Research of detonation products of RDX/Al from the perspective of composition
2023-07-04XinghnLiZhicongYiQijunLiuFushengLiuZetengZhngShenyunHouXinxuZhengXuZhngHongPei
Xing-hn Li , Zhi-cong Yi , Qi-jun Liu , Fu-sheng Liu , Ze-teng Zhng ,Shen-yun Hou , Xin-xu Zheng , Xu Zhng , Hong-o Pei ,*
a School of Physical Science and Technology, Southwest Jiaotong University, Chengdu, 610031, China
b National Key Laboratory for Shock Wave and Detonation Physics, Mianyang, 621900, China
Keywords:Aluminized explosive Detonation products EOS Rod-driven test Compositional evolution
ABSTRACT
1. Introduction
The equation of state (EOS) of detonation products describes their thermodynamic state and determines the detonation performance and explosion effect. However, researching the EOS of detonation products of aluminized explosives is challenging.Aluminized explosives are prepared by adding Al powders to CHNO high explosives and have been extensively used in military and industrial applications worldwide. These explosives exhibit outstanding blast and thermal effects [1,2] owing to the strong post-detonation reaction of Al powders [3] that enhances the thermodynamic state of aluminized detonation products(P,T,and so on).Because of the complex heat and mass exchange between Al powder and CHNO products, the EOS of aluminized detonation products has not yet been completely understood.
Various experiments such as metal acceleration test [3,4],detonation bomb test [5,6], air-pressure and temperature measurements [7,8], underwater test [9,10] and molecular dynamics(MD) calculations [11,12] have been conducted to analyze the Al reaction and the EOS of aluminized detonation products. The EOS depends on Al content,Al particle size,explosive formula,confiner and charge size,etc.Several studies have proposed experimentally calibrated phenomenological EOS for aluminized detonation products.The JWL—Miller EOS[13]is the most commonly used EOS that accounts for the contribution of the Al reaction to pressure.Recently, Yue et al. [14] proposed an EOS that considers the negative correlation between gas consumption during the Al reaction and pressure.Liu et al.[15]derived an EOS that takes into account gasification-dominated reaction of Al powders. Thermochemical EOSs, such as CHEQ [16], CHEETAH [17], KHT [18], VLW [19] and EXPLO5 [20], are based on the characteristics of components and are suitable for studying the EOS of detonation products. For example, CHEETAH has been coupled with the hydrodynamic program ALE3D to evaluate the detonation velocity and failure diameter of NM[21],the double shock response of LX-17[22],and the shock ignition of HXM [23]. However, the extremely long computational time of thermochemical EOSs limits their applicability.
Despite various achievements[24—26],previous studies have a few limitations. First, the understanding of the Al reaction is still not enough because composition is not accounted for in the phenomenological EOS. In addition, the general assumption of temperature equilibrium between Al powders and CHNO products may be invalid[27,28].Second,the EOS should be validated because of the weak predictive ability of the phenomenological EOS. At present,the EOS of aluminized detonation products and Al reaction process are calibrated simultaneously using the same experimental data; theoretically, the uniqueness of each one cannot be guaranteed. For example, we calibrated two sets of the JWL—Miller EOS and Al reaction process according to the same cylinder test result of RDX 70 wt%/Al 30 wt%,and the calibrated Al reaction time was less than 10 μs [29] and more than 30 μs [30],respectively. Finally,the thermodynamic state of Al powders is generally neglected in the EOS. Notably, the heat absorbed by Al powders and heat released during the Al reaction are considerably different at different temperatures, thereby notably affecting the EOS of aluminized detonation products. When the temperature of Al rises from room temperature(300 K)to its vaporization point(2767 K),the ratio of the heat absorbed by Al (per mass) to the detonation heat of TNT(per mass)is approximately 1,and it increases to approximately 4 if Al is completely vaporized. When Al is fully oxidized by H2O, CO2,and O2, which are the primary oxidizers in detonation flow, the ratio of reaction heat of hot Al (2000—2700 K) to reaction heat of cold Al(300 K)is 1.2—1.9 times,1.2 wt%1.8 times,1.15 wt%1.5 times(all substances except Al are 300 K),respectively.
This paper proposes a novel method to research the EOS of aluminized detonation products from the perspective of components. A two-phases model (Al powders and CHNO products) are introduced into the thermochemical code DLCHEQ,and it accounts for the exchange of energy and matter between the two phases.Experimental data are no longer used to simultaneously calibrate the EOS and Al reaction process. The EOS of CHNO products is reliably predicted using DLCHEQ,and the endothermic and reaction processes of Al powders are calibrated using a recently designed rod-driven test. The most important contribution of this study is the successful application of DLCHEQ to the hydrodynamic program,which was not extensively used before.
The remainder of this paper is organized as follows: the first section introduces the thermochemical code DLCHEQ and the endothermic and reaction model of Al;the second section describes the rod-driven test,and the experimental results of RDX,RDX 85 wt%/LiF 15 wt%,RDX 70 wt%/LiF 30 wt%, RDX 85 wt%/Al 15 wt%, RDX 70 wt%/Al 30 wt%.In the third section,the calculation model of the rod-driven test is described. The fourth section highlights the reliability of DLCHEQ and presents the calculation results of the rod-driven test.This section also discusses the key factors affecting the EOS of aluminized detonation products.
2. DLCHEQ and Al-related models
2.1. DLCEHQ code
Thermochemical code DLCEHQ is a framework of EOS that includes EOS of unreacted explosives, EOS of detonation products,reaction model,etc.EOS of unreacted explosives is based on the MD calculation [31] and Hugoniot experiment [32]. EOS of detonation products contains 16 components (CO2,O2, H2, H2O,CH4,NH3,NO,N2,CO,N,O, N2O,NO2,C,Al and Al2O3), and the EOS is developed on the basis of the CHEQ code (primary improvements are shown in Table 1).DLCHEQ can describe the thermodynamic states of detonation products from high pressure and temperature state(such as the VN point) to the normal state on the basis of the theoretical foundation and reliable database. We used DLCHEQ to calculate the shock ignition process, the CJ state, the isentropic curve and the quasi-static pressure,etc.,all of which were in good agreement with experimental values (see appendix for partial results).
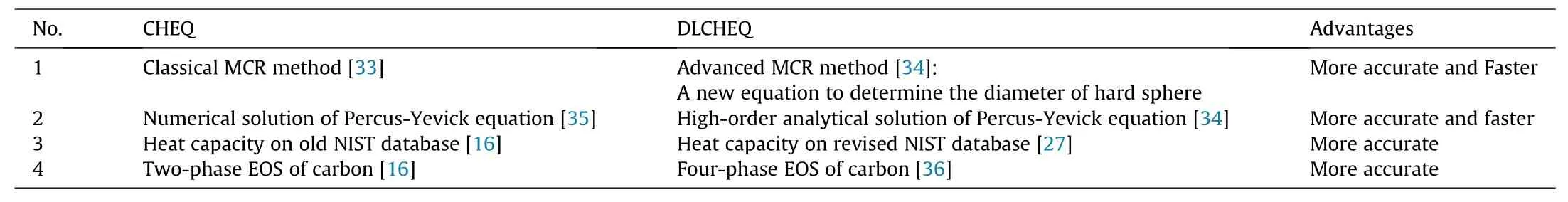
Table 1Primary developments of DLCHEQ.
