chemical Ecology, 2019, 45(11/12): 946-958.
[18] JIANG N J, TANG R, GUO H, NING C, LI J C, WU H, HUANG L Q, WANG C Z. Olfactory coding of intra- and interspecific pheromonal messages by the malein North China. Insect Biochemistry and Molecular Biology, 2020, 125: 103439.
[19] VENTHUR H, ZHOU J J. Odorant receptors and odorant-binding proteins as insect pest control targets: a comparative analysis. Frontiers in Physiology, 2018, 9: 1163.
[20] LI T T, LIU W C, ZHU J, YANG Y H, MA C, LU C, ZHANG K X. Crystal structure and ligand identification of odorant binding protein 4 in the natural predator. International Journal of Biological Macromolecules, 2019, 141: 1004-1012.
[21] LAUE M, STEINBRECHT R A. Topochemistry of moth olfactory sensilla. International Journal of Insect Morphology and Embryology, 1997, 26(3/4): 217-228.
[22] LEE D, DAMBERGER F F, PENG G, HORST R, GÜNTERT P, NIKONOVA L, LEAL W S, WÜTHRICH K. NMR structure of the unligandedpheromone-binding protein at physiological pH. FEBS Letters, 2002, 531(2): 314-318.
[23] STENGL M, FUNK N W. The role of the coreceptor Orco in insect olfactory transduction. Journal of Comparative Physiology A, 2013, 199(11): 897-909.
[24] ZENG F F, XU P X, LEAL W S. Odorant receptors fromandsensitive to floral compounds. Insect Biochemistry and Molecular Biology, 2019, 113: 103213.
[25] VOGT R G, RIDDIFORD L M. Pheromone binding and inactivation by moth antennae. Nature, 1981, 293(5828): 161-163.
[26] CHEN X L, LI G W, XU X L, WU J X. Molecular and functional characterization of odorant binding protein 7 from the oriental fruit moth(Busck) (Lepidoptera: Tortricidae). Frontiers in Physiology, 2018, 9: 1762.
[27] LARTIGUE A, GRUEZ A, SPINELLI S, RIVIÈRE S, BROSSUT R, TEGONI M, CAMBILLAU C. The crystal structure of a cockroach pheromone-binding protein suggests a new ligand binding and release mechanism. The Journal of Biological Chemistry, 2003, 278(32): 30213-30218.
[28] CHRISTAIN C, SILVIA S, AMANDINE L, MARIELLA T. Structural diversity of insects odorant binding proteins around a conserved core. Chemical Senses, 2011, 31: A11-A12.
[29] ZHOU J J, HUANG W S, ZHANG G A, PICKETT J A, FIELD L M. “Plus-C” odorant-binding protein genes in twospecies and the malaria mosquito. Gene, 2004, 327(1): 117-129.
[30] ZHENG Z C, LI D Z, ZHOU A M, YI S C, LIU H, WANG M Q. Predicted structure of a minus-C OBP from(Hope) suggests an intermediate structure in evolution of OBPs. Scientific Reports, 2016, 6: 33981.
[31] ZHANG X Y, ZHU X Q, GU S H, ZHOU Y L, WANG S Y, ZHANG Y J, GUO Y Y. Silencing of odorant binding protein geneby RNAi induces declining electrophysiological responses ofto six semiochemicals. Insect Science, 2017, 24(5): 789-797.
[32] CHEN X F, LEI Y B, LI H F, XU L, YANG H, WANG J J, JIANG H B. CRISPR/Cas9 mutagenesis abolishes odorant-binding protein BdorOBP56f-2 and impairs the perception of methyl eugenol in(Hendel). Insect Biochemistry and Molecular Biology, 2021, 139: 103656.
[33] Zhan H X, DEWER Y, ZHANG J P, TIAN J H, LI D, QU C, YANG Z, LI F Q, LUO C. Odorant-binding protein 1 plays a crucial role in the olfactory response ofto-curcumene. Journal of Agricultural and Food Chemistry, 2021, 69(43): 12785-12793.
[34] HAN W K, YANG Y L, SI Y X, WEI Z Q, LIU S R, LIU X L, YAN Q, DONG S L. Involvement of GOBP2 in the perception of a sex pheromone component in both larval and adultrevealed using CRISPR/Cas9 mutagenesis. Insect Biochemistry and Molecular Biology, 2022, 141: 103719.
