Enhancing energetic performance of metal-organic complex-based metastable energetic nanocomposites by spray crystallization
2023-07-04KexinWangLixiaoShenBinYuanYanLiShunguanZhuLinZhangZhenxinYiChenguangZhu
Ke-xin Wang, Li-xiao Shen, Bin Yuan, Yan Li, Shun-guan Zhu, Lin Zhang, Zhen-xin Yi,Chen-guang Zhu
School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210941, China
Keywords:Energetic metal-organic complexes Nano aluminum Energetic nanocomposites Spray crystallization Thermite reaction
ABSTRACT
1. Introduction
In the past few decades,nanothermites have always been one of the most popular research topics in the field of energetic materials.Because of the rapid energy release rate and high theoretic energy density, researchers around the world have already studied and reported many thermites prepared from aluminum (Al), metal oxides [1—5] or oxygen-containing metal salts [6—13], such as Al/Bi2O3, Al/CuO, Al/WO3and Al/Ba(SO4)2, etc. However, most of the traditional oxidants lack components that can generate high pressure, traditional preparation methods are also not flexible enough in customizing physical properties of product such as oxidant morphology and product uniformity,the reactivity and application of nanothermites are restricted.
Novel energetic nanocomposites composed of Al,high explosive nanoparticles[14]and energetic metal-organic complexes[15—17]have attracted widespread attention. The high-energy nanocomposites consist of nanothermites and RDX proved that the most reactive nanothermites are those whose reaction produces a large amount of gas that can offer high pressure [14]. The convection propagation mechanism [18,19] relative to the reaction of nanothermites can also support this view. Besides, researchers pointed out that less gas produced by nanothermites will possibly fail the transition from combustion to detonation of energetic materials [20,21]. Based on these considerations, energetic metalorganic complexes are investigated in energetic nanocomposites.For example, the organic ligands in [Mn(BTO) (H2O)2]n[15] and[Cu(atrz)3(NO3)2]n[22] can generate a large amount of gas during the thermal decomposition process and offer a high peak pressure.Furthermore, most energetic metal-organic complexes release numerous heat when they decompose, accompanied with the production of nanostructures such as metal oxides and metal nanoparticles [23,24], the metal oxide product from energetic metal-organic complexes can further react with n-Al to generate more heat energy within the composites [15,16]. Besides, the structure diversity and adjustable functions of energetic metalorganic complexes make it have great potential in the field of energetic materials [25—29]. These facts reflect that one of the most promising methods for current and future research in the field of nanothermites is to combine Al with high explosive energetic metal-organic complexes to prepare hybrid nanocomposites.
Apart from chemical properties of oxidants,physical properties of energetic nanocomposites also have a great influence on the overall reaction characteristics of the product. Both the diffusion limited mechanism of the classical combustion theory[30]and the melt dispersion mechanism [31] suggest that the transmission distance will be reduced with smaller particle size of reactants,thereby increasing the reaction speed [2,32,33]. Besides, studies show that specific surface area, porosity of the reactants, and the contact area of components also play important roles in enhancing reactivity [14,34—36]. These mean that the choice of preparation method is of great importance.
Traditional preparation method such as sol-gel synthesis is relatively safe, but the product is of low purity [3], and vapor deposition is a simple but cost-effective method to produce nanothermites with novel structures[37—39].There are some other novel methods. For example, spray drying has the advantages of spheroidization and refinement of products, but the loss on the dryer wall and cyclone separator caused by adhesion will reduce the yield, the process will also cause higher heat stress and mechanical stress of materials [40]. The spray crystallization (SC)method can well make up for the defects mentioned above,which can not only refine and spheroidize materials, but also optimize particle size and density by controlling the atomization process of solution. At the same time, it has a wider range of selectivity in terms of the target products and the use of solvents.What's more,the operation is safe and reliable since it can avoid potential safety hazards due to the necessary high temperature during the preparation process.In recent years,the application of SC technology has appeared in the field of energetic materials[41],the application and exploration of which are at present mainly focused on the refinement and spheroidization of single products,with fewer reports on the preparation of multi-component composites.
In this work, the high explosive energetic metal-organic complex[Ni(CHZ)3](ClO4)2(CHZ=1,3-diaminourea)was produced and mixed with n-Al through SC without adding additional non-energy additives that may reduce the energy density of the overall product.The exothermic properties, pressure output and detonation ability of n-Al/[Ni(CHZ)3](ClO4)2energetic nanocomposites prepared by SC were investigated by thermal analysis techniques, closed bomb experiments and lead plate test respectively.