The research of EOS of aluminized detonation products using DLCHEQ follows the plot shown in Fig.1. As indicated in Fig.1, inputs of the DLCHEQ areP,T,reaction degree of Al powder(λ,λ=0 means Al has not reacted,λ =1 means Al has completely reacted)and a molecular formula of high explosive (HE). Different models account for the thermodynamic state of CHNO products and Al powders, since a temperature imbalance between the two phases may exist. Temperature model and reaction rate model of Al powders, and EOS of Al and Al2O3are introduced in this paper to calculate the thermodynamic state of Al and Al2O3˙When DLCHEQ determines the composition and thermodynamic state of CHNO products by Gibbs free energy minimization, it is necessary to consider the change in the total amount of elements in the CHNO products due to oxygen depletion during the Al reaction. Considering the mass coupling rule[37]between the CHNO products and the Al powders, DLCHEQ can output volume (V), internal energy(E), component {ni}, enthalpy (H) and other thermodynamic quantities of aluminized detonation products.
2.2. Al temperature model
The pressure of Al and Al2O3should always be consistent with the CHNO products[38]because the stress wave travels fast in the detonation flow.As the reaction products of Al powders and CHNO products,Al2O3should remain in temperature equilibrium with the CHNO products. However, our previous study [27] found a long-time temperature disequilibrium between Al powders and CHNO products, and one reason is that the efficiency of the heat absorption of Al powders is limited. In this paper, we propose a simple model for Al temperature considering the mechanism of forced convection
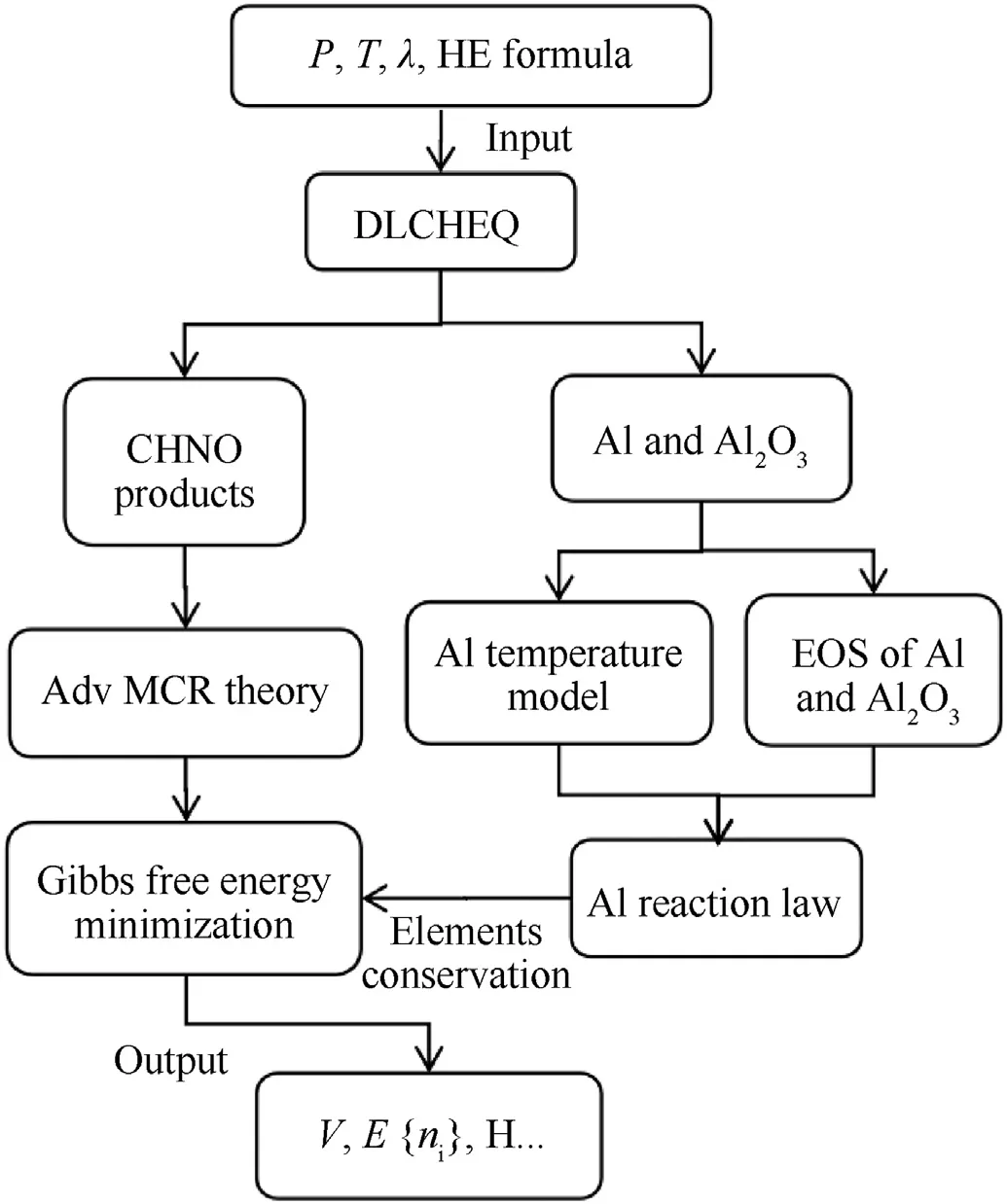
Fig. 1. The research schematic diagram of EOS of aluminized detonation products using DLCHEQ.
where thermal radiation is neglected due to the small contribution to Al temperature, and temperature gradient within Al particles is also neglected owing to the rapid internal heat transfer[27].In Eq.(1),the subscript Al stands for the Al particles, and the subscript g for the CHNO detonation products.CPis the specific heat capacity at constant pressure(replaced byCP0in Eq.(6)as the pressure studied in this paper is low),dthe Al particle diameter,ρ the density,Nuthe Nusselt number,tthe time,Kthe free convection coefficient (a function ofT) [39].Nudominates the forced convection process of Al particles,and it depends on the relative velocity between the Al particles and the CHNO products(Δu).Nuis defined as follows[40]:
where μ is the viscosity (a function ofT[39]), the value of Prandtl Number (Pr) approaches 1 in the detonation flow[39].
2.3. EOS of Al and Al2O3
The EOS of Al and Al2O3is divided into high-pressure form (>2—3 GPa) and low-pressure (< 2—3 GPa) form. The high-pressure EOS of Al [41] and Al2O3[42] was built on the Hugoniot results.Based on the thermodynamic relationwe established EOS of Al and Al2O3at low pressure.
whereT0is the room temperature,P0the atmospheric pressure.The subscript 0 represents room condition. γ0is the Grunisen parameter, α the isobaric expansion coefficient,KTthe isothermal compression coefficient. According to Eq. (3), Gibbs energy (G)expression can be obtained.
where ΔH0298˙15and ΔS0298˙15are the standard formation enthalpy and standard entropy,respectively.Ris the ideal gas constant,aandbare the fitting parameters derived from the NIST database. Solid and liquid phases of Al and Al2O3are introduced to construct a more realistic model.The gas phase of Al and Al2O3are omitted in this paper,because only a small percentage of Al and Al2O3can be vaporized in the detonation flow due to their extremely high vaporization temperature and enthalpy (as shown in Table 4). All parameters of Al and Al2O3EOS are listed in Table 2.Since α of the liquid phase is absent, it is replaced by the solid value due to the small contribution of the second term on the right-hand side of Eq.(4) toG(P,T).