[35] VANDESOMPELE J, DE PRETER K, PATTYN F, POPPE B, VAN ROY N, DE PAEPE A, SPELEMAN F. Accurate normalization of real-time quantitative RT-PCR data by genometric averaging of multiple internal control genes. Genome Biology, 2002, 3(7): research0034.1-0034.11.
[36] KELMANSKY D M, MARTÍNEZ E J, LEIVA V. A new variance stabilizing transformation for gene expression data analysis. Statistical Applications in Genetics and Molecular Biology, 2013, 12(6): 653-666.
[37] CALVELLO M, GUERRA N, BRANDAZZA A, D’AMBROSIO C, SCALONI A, DANI F R, TURILLAZZI S, PELOSI P. Soluble proteins of chemical communication in the social wasp. Cellular and Molecular Life Sciences, 2003, 60(9): 1933-1943.
[38] ZHANG T T, MEI X D, FENG J N, BERG B G, ZHANG Y J, GUO Y Y. Characterization of three pheromone-binding proteins (PBPs) of(Hübner) and their binding properties. Journal of Insect Physiology, 2012, 58(7): 941-948.
[39] GUERIN P M, ARN H, BUSER H R, CHARMILLOT P, TÓTH M, SZIRÁKI G. Sex pheromone ofoccurrence of-8- and-10-tetradecenyl acetate as secondary components. Journal of Chemical Ecology, 1986, 12(6): 1361-1368.
[40] VALLAT A, DORN S. Changes in volatile emissions from apple trees and associated response of adult female codling moths over the fruit-growing season. Journal of Agricultural and Food Chemistry, 2005, 53(10): 4083-4090.
[41] CASADO D, GEMENO C, AVILLA J, RIBA M. Day-night and phenological variation of apple tree volatiles and electroantennogram responses in(Lepidoptera: Tortricidae). Environmental Entomology, 2006, 35(2): 258-267.
[42] CHEN X L, SU L, LI B L, LI G W, WU J X. Molecular and functional characterization of three odorant binding proteins from the oriental fruit moth(Busck) (Lepidoptera: Tortricidae). Archives of Insect Biochemistry and Physiology, 2018, 98(2): e21456.
[43] LI G W, CHEN X L, LI B L, ZHANG G H, LI Y P, WU J X. Binding properties of general odorant binding proteins from the oriental fruit moth,(Busck) (Lepidoptera: Tortricidae). PLoS ONE, 2016, 11(5): e0155096.
[44] HUANG G Z, LIU J T, ZHOU J J, WANG Q, DONG J Z, ZHANG Y J, LI X C, LI J, GU S H. Expressional and functional comparisons of two general odorant binding proteins in. Insect Biochemistry and Molecular Biology, 2018, 98: 34-47.
[45] YAN Q Y, YANG J W, YAO Y W, JIA Z, WANG Y Q, CHENG M, YAN X B, XU Y F. Research of the active components and potential mechanisms of Qingfei Gujin decoction in the treatment of osteosarcoma based on network pharmacology and molecular docking technology. Computational and Mathematical Methods in Medicine, 2022, 2022: 7994425.
[46] LAGARDE A, SPINELLI S, QIAO H L, TEGONI M, PELOSI P, CAMBILLAU C. Crystal structure of a novel type of odorant-binding protein from, belonging to the C-plus class. The Biochemical Journal, 2011, 437(3): 423-430.
[47] SANDLER B H, NIKONOVA L, LEAL W S, CLARDY J. Sexual attraction in the silkworm moth: structure of the pheromone- binding-protein-bombykol complex. Chemistry and Biology, 2000, 7(2): 143-151.
[48] FALCHETTO M, CIOSSANI G, SCOLARI F, DI COSIMO A, NENCI S, FIELD L M, MATTEVI A, ZHUO J J, GASPERI G, FORNERIS F. Structural and biochemical evaluation ofodorant-binding protein 22 affinity for odorants involved in intersex communication. Insect Molecular Biology, 2019, 28(3): 431-443.
[49] XU W, LEAL W S. Molecular switches for pheromone release from a moth pheromone-binding protein. Biochemical and Biophysical Research Communications, 2008, 372(4): 559-564.
[50] XU W, XU X, LEAL W S, AMES J B. Extrusion of the C-terminal helix in navel orangeworm moth pheromone-binding protein (AtraPBP1) controls pheromone binding. Biochemical and Biophysical Research Communications, 2011, 404(1): 335-338.
[51] DAMBERGER F F, ISHIDA Y, LEAL W S, WÜTHRICH K. Structural basis of ligand binding and release in insect pheromone- binding proteins: NMR structure ofPBP1 at pH 4.5. Journal of Molecular Biology, 2007, 373(4): 811-819.