2. Experiment
2.1. Materials
n-Al with the size distribution of 80—100 nm was purchased from Jiaozuo Nanomaterial Co.,Ltd.(China),the active content of Al is approximately 80 wt%. The nickel (II) perchlorate hexahydrate(98%) was commercially available from Meryer Chemical Technology Co., Ltd. (China). Carbohydrazide (CHZ) was supplied by Aladdin Technology Co., Ltd. (China). Ethanol was purchased from Sinopharm Chemical Reagent Co., Ltd., which was used as one of the solvents without further purification.Deionized water was labmade,used as another component of the mixed solvent applied in the experiment.
2.2. Synthesis of energetic metal-organic complex by conventional method
The energetic metal-organic complex used in this study was conventionally synthesized by an improved method based on a previous study[42].The precursor(i.e.CHZ)was first dissolved in a certain volume ethanol with stirring and heated to around 78◦C.Then nickel perchlorate inorganic salt aqueous solution was prepared,in which the volume of deionized water was three times that of ethanol used with the precursor.The two components were then combined by dropping the inorganic salt solution into the precursor ethanol suspension. After about 5 min’ stirring, the clear blue solution was moved to another magnetic stirrer at room temperature. The blue crystalline product was separated by filtration after stirring slowly for 12 h.
2.3. Synthesis of energetic metal-organic complex by spray crystallization
The preparation methods of the precursor and the inorganic salt solution were the same as the conventional one. After combining the two liquid systems,the clear blue solution was then transported to the anti-solvent (i.e. ethanol) of product through a SC device,which was mainly composed of a peristaltic pump,a nozzle,and a gas cylinder that provides the power of gas pump. The schematic diagram of the device is shown in Fig.1. The mixture was sprayed into the anti-solvent with a pump rate of 25 mL/min, a nozzle air flow rate of 15 L/min, and a stirring speed of 80 r/min. The blue product was separated by filtration after stirring for around 1 h.
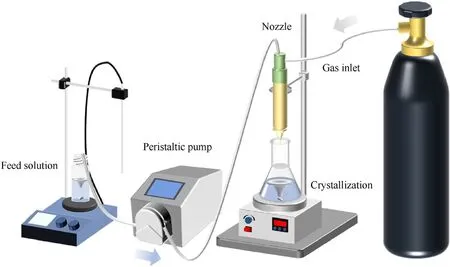
Fig.1. The schematic diagram of SC device.
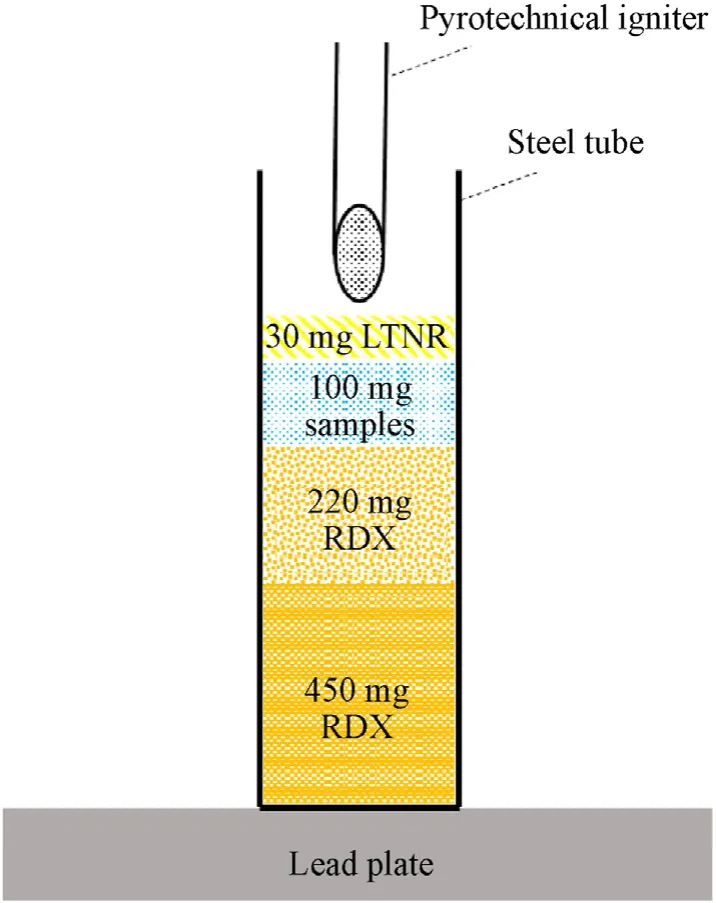
Fig. 3. Schematic diagram of initiating device.