Table 2EOS parameters of Al and Al2O3 under low pressure [43].
2.4. Irreversible reaction model of Al
A large number of studies have focused on the chemical kinetics of Al at low pressure, but few studies have focused on high pressure. Thus, the irreversible reaction is a general assumption to study the oxidation of Al powders. In this paper, the reaction mechanism of Al powders is described as follows:
Al takes the oxygen atom(O)from the CHNO products,and the specific oxygen-suppliers are determined by Gibbs free energy minimization.We assume that the classical pressure control model governs the reaction rate of Al powders.
According to the theoretical and experimental analysis [44,45],values(under the basic unit of cm-g-μs)offandzare 1/2 and 1/6,respectively. Once the parameter para is calibrated, the reaction process of the Al powders is determined.
3. Rod-driven test
To research the EOS of aluminized detonation products for a long duration over a simple flow field,we designed an experiment using a rod that was accelerated by detonation products(Fig.2).As illustrated in Fig. 2, the detonation products expanded freely after explosion and caused the rod to accelerate in the smooth pipe after contacting it.PDV probes were placed at the center of the outlet of the pipe to measure the free-surface velocity of the rod. The detonation products underwent quasi one-dimensional expansion along the axial direction of the pipe because only the right boundary (rod)of the detonation products could move. The acceleration time of the rod was estimated from a simple solution[46],which is based on the momentum conservation law. When the mass of the rod was approximately 80 times that of RDX, the predicted acceleration time was approximately 1 ms, which was considerably greater than that of the tube test and flyer test.
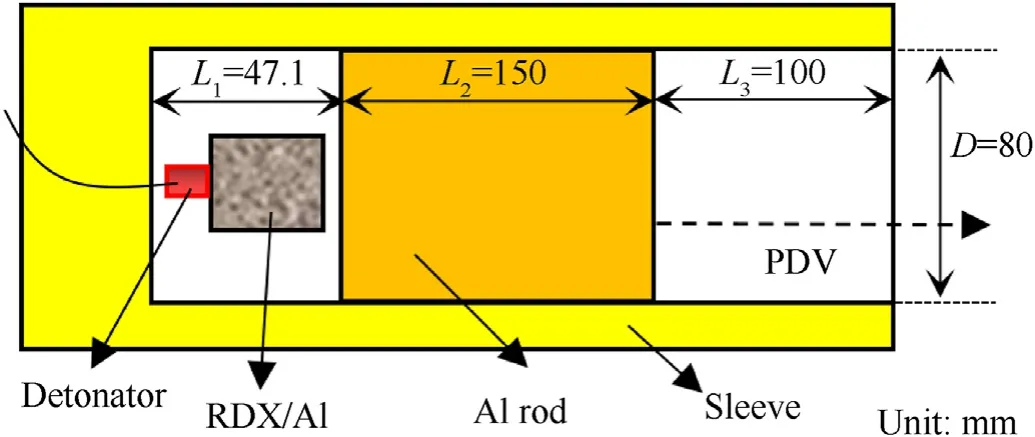
Fig. 2. The experimental device of rod-driven test.

Fig.3. The rod-driven test results:(a)The free-surface velocity of RDX 85 wt%/Al 15 wt%and RDX 85 wt%/LiF 15 wt%;(b)The free-surface velocity of RDX 70 wt%/Al 30 wt%and RDX 70 wt%/LiF 30 wt%. The results of the rod-driven tests can be summarized as follows.
To minimize the peaks of the free-surface velocity due to the strong off-body shock entering the rod,the rod was designed such that it did not make direct contact with the explosive.The samples used in the tests are described in Table 3 the samples of rod-driven test. LiF is often considered as inert Al because its density and impact properties are similar to those of Al.The results of the roddriven test are illustrated in Fig. 3.

Table 3The samples of rod-driven test.
(1) The free-surface velocity of the rod increased in the form of oscillations,which were caused by the propagation of shock and stress waves. The strong off-body shock and the continuous beat of the detonation products against the rod contributed to the shock and stress waves,while the off-body shock disappeared rapidly and the beat persisted for a long time.
(2) The Al powders in RDX 70 wt%/Al 30 wt%and RDX 85 wt%/Al 15 wt%reacted because the free-surface velocity of the rod in RDX/Al was higher than that of the corresponding RDX/LiF.The effect of Al reaction on the free-surface velocity of the rod increases with Al content.
(3) The Al powders in the rod-driven test should ignite after 100 μs because the free-surface velocity of the rods in RDX 85 wt%/LiF 15 wt% and RDX 85 wt%/Al 15 wt% exhibited a difference of more than 20% at 117 μs, and the velocity in RDX 70 wt%/LiF 30 wt% and RDX 70 wt%/Al 30 wt% exhibited a difference of more than 50% at 140 μs. Ripley et al. [47]analyzed Al powders in the free expansion flow field of NM products and discovered that micron-scale Al powders did not react within tens of microseconds, which is consistent with our experimental results.
4. Calculation method
We successfully tightly coupled the thermochemical code DLCHEQ with the hydrodynamic program SSS by replacing the JWL EOS with the complete thermodynamic database generated by DLCHEQ. The combination of DLCHEQ and SSS allows us to study EOS of aluminized detonation products from the perspective of components, and the corresponding strategy and approaches are listed below.
4.1. Computing strategy
The research of EOS of aluminized products is divided into three steps (Fig. 4). Firstly, DLCHEQ is validated by comparing the calculated rod free-surface velocity of RDX with the measured value.Secondly,the parameterNuis calibrated through comparing the calculated rod free-surface velocity of RDX/LiF with the measured velocity (the reliability of replacing inert Al powders with LiF powders is shown below). Finally, the parameter para is calibrated by comparing the calculated rod free-surface velocity of RDX/Al with the measured velocity,and the thermodynamic states of aluminized detonation products can be obtained.

Fig. 4. Research strategy for the EOS of RDX/Al products.
4.2. Application of DLCHEQ in SSS
SSS is a one-dimensional Lagrangian hydrodynamic program[48],which uses the JWL or ideal gas EOS to describe the detonation products.We used DLCHEQ to generate a complete thermodynamic database(P,T,V,E,λ,{ni},etc.)for a certain range,which replaced the JWL and ideal gas EOS in SSS.SSS transmittedVin,Ein,λinto the thermodynamic database, which returned the correspondingVout,Eout,λout,Pout,Tout,{ni}out,etc.back to SSS.The calculation error of DLCEHQ was defined by the maximum relative deviation between(Vin,Vout), (Ein,Eout) and (λin,λout), and a value of less than 1% canmeet the requirement.The time cost of using the DLCHEQ database is currently 2—5 times that of the JWL EOS and can be reduced by further optimization.