[52] THODE A B, KRUSE S W, NIX J C, JONES D N M. The role of multiple hydrogen-bonding groups in specific alcohol binding sites in proteins: insights from structural studies of LUSH. Journal of Molecular Biology, 2008, 376(5): 1360-1376.
[53] ZHOU J J, ROBERTSON G, HE X, DUFOUR S, HOOPER A M, PICKETT J A, KEEP N H, FIELD L M. Characterization ofodorant-binding proteins reveals that a general odorant-binding protein discriminates between sex pheromone components. Journal of Molecular Biology, 2009, 389(3): 529-545.
[54] TSITSANOU K E, DRAKOU C E, THIREOU T, VITLIN GRUBER A, KYTHREOTI G, AZEM A, FESSAS D, ELIOPOULOS E, IATROU K, ZOGRAPHOS S E. Crystal and solution studies of the “Plus-C” odorant-binding protein 48 from: control of binding specificity through three-dimensional domain swapping. The Journal of Biological Chemistry, 2013, 288(46): 33427-33438.
[55] 孙亚兰, 吕琪卉, 杨海博, 胡镇杰, 李定旭, 董钧锋. 疆夜蛾Plus-C气味结合蛋白PsauOBP7的组织表达谱及配体结合特性分析. 昆虫学报, 2020, 63(7): 807-816.
SUN Y L, Lü Q H, YANG H B, HU Z J, LI D X, DONG J F. Tissue expression profiling and ligand binding characterization of the Plus-C odorant binding protein PsauOBP7 of(Lepidoptera: Noctuidae). Acta Entomologica Sinica, 2020, 63(7): 807-816. (in Chinese)
[56] ZHANG H, CHEN J L, LIN J H, LIN J T, WU Z Z. Odorant-binding proteins and chemosensory proteins potentially involved in host plant recognition in the Asian citrus psyllid,. Pest Management Science, 2020, 76(8): 2609-2618.
[57] QU Y F, LIU X Y, ZHAO X, QIN J H, CAO Y Z, LI K B, ZHOU J J, WANG S S, YIN J. Evidence of the involvement of a Plus-C odorant-binding protein HparOBP14 in host plant selection and oviposition of the scarab beetle. Insects, 2021, 12(5): 430.
[58] JING D P, ZHANG T T, PRABU S, BAI S X, HE K L, WANG Z Y. Molecular characterization and volatile binding properties of pheromone binding proteins and general odorant binding proteins in(Lepidoptera: Crambidae). International Journal of Biological Macromolecules, 2020, 146: 263-272.
[59] ZHANG X Q, MANG D Z, LIAO H, YE J, QIAN J L, DONG S L, ZHANG Y N, HE P, ZHANG Q H, PURBA E R, ZHANG L W. Functional disparity of three pheromone binding proteins to different sex pheromone components in(Drury). Journal of Agricultural and Food Chemistry, 2021, 69(1): 55-66.
[60] QU C, YANG Z K, WANG S, ZHAO H P, LI F Q, YANG X L, LUO C. Binding affinity characterization of four antennae-enriched odorant- binding proteins from(Coleoptera: Coccinellidae). Frontiers in Physiology, 2022, 13: 829766.
[61] TANG H Y, XIE J X, LIU J T, KHASHAVEH A, LIU X X, YI C Q, ZHAO D Y, HE L, SUN Y, ZHANG Y J. Odorant-binding protein HvarOBP5 in ladybirdregulates the perception of semiochemicals from preys and habitat plants. Journal of Agricultural and Food Chemistry, 2023, 71(2): 1067-1076.
[62] MEILLOUR P N L, LAGANT P, CORNARD J P, BRIMAU F, Danvic C L, Vergoten G, Michalski J C. Phenylalanine 35 and tyrosine 82 are involved in the uptake and release of ligand by porcine odorant-binding protein. Biochimica et Biophysica Acta, 2009, 1794(8): 1142-1150.
[63] NORTHEY T, VENTHUR H, DE BIASIO F, CHAUVIAC F X, COLE A, JUNIOR K A, GROSSI G, FALABELLA P, FIELD L M, KEEP N H, ZHOU J J. Crystal structures and binding dynamics of odorant-binding protein 3 from two aphid speciesand. Scientific Reports, 2016, 6: 24739.