2.4. Preparation of n-Al/energetic metal-organic complex
According to the yield of[Ni(CHZ)3](ClO4)2crystallized by spray or conventional method, the amount of n-Al added is determined,whose mass ratio is n-Al/[Ni(CHZ)3](ClO4)2=0.55,which is decided based on the zero balance of oxygen. Physically mixed (PM) n-Al/[Ni(CHZ)3](ClO4)2was obtained afterwards by complying with the conventional process.Comparatively,after preparing the precursor solution as previous, n-Al was added in the inorganic salt aqueous solution with constant stirring. The homogeneous suspension was then added dropwise to the precursor solution that has been heated to required temperature of 75◦C under continuous stirring.The mixture was then sprayed into the anti-solvent of the same volume as preparing the energetic metal-organic complex through SC. After about 1 h’s stirring, the product was available after filtration and drying.The schematic representation of the process is shown in Fig.2.Note that the stoichiometry of components in n-Al/[Ni(CHZ)3](ClO4)2(PM) was consistent with that of spray crystallized nanocomposites, which were demonstrated by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES)experiment.
2.5. Characterization
The morphologies and element distribution analyses of all the samples were examined by a field emission scanning electron microscopy (FESEM, S-4800, Hitachi) and energy dispersive spectrometer(EDS).Powder X-ray diffraction(PXRD,λ=1.5406 Å for Cu Kα-radiation, Bruker D8-ADVANCE, Germany) and Fourier Transform Infrared Spectroscopy (FT-IR) (Thermo Fisher NicoletiS10 facilities instrument) were utilized to demonstrate the successful preparation of all the products. In addition, nitrogen adsorption measurements were conducted on a Micromeritics ASAP 2050 analyzer at 77 K, samples were heated at 80◦C under vacuum for 12 h prior to recording the isotherms.Besides,Inductively Coupled Plasma-Optical emission spectrometer Varian (ICP-OES) 720-ES served to further determine the content of n-Al and the energetic metal-organic complex quantitatively in the sample.PXRD,Raman spectra (LabRAM HR Evolution, HORIBA Scientific, France) and Xray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Scientific, USA) were also exploited to analyze the calcined products of n-Al/energetic metal-organic complex and the energetic metal-organic complex respectively to explore the reaction mechanism.
Differential scanning calorimetry (DSC) and thermogravimetric(TG) analysis was implemented on a NETZSCH STA 449C Simultaneous Thermal Analysis (NETZSCH Group, Germany) to determine the thermal reaction of the prepared samples, during which a weight of 0.3—0.5 mg was loaded into an 85 μL alumina crucible inside the apparatus and analysis was conducted under flowing Argon. The temperature increased from 30 to 1000◦C at a typical heating rate of 10◦C/min.
The pressure status of reactions was explored by measurement in a confined combustion cell with a constant volume (25 mL). To obtain the peak pressures and boost rates,100 mg of loose powder was loaded inside the combustion cell. After igniting the sample,the real-time pressure values from reactions were recorded by a pressure sensor (Fst800-ZQ-BZ, Fistrate) and a digital oscilloscope(MDO 3034, Tektronix). Due to the linear relationship between pressure and electrical signals,the electrical signals recorded by the oscilloscope can be converted into pressure values, thus forming the experiment.
According to von Franz, spinning is connected to wishful thinking as well (172). Von Franz continues, Both the spinning wheel and the act of Spinning are proper to Wotan, and in our tale [ King Thrushbeard ] the girl has to spin to support her husband. The animus has taken possession of her own properly feminine activity (172-173).Return to place in story.
2.6. Detonation performance test
The evaluation and comparison of the detonation ability of all samples in the study were explored through the perforation of lead plates, which is a typical method of rating the performance of primary explosives.As shown in Fig.3,450 mg hexogen(RDX)was first pressed into a steel tube with a diameter of 6 mm and a height of 25 mm using a pressure of 40 MPa,after which 220 mg RDX was placed with a pressure of 30 MPa.Then,100 mg samples including[Ni(CHZ)3](ClO4)2, n-Al/[Ni(CHZ)3](ClO4)2(PM), n-Al/[Ni(CHZ)3](-ClO4)2(SC) and lead azide (LA) were pressed with a pressure of 30 MPa into the tube for comparison.30 mg lead styphnate(LTNR)was used in all test groups because of its good flame sensitivity.The steel tubes were finally placed tightly on a 5 mm thick lead plate.
2.7. Electrostatic sensitivity experiment
For comparison, the electrostatic sensitivity of pure[Ni(CHZ)3](ClO4)2and n-Al/[Ni(CHZ)3](ClO4)2(SC) were measured using electrostatic spark sensitivity tester (JGY-50III), and the minimum firing energy was obtained by using Neyer D-Optimal method, referring to the military specifications of China GJB 891.27-2006. The charging capacitor was 500 pF, and the gap between the electrodes was 0.12 mm.Around 20 mg of powder were loaded and twenty-five samples of each product were tested.