A one-dimensional model was built in the SSS to simulate the rod-driven experiments, with the fixed boundary, the detonation products and the rod from left to right.The Gruneisen EOS and the Johnson-Cook constitution[49]were adopted for Al rod.The spatial and time step sizes were 0.05 cm and 50ns, respectively, which resulted in a good convergence of the calculations.
4.3. Thermal equivalence between Al and LiF powders
The endothermic process of Al powders, which may have a significant effect on the flow field of aluminized detonation products, is primarily determined byNu. The preliminary scope ofNushould be within 2—200 according to its definition(Eq.(2)),and the specific value ofNucan be calibrated by the rod-driven experiment.However,a direct research into the rod-driven test of RDX/Al can't decouple the endothermic and the reaction process of Al powders. Based on the thermal equivalence between Al and LiF powders,the rod-driven test of RDX/LiF was used to calibrateNuof the corresponding RDX/Al.
LiF is considered as inert Al because LiF has a similar density and hugoniot parameters to that of Al. In addition, the melting point,the melting enthalpy, the vaporization point and vaporization enthalpy of Al are similar to that of LiF (Table 4), too. When the value ofNuis much greater than 2(like the situation in this paper),the rate of powder temperature rise can be achieved according to Eq. (2)

Table 4Thermodynamic quantities of Al and LiF [43].
where Δuis proportional todr,andris 1—2.Therefore,Eq.(9)can be simplified
According to the diameter of Al and LiF powders, and the properties in Table 4, the value ofis 12.9%—52.9% from 300—3000 K. Therefore, it is roughly reliable to use 3 μm LiF powders to research theNuof 50 μm inert Al powders.
The feasibility of using 3 μm LiF powders to investigate the endothermic process of 50 μm inert Al powders can be validated by a comparison of the detonation velocity between RDX/Al and RDX/LiF(Table 7).In the ZND zone,the endothermic process of Al and LiF powders has an effect on the detonation velocity of the explosives.When Al powder size changes from 3 to 50 μm in RDX 85 wt%/Al 15 wt%and RDX 70 wt%/Al 30 wt%,the detonation velocity of 50 μm Al powders is always closest to that of corresponding RDX/liF with 3 μm LiF powders [30].
4.4. Initial conditions
The timeline of events in the rod-driven test is shown in Fig.5.As displayed in Fig. 5, the detonation products expand freely after the explosion and take several microseconds to fill the reserved space. The initial time of the rod-driven test (t0) is defined as the moment the detonation products first reach the uniform thermodynamic state.t0is approximately 20—40 μs after the CJ state because the propagation of shock and stress waves through the reserved space costs time. The motion delay of the rod (approximately 50—70 μs)corresponds to the first travel of the shock wave through the rod,and the rod stops moving at about 1 ms.
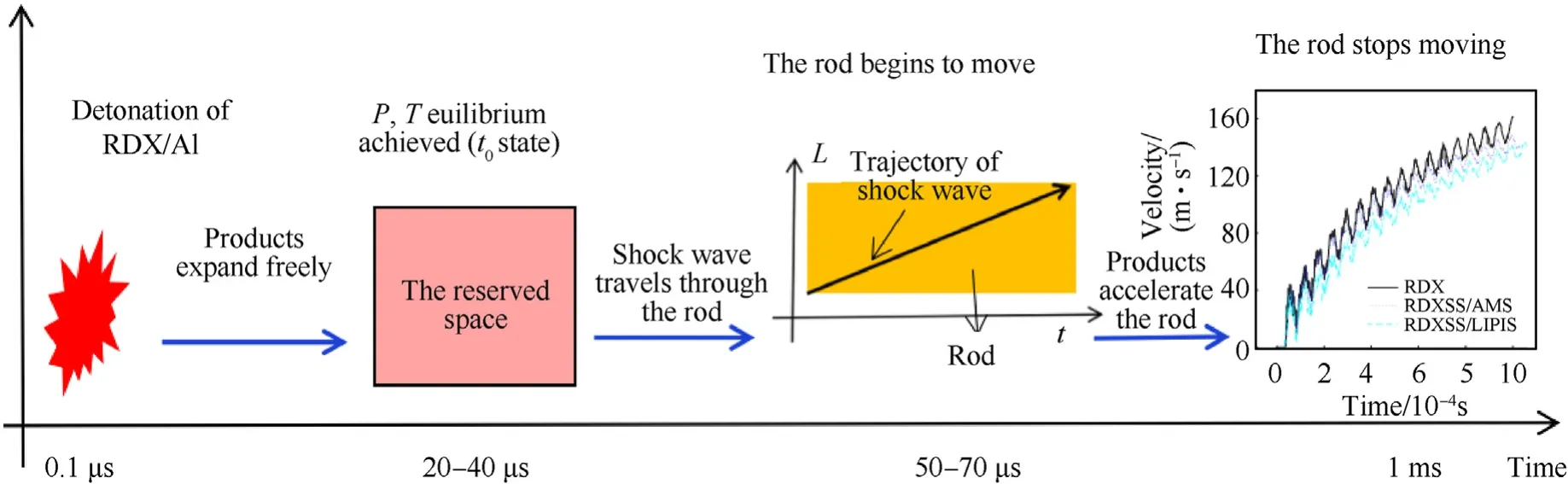
Fig. 5. The timeline of events in the rod-driven test.
The thermodynamic state of the detonation products att0can be obtained by 3 steps. First, the CJ state of the explosives is computed. We have proposed a CJ method that uses JWL EOS to calculate the CJ state of aluminized explosives[37].By replacing the JWL EOS with DLCHEQ,we can obtain the CJ parameters of RDX/Al,and the results are shown in appendix.
Second, in the free expansion process of the detonation products, the heat absorbed by Al powders from the CHNO products is computed by applying the Al temperature model into the analytical solution of one-dimensional flow field [27]. The calculated results illustrate that the temperature of the Al powders att0is 679.7 K and 650.2 K for RDX 85 wt%/Al 15 wt% and RDX 70 wt%/Al 30 wt%,respectively, with a slight temperature gradient within the Al powders. The results are verified qualitatively. The calculated Al temperature(lower than the Al melting point)indicates that the Al powders are not ignited att0,which are consistent with the above experimental conclusions.
At last,the thermodynamic states of the detonation products att0are obtained by the first law of thermodynamics [50]. When neglecting heat transfer between the detonation products and environment,the internal energy of the CHNO products att0(Egto)should satisfy
where subscriptt0denotes thet0state, CJ denotes the CJ state,gdenotes the CHNO products. In the free expansion process of the detonation products,Qrerepresents the heat released by the reactions within the CHNO products,QAlt0represents the heat absorbed by the Al powders from the CHNO products, andAworkrepresents the work done by the CHNO products to compress the ambient air.EgCJandQAlt0can be computed in advance, whileEgto Qre (Qre= Σ(ni,t0-ni,CJ)•Hni,t0) andAwork(Awork=Pt0•Vt0) are related to thet0state.Considering thatVt0equals to the volume of the reserved space and λt0= 0, onlyTt0is unknown to determine thet0state. We calculatedEgto,QreandAworkunder different temperatures, and the calculation errors were obtained by substituting their values into Eq.(11).Tt0was determined when the corresponding absolute value of error was less than 0.1%, and the calculatedt0state of RDX/Al (Table 5) was obtained by weighting the thermodynamic quantities of the Al powders and the CHNO products by mass.