[64] JIANG Q Y, WANG W X, ZHANG Z D, ZHANG L. Binding specificity of locust odorant binding protein and its key binding site for initial recognition of alcohols. Insect Biochemistry and Molecular Biology, 2010, 39(7): 440-447.
[65] ZHANG Y N, ZHANG X Q, ZHANG X C, XU J W, LI L L, ZHU X Y, WANG J J, WEI J Y, MANG D Z, ZHANG F, YUAN X H, WU X M. Key amino acid residues influencing binding affinities of pheromone-binding protein fromto two sex pheromones. Journal of Agricultural and Food Chemistry, 2020, 68(22): 6092-6103.
Expression and ligand binding characteristics of GfunOBP2 from
NIAN HeFen1,2, ZHANG YuXi2, LI BoLiao1,2, CHEN XiuLin1,2, LUO Kun1,2, LI GuangWei1,2
1Shaanxi Key Laboratory of Chinese Jujube (Yan’an University), Yan’an 716000, Shaanxi;2College of Life Sciences, Yan’an University, Yan’an 716000, Shaanxi
【Objective】The objective of this study is to determine the binding affinities of the Plus-C odorant binding protein 2 of(2) to sex pheromones and volatile compounds from apple trees, and to provide a basis for explaining the olfactory molecular mechanism of locating the host plantsof.【Method】The ORF of2 was cloned by RT-PCR, and GfunOBP2 was identified as a Plus-C OBP subfamily protein through homology annotation and alignment of cysteine distribution patterns in amino acid sequences. The relative expression level of2 in the antenna, head, thorax, leg, wing, abdomen, and sex gland of the 3-day-old adults ofwas detected by RT-qPCR. The prokaryotic expression vector pET30a(+)/GfunOBP2 was constructed, and the recombinant GfunOBP2 protein was expressed inBL21 (DE3) cells. The binding affinity of recombinant GfunOBP2 protein to five sex pheromones and 35 plant volatiles of apple trees was determined by using a fluorescence competitive binding assay. The interaction force and key amino acid residues of GfunOBP2 interacting with odorant ligands with strong binding affinities were predicted by molecular docking.【Result】The full-length ORF sequence of2 (GenBank number: OQ054799.1) was cloned, encoding 183 amino acids. It was found that GfunOBP2 has 12 conserved cysteines, and the distribution motif of cysteine residues indicated that GfunOBP2 belongs to the Plus-C OBP subfamily.2 was mainly expressed in the antennae of adults, and the relative expression level in male antennae was significantly higher than that in female antennae (<0.05). Recombinant GfunOBP2 protein exhibited strong binding affinities to ()-2-hexen-1-ol, benzyl alcohol, 1-heptanol, 1-decanol, hexanal, heptanal,-3-hexenyl acetate,-3-hexenyl 2-methylbutanoate,-ocimene,-caryophyllene,-pinene and limonene, and the inhibition constant (Ki) for each ligand above was less than 5.0 μmol·L-1. The molecular docking results showed that hydrogen bonds, donor-donor interactions, and alkyl interactions are the main weak interactions between GfunOBP2 and ()-2-hexen-1-ol, 1-heptanol, and 1-decanol. The conventional hydrogen bonds and carbon hydrogen bonds are the main weak interactions between GfunOBP2 and-3-hexenyl acetate and-3-hexenyl 2-methylbutanoate. The alkyl interaction is the only weak force of GfunOBP2 interacting with-ocimene and-caryophyllene. Several hydrophobic amino acid residues, including Ile, Pro, Phe, Ala, Leu, and Val, play an important role in GfunOBP2’s binding to odorant ligands.【Conclusion】2 is mainly expressed in the antennae of adults ofand the corresponding recombinant protein has strong binding affinities to 12 of the 35 volatile compounds of apple trees, and has moderate binding affinities to 10 compounds, indicating that GfunOBP2 plays an important role in the process of perceiving and recognizing the volatile compounds of host plants. This study provides a theoretical basis for confirming that Plus-C OBP was involved in the peripheral olfactory communication of.
; chemoreception; odorant binding protein (OBP); host-plant volatile; molecular docking

10.3864/j.issn.0578-1752.2023.12.006
2023-03-15;
2023-04-21
国家自然科学基金(32160636)、延安大学产学研合作培育项目(CXY202118)、2021年延安大学校级大学生创新创业计划训练项目(D2021099)
年和粉,E-mail:nhf762786194@163.com。通信作者李广伟,E-mail:liguangwei@yau.edu.cn
(责任编辑 岳梅)