3. Results and discussions
3.1. Morphology and composition analysis
As shown in Fig. 4(a), complex particles produced through conventional method shows a shape of broken cube with sizes ranging from 50 to more than 100 μm. Fig. 4(b) shows the morphology of the sample prepared by SC. Compared with the former, the size of particles is significantly reduced, showing a shape of flower cluster with a diameter of approximate 2 μm. It is worth mentioning that the total pore surface area and porosity of the sample increase visibly, proved by the evidence from the nitrogen adsorption measurements as shown in Fig.4(f)and Table 1.The N2adsorption-desorption isotherms for the complexes prepared by different method show type IV and H3hysteresis loop,which imply that particles have internal mesoporous structures of irregular shape such as slits and wedges.The surface area,pore size and pore volume of the prepared complexes are presented in Table 1. BET surface area analysis of the results shows that[Ni(CHZ)3](ClO4)2prepared by conventional method has an area of 86.05 m2/g,which increases to 106.80 m2/g after preparation with SC. The trend was found to be opposite for the pore volumes and the average pore sizes, indicating that the number of pore structures per unit mass increases. Thus, it can be inferred that the SC method significantly increased the surface area and porosity of the complex, which is beneficial to the reactivity of nanothermites.

Table 1Comparison of surface area and porosity between [Ni(CHZ)3](ClO4)2 prepared by different methods.
Physically mixed n-Al/[Ni(CHZ)3](ClO4)2present a state of uneven mixing, as shown in Fig. 4(c). However, Fig. 4(d) show that most of the surface of complex is evenly covered by n-Al powder after compounding n-Al/[Ni(CHZ)3](ClO4)2by SC, with some n-Al embedded in the pores.Elemental mappings confirm that Ni(blue),Cl (green), which are the main elements of [Ni(CHZ)3](ClO4)2, and Al (red) with strong detection signals were almost completely compatible. It indicates that [Ni(CHZ)3](ClO4)2and n-Al are more widely contacted, reducing aggregation of n-Al to some extent.Besides, the actual contents of n-Al and [Ni(CHZ)3](ClO4)2in nanocomposites were calculated by ICP-OES as shown in Fig. 4(e).The result revealed that the mass percentage of n-Al and[Ni(CHZ)3](ClO4)2were 33.2% and 66.8% respectively, which was basically identical with the feeding ratio.
To confirm the successful synthesis of[Ni(CHZ)3](ClO4)2and the n-Al/complex energetic nanocomposites through both conventional method and SC, PXRD patterns (Fig. 5(a)) were obtained to assess the samples.Characteristic diffraction peaks of the complex in both n-Al/[Ni(CHZ)3](ClO4)2(PM) and n-Al/[Ni(CHZ)3](ClO4)2(SC)are consistent with standard product in the previous reference[42],indicating that[Ni(CHZ)3](ClO4)2was successfully synthesized without phase transition by both methods. The significant reflection peaks in n-Al/[Ni(CHZ)3](ClO4)2(PM)or n-Al/[Ni(CHZ)3](ClO4)2(SC)also correspond to the powder diffraction standards pattern of n-Al completely.

Fig. 5. The composition and ingredient analysis of n-Al/energetic metal-organic complex: (a) PXRD pattern of the energetic complex and n-Al、complex composites;(b) FT-IR spectra of the energetic complex and the pristine materials.
The composition and ingredient analysis of samples were further verified through FT-IR. Fig. 5(b) shows the spectra of pristine carbohydrazide and the ligand carbohydrazide in the energetic metal-organic complex, some characteristic vibrations are distinguishable from the pristine materials. The free organic ligand exhibits typical N—H stretching vibrations in the range from 3168 to 3348 cm-1.Due to the formation of associations through hydrogen bonds in the solid state,multiple peaks and sharp absorption bands appear in this region. A weak absorption band between 3110 and 3060 cm-1also be detected,which is the double frequency peak of the N—H deformation vibration(1531 cm-1).Besides,there is a very strong absorption band at 1629 cm-1, with shoulders located at 1660 and 1623 cm-1because of N—H deformation and C═O stretching vibrations.At 1447 cm-1a N—H bending vibration can be observed and N—H rocking vibration with strong intensity along with C—N valence vibrations can be detected at 1340 and 1318 cm-1.In comparison,the CHZ ligand vibrations are shifted and changed in the complex due to coordination,which can be assigned according to the reference [42]. The υ(Cl—O) can be found characteristically at 1087 cm-1apart from vibrational combinations of carbohydrazide.
3.2. Thermal and reaction analysis

Fig. 6. Thermal analysis of the pure complex, n-Al/complex (PM) and n-Al/complex(SC): (a) DSC curve of the pure complex; (b) DSC curve of n-Al/complex (PM); (c) DSC curve of n-Al/complex (SC).
To verify the decomposition products, pure complex and n-Al/complex(SC)nanocomposites were heated from room temperature to 390◦C at a rate of 10◦C/min in Ar atmosphere respectively,which were characterized by PXRD afterwards. As shown in Fig. 7(a), characteristic diffraction peaks of the complex disappear in the calcined product of nanocomposites, compared to Fig. 5(a).Meanwhile,new diffraction peaks arise,which highly coincide with the diffraction peaks of products when the complex was calcined alone. It can be inferred that the new peaks belong to the decomposition product of the complex,which can be preliminarily judged as NiO by querying Crystallography Open Database (COD).