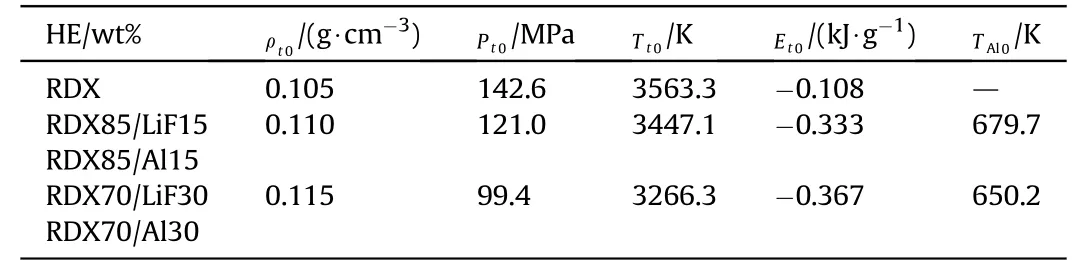
Table 5The calculated t0 state of RDX/Al and RDX/LiF.
ThePt0corresponds to the quasi-static pressure in the closedspace explosion experiment, which has been most extensively studied for TNT.The calculatedt0state can be validated by that the calculatedPt0of TNT is in good agreement with the experimental values over a wide range(Table 8).
As shown in Table 5,the calculatedt0state of RDX/LiF equals to that of the corresponding RDX/Al because the endothermic process of 3 μm LiF powders is considered equivalent to that of 50 μm inert Al powders.
5. Calculation results
5.1. Reliability of DLCHEQ
Using thet0states of RDX displayed in Table 5 as initial conditions, the rod-driven tests of RDX were performed based on DLCHEQ, JWL EOS (calibrated according to tube test results using the same formula), and the ideal-gas EOS (γ=1˙4 and 3), and the results are displayed in Fig. 6. As illustrated in Fig. 6, the freesurface velocity calculated using DLCHEQ was less than the experimental value before 200 μs because the off-body shock wave was neglected in the calculations. After 200 μs, the DLCHEQcalculated velocity was close to the experimental value, with a less deviation (< 10%) than that calculated using the JWL EOS (<66%) and the ideal-gas EOS (< 94% and 96%). Therefore, the reliability of DLCHEQ was validated.

Fig. 6. Calculation results of rod-driven test of RDX: (a) The free-surface velocity of rod;(b) Pressure of detonation products; (c) Temperature of detonation products; (d) Primary components of detonation products; (e) Calculation error.
Figs.6(b)and 6(c)depict thatPandTat three Lagrangian points(left boundary, middle point and right boundary) were similar to each other within 1 ms, and oscillations were caused by the propagation of the shock and stress waves. The results indicated that the thermodynamic state of the flow field of the rod-driven test was uniform throughout the experiment. In the following paragraphs, the calculated values ofP,T, {ni}, and energy did not correspond to spatial locations.
According to Fig.6(d),the primary components of RDX products were CO2, H2, H2O, NO, N2, and CO˙The compositional evolution curves were not smooth because of the propagation of shock and stress waves. An overall reaction in 1 g RDX detonation products within 1 ms was illustrated in Fig. 6(d).
whereQchemis the reaction heat(exothermic is positive),and ΔVgasis the change of gas molar quantity after the reaction. Fig. 6(d)indicated that the CHNO detonation products proceeded a gasconsumed and exothermic reaction after the CJ point, which was consistent with the previous studies[50,51].As shown in Fig.6(e),the calculation error ranged from 5 ×10-4to 2×10-3in the RDX case,which was the same order of magnitude as that of the RDX/Al case in the following paragraphs. The small computational error indicates a good application of the DLCEQ database in the SSS,and hence the error analysis is omitted later.
5.2. Endothermic process of Al powders
The rod-driven test of RDX/LiF under different values ofNuwere calculated(Fig.7)using the parameters of 50 μm Al powders(inert)to replace those of 3 μm LiF powders.As exhibited in Figs.7(a)and 7(b), the free-surface velocity of the rod increased with a decreasingNu,andNuexhibited more notable influence within the range 2—25 than that within the range 25—50.A largerNuindicated higher absorption of energy from the CHNO products and thus a faster decrease in the work ability of the CHNO products.
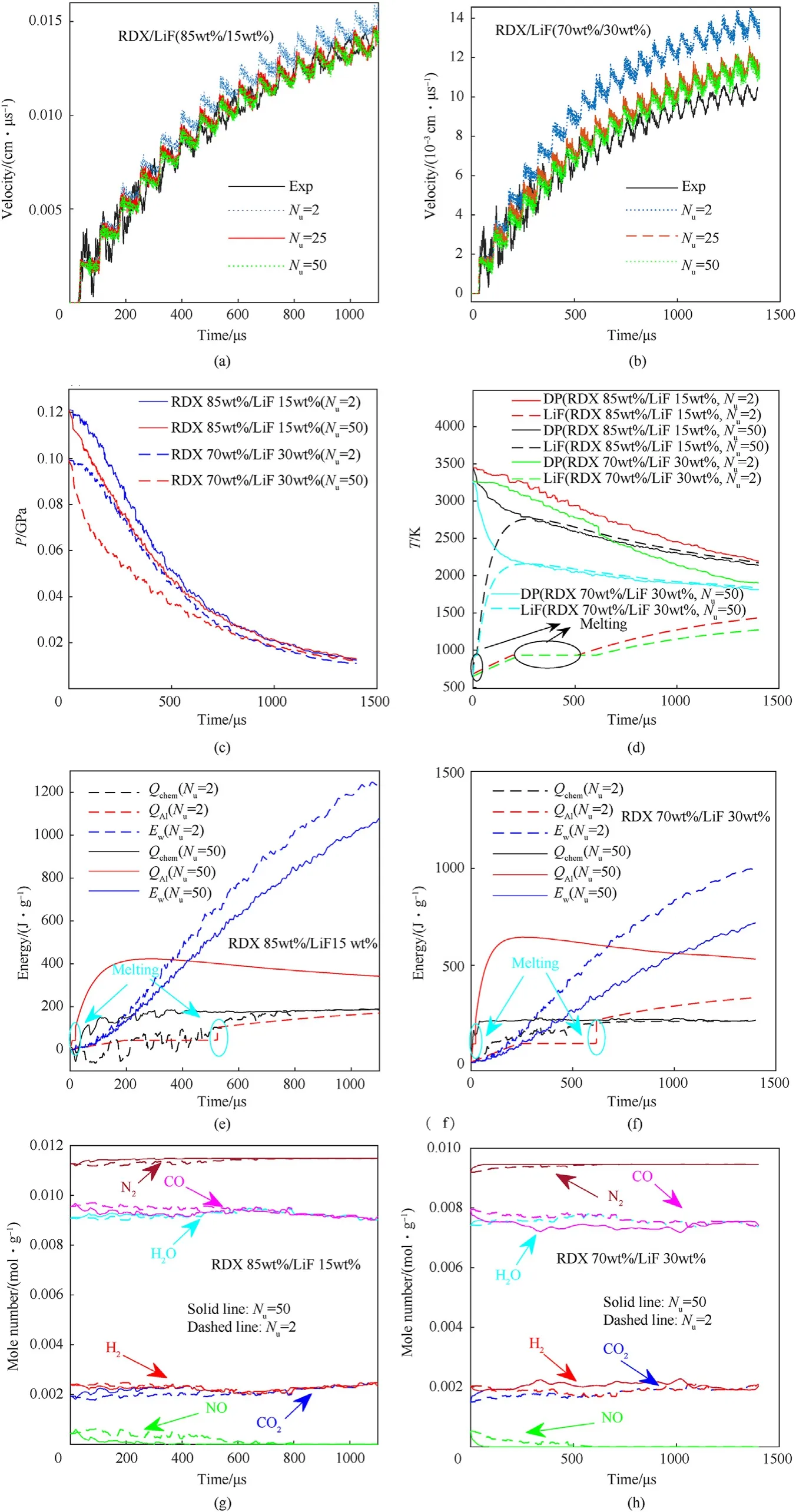
Fig.7. Calculated rod-driven tests of RDX/LiF under different values of Nu:(a)Free-surface velocity of rod of RDX85/lif15;(b)Free-surface velocity of rod of RDX 70 wt%/LiF 30 wt%;(c)Pressure of detonation products;(d)Temperature of CHNO products and Al powders;(e)Energy evolution of RDX 85 wt%/LiF 15 wt%detonation products;(f)Energy evolution of RDX 70 wt%/LiF 30 wt% Detonation products; (g) Compositional evolution of RDX85/lif15 Products; (h) Compositional evolution of RDX70/LiF 30 products.