To further confirm the product composition, calcined complex was characterized by Raman Spectra. Fig. 7(b) shows the second order magnon (2 M) scattering peak of nickel oxide at around 1500 cm-1, with Raman peaks of phonon scattering at ~300-600 cm-1,~720 cm-1,~900 cm-1,and~1100 cm-1.The Raman peak at ~300-600 cm-1contains the first-order transverse optical phonon (TO) peak and the first-order longitudinal optical phonon(LO) peak, which are caused by rhomboid lattice distortion and crystal defects [43]. The peaks at ~720 cm-1, ~900 cm-1and~1100 cm-1correspond to the second-order transverse optical phonon(2TO)peak,the combination of transverse and longitudinal optical phonons(TO +LO)peak,and the second-order longitudinal optical phonon (2LO) peak respectively [44,45]. Taking the 2LO scattering at ~1100 cm-1as a reference, the high temperature annealing treatment will significantly increase the scattering intensity of the 2 M scattering peak in nickel oxide[43],which proves that the complex calcined product is indeed nickel oxide and that very high temperature was generated during the complex decomposition process.
To further explore the reaction process of n-Al/[Ni(CHZ)3](ClO4)2(SC),nanocomposites were heated to 790◦C at a rate of 10◦C/min in an Ar atmosphere.The calcined product was then characterized by PXRD and XPS. Fig. 7(c) shows diffraction peaks that are completely different from that of Fig.7(a),which can be attributed to Ni, Al2O3and NiAl2O4initially according to the information in COD.To further prove the components,the valence information of related elements is obtained from XPS characterization. The deconvoluted nickel spectrum (Fig. 8(a)) shows the multiple splitting peaks,which can be assigned to NiAl2O4(856.0 eV for Ni 2p3/2,873.6 eV for Ni 2p1/2)and Ni(852.9 eV for Ni 2p3/2)along with their main satellites according to the references [46—48]. As shown in Fig. 8(b), the Al 2p signal (73.7 eV for Al2O3, 74.5 eV for NiAl2O4)corresponding to Al3+are represented [46]. Fig. 8(c) shows O 1s signal,in which the peaks with binding energies of around 531.1 eV and 532.7 eV can be assigned to Al2O3and NiAl2O4respectively[46,49].The joint analysis results of XRD,Raman spectrum and XPS on the calcined product of n-Al/complex (SC) nanocomposites confirmed the occurrence of the thermite reaction, the high temperature environment generated by which even caused the formation of NiAl2O4spinel during the reaction process.
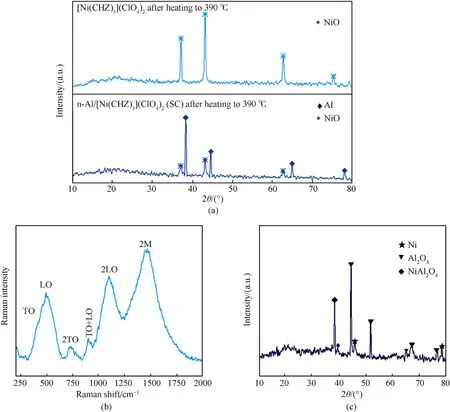
Fig.7. Reaction analysis of the pure complex and n-Al/complex(SC):(a)PXRD of the complex and n-Al(SC)after heating to 390 ◦C;(b)Raman spectra of the complex after heating to 390 ◦C; (c) PXRD of n-Al/complex (SC) after heating to 790 ◦C.
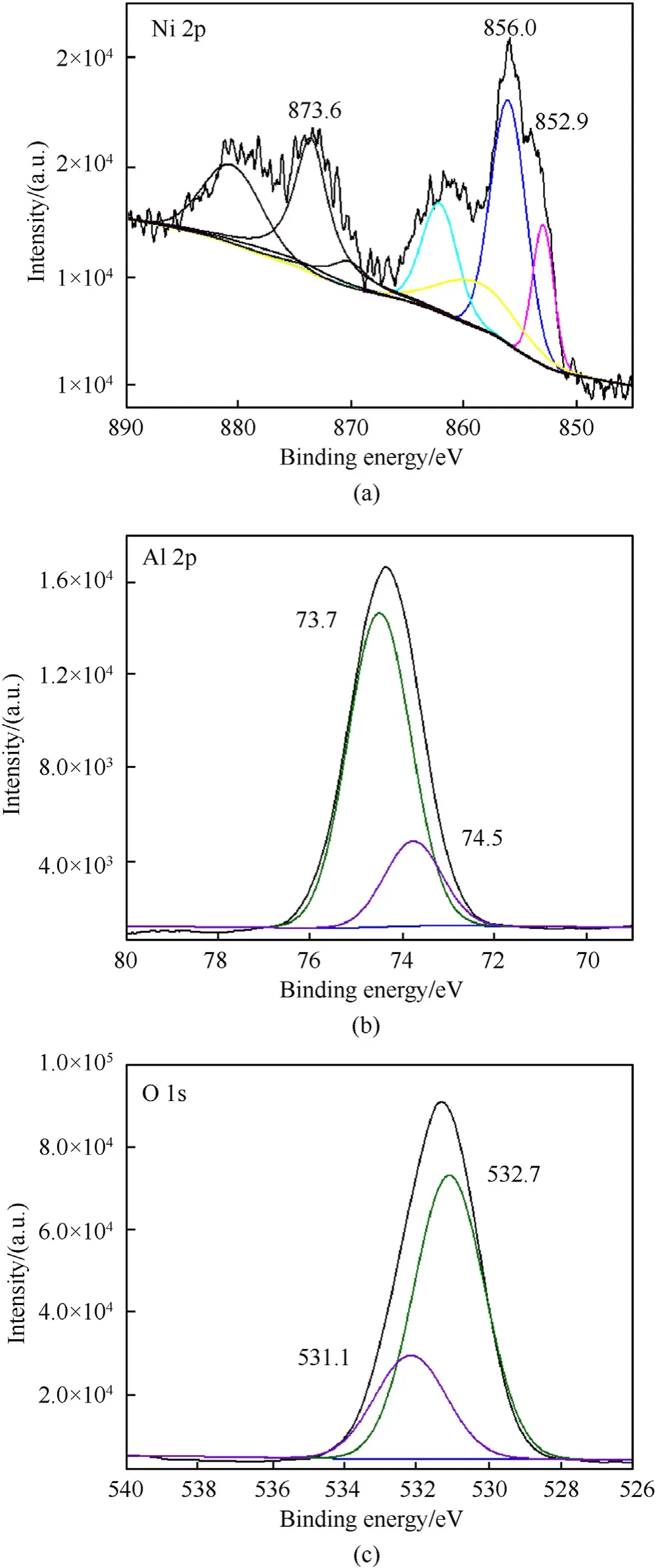
Fig.8. XPS spectra and deconvolution results for the calcined product of n-Al/complex(SC) from room temperature to 790 ◦C in Ar atmosphere: (a) XPS spectra and deconvolution results of Ni 2p;(b) XPS spectra and deconvolution results of Al 2p;(c)XPS spectra and deconvolution results of O 1s.
Based on the experiments and characterizations above, a possible reaction process of the energetic nanocomposites prepared by SC can be proposed with the following three steps:
3.3. Confined bomb experiment
According to the convection propagation mechanism, the gas pressure produced is closely related to the reaction state of nanocomposites.A piezoelectric sensor was used to record the change of pressure in the confined bomb to make dynamic pressure curves as shown in Fig. 9(a). The experimental samples were the energetic metal-organic complex prepared by conventional method, spray crystallized complex, n-Al/complex (PM) and n-Al/complex (SC)respectively. Fig. 9(b) shows the peak pressures and boost rates calculated from the Pressure-time(P-t)curves.The peak pressure of n-Al/complex(SC)is 3.91 MPa,which is 1.67,1.54 and 1.26 times of the complex prepared through conventional method, the complex prepared by SC and n-Al/complex (PM) respectively. At the same time, the n-Al/complex (SC) has the highest pressurization rate,which is about 1324.06 MPa/s. The results shows that both the preparation method of SC and the addition of n-Al powder can effectively increase the peak pressure and pressurization rate of the energetic nanocomposites.