As illustrated in Figs. 7(a) and 7(b), the measured free-surface velocities of RDX 85 wt%/LiF 15 wt% and RDX 70 wt%/LiF 30 wt%
were similar to those calculated whenNuwas 25—50.After 0.8 ms,the calculated free-surface velocity of RDX 70 wt%/LiF 30 wt% for the values ofNuwithin the range 25—50 was approximately 10%higher than the experimental value, which could be attributed to the advanced gas leakage due to the exceedingly long acceleration time.Therefore,the value ofNuwas calibrated to be within 25—50 for the detonation products of RDX/LiF. Since a change inNufrom 25 to 50 caused only a small difference,Nuwas fixed at 50 for RDX/Al in the calculations.
As displayed in Figs.7(c)and 7(d),Nuhad a similar effect on the temperature and pressure of RDX 85 wt%/LiF 15 wt%and RDX 70 wt%/LiF 30 wt% products. The increase inNuresulted in a pressure reduction of the detonation products and a closer approach to the temperature equilibrium between the Al powders and the CHNO products. As depicted in Fig.6(d), the temperature of the powders were at least 500 K lower than that of the CHNO products whenNu= 2, and the temperature equilibrium was rapidly established whenNu= 50.
Energy change of the RDX/Al detonation products follows the first law of thermodynamics
where ΔEgdenotes the increase in the internal energy of the CHNO products,QAldenotes heat absorbed by powders (Al or LiF),EWdenotes the increase in the kinetic energy of the rod.Figs.7(e)and 7(f) illustrated that for RDX/LiF, a largerNunotably enhancedQAland reducedEW,whereas the change inQchemwas not substantial.WhenNu=2,QchemandQAlof RDX/LiF were of the same order of magnitude;thus,ΔEg≈-EW,indicated that the energy released by the CHNO products was utilized to accelerate the rod.WhenNufor RDX/LiF increased from 2 to 50,Qchemchanged slightly andQAlincreased substantially. Therefore, ΔEg≈-QAl-EW, which indicates that part of the energy released by the CHNO products was absorbed by the powders. The energy of the CHNO products decreased with increasedNuand powder content because of greater heat absorption of the powders.Therefore,the temperature model and phase change should not be neglected for aluminized detonation products.
The compositional evolution curves of the RDX/LiF products(Figs. 7(g) and 7(h)) were not smooth over time because of the effects of the propagation of shock and stress waves.The reaction of the CHNO products was primarily affected by the powder content,whereas theinfluence ofNuwas not significant. The overall reactions in 1 g RDX/LiF within 1 ms were summarized below.
RDX 85 wt%/LiF 15 wt% (Nu= 50):
Eqs. (14) and (15) indicated that an exothermic and gasconsumed reaction occurs in the detonation products of RDX 85 wt%/LiF 15 wt% and RDX 70 wt%/LiF 30 wt%. The primary difference between the two reactions was that H2is consumed to produce H2O in RDX 85 wt%/LiF 15 wt%and reversed in RDX 70 wt%/LiF 30 wt%.
5.3. Reaction process of Al powders
We calculated the rod-driven tests of RDX 85 wt%/Al 15 wt%and RDX 70 wt%/Al 30 wt% for different values of para (Fig. 8). As depicted in Figs. 8(a) and 8(b), the higher the value of para, the quicker the reaction of the Al powders,and the faster the motion of the rod. para can be preliminarily determined if the calculated velocity (the middle and later periods) had a mean deviation less than 2% (the measurement uncertainty of velocity). The preliminary values of para for RDX 85 wt%/Al 15 wt% and RDX 70 wt%/Al 30 wt%were 5×10-4—15×10-4and 10 × 10-4—20 × 10-4,respectively.
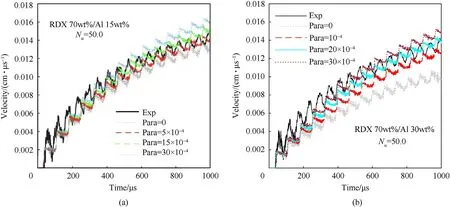
Fig. 8. Calculated free-surface velocity of rod in (a) RDX 85 wt%/Al 15 wt% and (b) RDX 70 wt%/Al 30 wt% upon different values of para.
Because the pressure and temperature of the RDX 70 wt%/Al 30 wt%products att0were lower than those of the RDX 85 wt%/Al 15 wt% products, Al powders in RDX 85 wt%/Al 15 wt% should proceed a reaction at a higher rate. Therefore, the most feasible reactive ranges of the Al powders were calibrated by overlapping the preliminary reaction process of the Al powders in RDX85/Al15 and RDX 70 wt%/Al 30 wt%. The calibrated values of para were within the range from 10 ×10-4to 15 ×10-4for RDX/Al (Fig. 9),and the corresponding reaction degree of Al powders was 25—38%at 1000 μs.The calculated results indicated a low reaction degree of Al powders (approximately 3—5%) at 100 μs; this is in agreement with the experimental result that the ignition delay of Al powders was greater than 100 μs.
Fig. 9. Most feasible reactive regions of Al powders in RDX/Al.
The influence of para on pressure, temperature, composition and energy evolution of RDX/Al products was shown in Fig.10.

Fig.10. Calculated rod-driven tests of RDX/Al under different values of para: (a) Pressure of detonation products; (b) Temperature of CHNO products and Al powders; (c) Energy evolution of RDX 85 wt%/Al 15 wt% products; (d) Energy evolution of RDX 70 wt%/Al 30 wt% products; (e) Compositional evolution of RDX 85 wt%/Al 15 wt% products; (f)Compositional evolution of RDX 70 wt%/Al 30 wt% products.
As illustrated in Figs. 10(a) and 10(b), the increasing value of para caused greater conversion of the Al reaction heat into the internal energy of the CHNO products,thereby considerably reducing the rate of decrease in the temperature and pressure of the CHNO products. For example, the temperature of the CHNO products of RDX 70 wt%/Al 30 wt% even increased after 200 μs. Generally, the temperatures of Al powders and CHNO products are assumed to be in equilibrium. However, Fig. 10(b) displayed a disequilibrium of temperature between the Al powders and the CHNO products in the present study. The temperature disequilibrium was more obvious with a larger value of para, because the highest temperature of Al powders did not exceed their boiling point owing to its high vaporization enthalpy,whereas the temperature of the CHNO products exceeded the boiling temperature of Al by absorbing the reaction heat of the Al powders.
According to Fig.10(c) and (d), a larger para corresponded to a greater increase inQchemandEWand a smaller increase inQAl.Qchem,QAl,andEWin RDX/Al were of the same order of magnitude,so they dominated ΔEgsimultaneously according to Eq. (13). For RDX 85 wt%/Al 15 wt%,when para increased from 0 to 15 × 10-4,ΔEgof RDX85/Al15 remained negative because (QAl+EW) was greater thanQchem, thereby causing the continued decrease in temperature and pressure of the CHNO products. For RDX 70 wt%/Al 30 wt%,QchemandQAlconsiderably increased owing to higher Al content,whereas the increment ofEWwas not significant.When the value of para increases from 10×10-4to 20×10-4for RDX70/Al30,the temperature of the CHNO products increased after 200 μs because the value of (Qchem-QAl) first decreased and then increased.
When the value of para increased from 0 to 15×10-4for RDX85/Al15, the values ofQAl/QchemandQAl/EWat 1 ms were1.93—0.528 and 0.353—0.342, respectively; the corresponding values were 2.54—0.438 and 1.0—0.84 for RDX 70 wt%/Al 30 wt%when the value of para increased from 0 to 20 ×10-4.At least approximately half of the Al reaction heat was utilized for heating only, thereby decreasing the internal energy of the aluminized products. This mechanism has rarely been discussed before to explain the weakened work ability of aluminized explosives.
Figs.10(e)and 10(f)indicated that Al primarily captured oxygen atoms from CO2and H2O, which is roughly consistent with the current studies. The overall reactions of 1 g RDX/Al within 1 ms were summarized as follows.
RDX 85 wt%/Al 15 wt% (para= 5× 10-4):
From the above equations,it can be concluded that Al reaction in RDX/Al was exothermic and gas-consumed (Al + H2O + NO +CO2→CO +H2+N2+Al2O3) and was accelerated by a higher Al content and a larger value of para.The molar consumption ratio of H2O to CO2(HCR) is an important indicator to study the reaction kinetics of Al because H2O and CO2are considered as the primary oxygen-suppliers for Al powders.Under atmospheric pressure,HCR is approximately 2 because the measured reaction rate between Al and H2O is approximately 2 times that between Al and CO2[52].In this paper, the calculated HCR of RDX 85 wt%/Al 15 wt% was approximately 2, and para had little effect on it. For RDX70/Al30,the calculated HCR increased from 2.9 to 4.2 when para increased from 10 ×10-4to 20× 10-4. Therefore, there should be a compli-cated dependence of the Al reaction rate on pressure and Al content.
A new reaction kinetics of Al under high pressure. The emergence of the new mechanism may involve NO,which is commonlyneglected in the analysis of Al reactions in the detonation flow.The gas-consumption process during the Al reaction is the general explanation for the reduced work ability of aluminizedexplosives.In this paper,however,we found that the gas depletion is negligible in RDX/Al.The reliability of this conclusion to the highcarbon aluminized explosives (such as TNT/Al) needs further research, as much condensed carbon may be produced during the Al reaction in TNT/Al, which is little in RDX/Al.
6. Conclusions
To research the EOS of aluminized detonation products from the perspective of composition,the thermochemical code DLCHEQ was applied in the present study to perform the rod-driven tests of RDX,RDX/LiF, and RDX/Al. The main conclusions of the study are summarized as follows:
(1) rod-driven test was designed to analyze the metal acceleration ability of the detonation products of RDX, RDX 85 wt%/LiF 15 wt%,RDX 70 wt%/LiF 30 wt%,RDX 85 wt%/Al 15 wt%,and RDX 70 wt%/Al 30 wt% within approximately 1 ms, and the free-surface velocity of the rod was recorded using PDV.The results indicated a notable reaction of Al powders in RDX 85 wt%/Al 15 wt% and RDX 70 wt%/Al 30 wt%, and the corresponding ignition delay was longer than 100 μs.
(2) We performed the rod-driven tests by coupling DLCHEQ with the hydrodynamic program SSS. The multi-phase EOS of Al and Al2O3, the Al temperature model and the Al reaction model were incorporated into DLCHEQ. We used the complete thermodynamic database generated using DLCHEQ instead of the phenomenological EOS to realize the engineering application of the thermochemical program, which has not been used extensively.DLCEHQ was validated by the small deviation between the experimental rod free-surface velocity of RDX and the calculated result (< 10%), which was considerably less than that calculated using the JWL EOS(< 66%) and ideal-gas EOS (< 94% and 96%).
(3) We analyzed the feasibility of using 3 μm LiF powders to investigate the endothermic process of 50 μm inert Al powders. We also calculated the rod free-surface velocity of RDX/LiF to calibrate the key parameterNuto 50 in the Al temperature model for the detonation products of RDX/Al.The results indicated that a largerNu, which corresponds to greater heat absorption by Al powders, causes a pressure reduction of the detonation products and a closer approach to the temperature equilibrium between the Al powders and the CHNO products.
(4) We calculated the rod free-surface velocity of RDX 85 wt%/Al 15 wt% and RDX 70 wt%/Al 30 wt% to calibrate the critical parameter para in the Al irreversible reaction model. The values of para were calibrated to be within the range from 10×10-4to 15 ×10-4for RDX/Al, and the corresponding reaction degree of Al was 25—38% at 1000 μs. Al reaction in RDX/Al was exothermic and gas-consumed (Al + H2O +NO +CO2→CO +H2+N2+Al2O3)and was accelerated by a higher Al content and a larger value of para.A larger para also caused a more pronounced temperature disequilibrium between the Al powders and CHNO products.