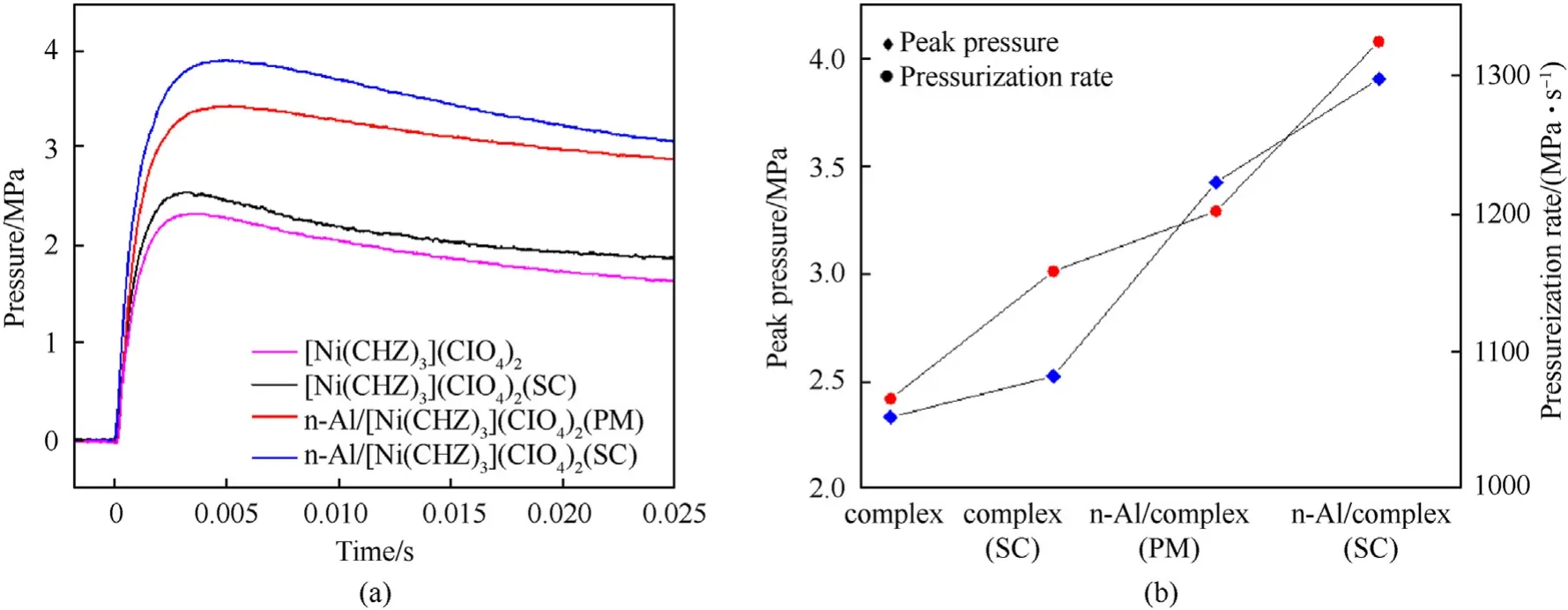
Fig. 9. Gas production performance of the complex, n-Al/complex (PM) and n-Al/complex (SC): (a) Pressure-time curves; (b) Peak pressures and boost rates.
This is because the SC method significantly reduced the particle size of the complex and its reaction products,which increased the specific surface area and porosity of product,making gaps and slits of the complex be uniformly and tightly filled and covered by n-Al particles. The reduced restriction on diffusion and transport between particles was conducive to mass and heat transfer,benefiting the acceleration of the convection process. Besides, the oxide particles decomposed from the complex could further react with n-Al powder. Although the addition of n-Al powder did not provide more gaseous product according to the TG curves of pure complex and n-Al/complex (SC) nanocomposites as shown in Fig. 10, the extra heat release because of thermite reaction was conducive to the improvement of gas pressure. Overall, the high peak pressure and high boost rate make the n-Al/complex (SC) energetic nanocomposite have greater application potential.
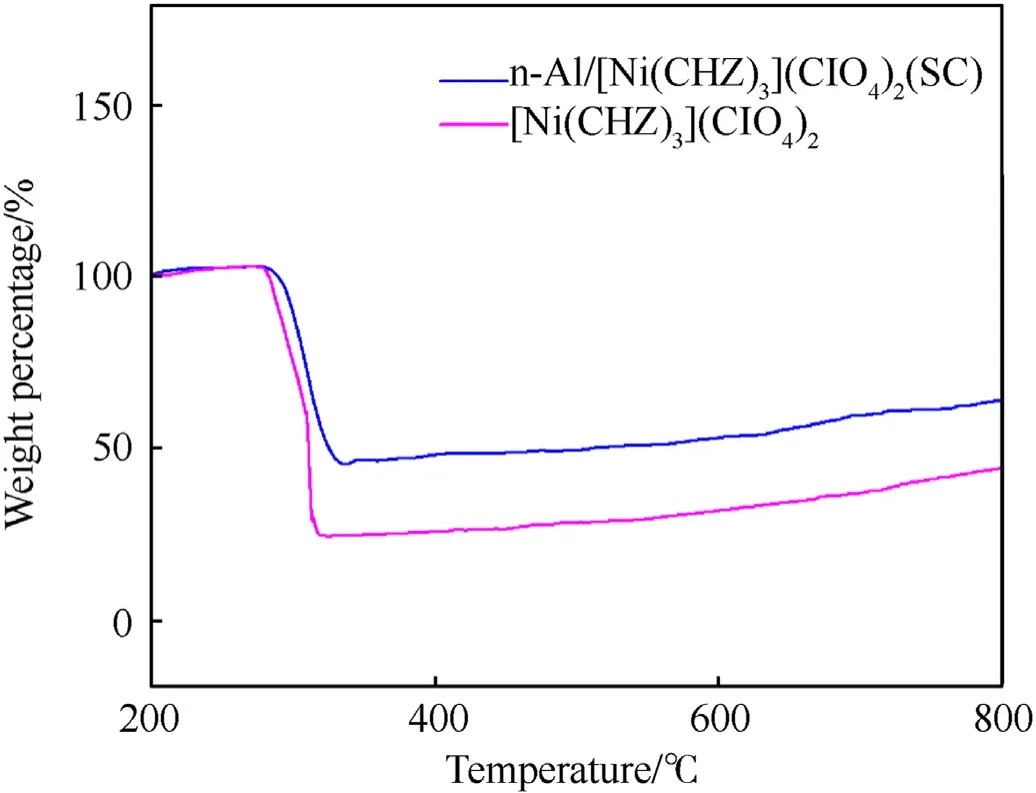
Fig. 10. TG curves of the pure complex and n-Al/complex (SC) energetic nanocomposites.
3.4. Lead plate test
Lead plate perforation is a simple qualitative method used to evaluate the detonation performance of agents.As shown in Fig.3,the lead plate is in contact with the bottom of the steel pipe, the diameter of the hole is an indicator of the detonation capability.The experimental results are shown in Fig.11,the pore diameters of the energetic metal-organic complex, n-Al/complex (PM) and n-Al/complex (SC) are 9.92 mm, 9 mm, and 10.14 mm respectively. It proves that the SC method and the additional thermite reaction enhanced the detonation performance of the energetic metalorganic complex. The high reactivity of n-Al/complex (SC) nanocomposites can be reasonably attributed to the strong heat release and gas products generated in large quantities at a high rate,since more heat and gas could produce higher temperature and pressure zones. Besides, the SC method leads to morphological characteristics of the complex that were conducive to convection propagation mechanism, which allows these heat and gas to enter RDX more effectively according to convection heat transfer mechanism,which contributes to the explosion of RDX.Meanwhile,the effect of welldistributed and uniformly mixed product structure provided by SC cannot be ignored. A comparative test of LA as the initiator was carried out under the same experimental conditions, whose hole diameter is 9.07 mm, further illustrating the good detonation ability of n-Al/complex (SC) nanocomposites.

Fig.11. Pictures of 5 mm thick lead plates detonated by (a) the complex 9.92 mm; (b) N—Al/complex (PM) 9 mm; (c) N—Al/complex (SC) 10.14 mm and (d) LA 9.07 mm.
3.5. Electrostatic safety
As shown in Table 2,the n-Al/complex(SC)nanocomposites did not fire at the maximum output energy (100 mJ) of instrument.Comparatively, the pure complex prepared by conventional method was greatly more sensitive to ESD,which may attribute to the increase of conductivity in n-Al/complex (SC) energetic nanocomposites about electrostatic discharge[50],and the reduction in charge accumulation because of the good dispersion of the product prepared through SC[51].This performance demonstrates a wider application prospect of the n-Al/complex (SC) nanocomposites because of their outstanding insensitivity to ESD.

Table 2Comparison of the ESD sensitivity of two samples.
4. Conclusions
Feasibility of Spray Crystallization (SC) has been demonstrated for preparation of the n-Al/energetic metal-organic complex nanocomposites. n-Al/[Ni(CHZ)3](ClO4)2(SC) were prepared and characterized for structure, thermal properties and reaction mechanism.SEM confirmed that the complex prepared through SC possessed flower-like structure with a significantly reduced particle size of around 2 μm, the n-Al/complex (SC) energetic nanocomposites also presented more uniform and tight mixing.N2adsorption-desorption isotherms indicated visibly increased total pore surface area as well as porosity of the n-Al/complex(SC).DSC measurement confirmed a high heat release of 2977.6 J/g from n-Al/complex (SC) and the existence of thermite reaction, which was further verified through PXRD,Raman and XPS measurements.The peak pressure, pressurization, as well as the detonation ability of the n-Al/complex(SC)energetic nanocomposites were also greatly improved compared with the complex alone and the n-Al/complex(PM). Meanwhile, the outstanding safety of the n-Al/complex (SC)to ESD was compared with the pure complex prepared by conventional method, making the n-Al/complex (SC) energetic nanocomposites more widely applicable. The excellent energetic properties of the n-Al/complex (SC) energetic nanocomposites demonstrated the positive significance of preparing n-Al/energetic metal-organic complexes nanocomposites through SC method.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by National Natural Science Foundation of China (project no. 51676100).
杂志排行
Defence Technology的其它文章
- A review on lightweight materials for defence applications: Present and future developments
- Study on the prediction and inverse prediction of detonation properties based on deep learning
- Research of detonation products of RDX/Al from the perspective of composition
- Anti-sintering behavior and combustion process of aluminum nano particles coated with PTFE: A molecular dynamics study
- Microstructural image based convolutional neural networks for efficient prediction of full-field stress maps in short fiber polymer composites
- Modeling the blast load induced by a close-in explosion considering cylindrical charge parameters