(5) The calculation results indicated that the thermodynamic state of the Al powders has notable influence on the EOS of aluminized detonation products, and the findings were compared with those of previous studies. First, the temperature equilibrium between the Al powders and CHNO products is not always feasible, and the disequilibrium is more obvious when the reaction of the Al powders is stronger.However,the temperature equilibrium assumption is commonly used to simplify calculations related to Al reactions.Second,there is a complicated dependence of the Al reaction rate on pressure and Al content, as the HCR of RDX 70 wt%/Al 30 wt% is significantly different from the HCR under low pressure. Finally, the endothermic process of Al powders has the highest contribution to the decrease in the work ability of RDX/Al because the heat absorbed by the Al powders is greater than half the reaction heat. The gasconsumption mechanism of Al is insignificant in the case of RDX/Al, but it is commonly used to explain the decrease in the work ability of aluminized products.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful for the financial support provided by the National Natural Science Foundation of China (Grant No.11902276), the Natural Science Foundation of Sichuan Province(Grant No.2022NSFSC1802),the National Key Laboratory for Shock Wave and Detonation Physics of China (Grant No.JCKYS2019212007),the Original Scientific Research Instrument and Equipment Development Project of Southwest Jiaotong University(Grant No. XJ2021KJZK055) and Sichuan Science and Technology Development Project (Grant No.2021ZYD0027).
Appendix
CJ parameters of some explosives calculated using DLCHEQ are shown in Table 6.
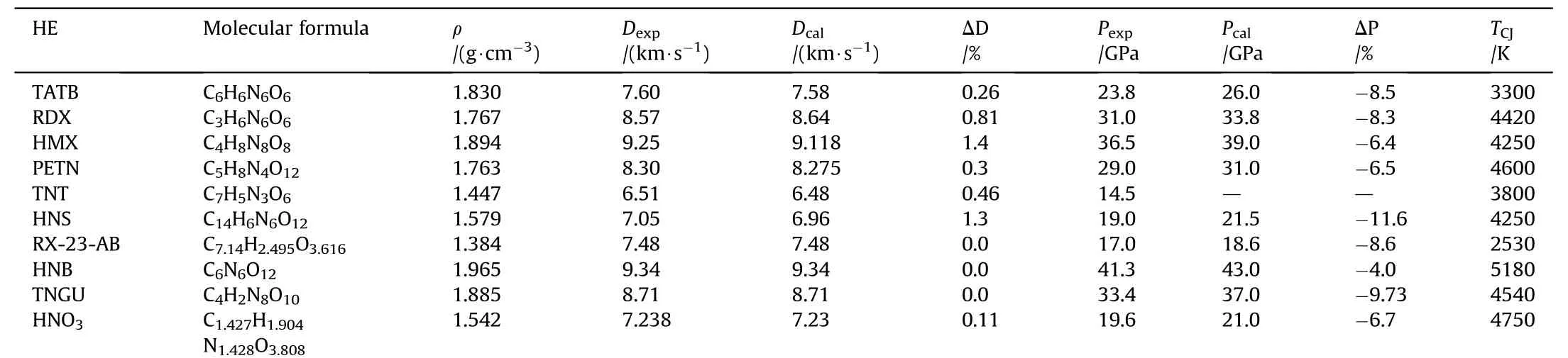
Table 6CJ parameters of some explosives calculated by DLCHEQ.
The calculated CJ states of RDX/Al and RDX/LiF are listed in Table 7.Both the contributions of bulk compression and convection are considered to compute the temperature of Al particles at CJ plane.
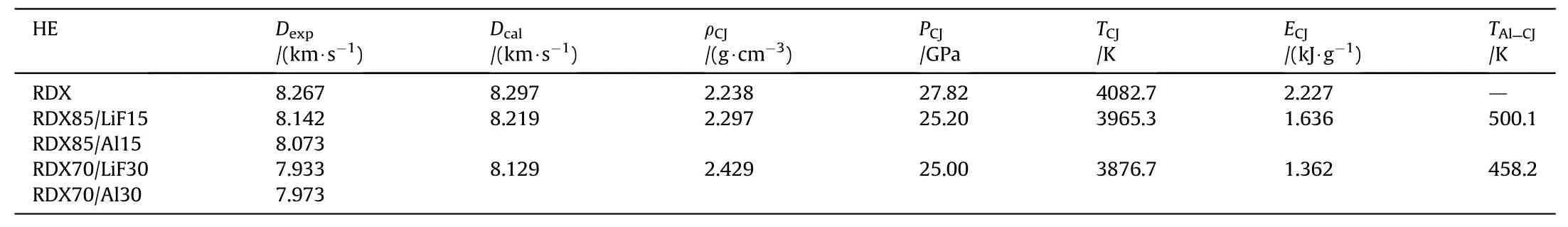
Table 7The calculated CJ states of RDX/Al and RDX/LiF.
where the subscript s denotes the state after shock, the subscript ZND denotes the mean value in the ZND zone.mandnare the parameters of Monaham EOS of Al,ΔtZNDis the time length,and ΔTZNDis the temperature difference between Al powders and the CHNO products. The value of parameters mentioned above can be referred in paper [37].
The calculated quasi-static pressures of TNT are listed in Table 8.As depicted in Table 8, DLCHEQ can accurately predict the quasistatic pressure with an average error of 6.9%, which is far less than that using B—W, L-C and LH method.
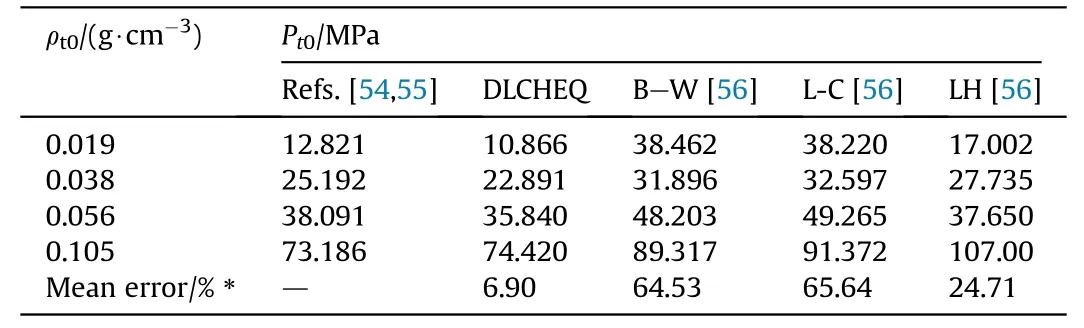
Table 8The quasi-static pressure of TNT(ρ0 =1.59).
The isentropic line of RDX and TATB products calculated using DLCHEQ are shown in Fig.A1.As displayed in Fig.A1,the calculated isentropic lines are in good agreement with the experimental results [53].

Fig. A1. The isentropic line of (a) RDX (ρ0 = 1˙709) and (b) TATB (ρ0 = 1.895) products.
杂志排行
Defence Technology的其它文章
- Defence Technology
- Joint target assignment and power allocation in the netted C-MIMO radar when tracking multi-targets in the presence of self-defense blanket jamming
- Design and dynamic analysis of a scissors hoop-rib truss deployable antenna mechanism
- Structural design and modal behaviors analysis of a new swept baffled inflatable wing
- Cooperative trajectory optimization of UAVs in approaching stage using feedback guidance methods
- An improved four-dimensional variation source term inversion model with observation error regularization
