Synthesis, crystallographic characterization, and potential application of fullerene anisole derivatives as nitrocellulose stabilizer
2023-07-04JieXiongBoJinXuemeiYuLingLiaoRufangPeng
Jie Xiong, Bo Jin, Xue-mei Yu, Ling Liao, Ru-fang Peng
State Key Laboratory of Environment-friendly Energy Materials, School of Materials Science and Engineering, Southwest University of Science and
Technology, Sichuan, Mianyang, 621010, China
Keywords:Fullerene Nitrocellulose Stability Propellant Mechanism
ABSTRACT
1. Introduction
Nitrocellulose (NC), also called cellulose nitrate, is a sort of energetic material extensively devoted to the military and civil fields[1—5]. NC with low nitration degree is used in inks, varnishes,lacquers, and paints, whereas NC with high nitration degree(>12.5%) can serve as the basic power source of single- or doublebase propellants [6—9]. Nevertheless, NC, which is inherently unstable and easily undergoes autocatalytic decomposition due to the energy-poor bond of nitrate groups,has potential thermal hazards during actual storage. The autocatalytic decomposition of NC is attributed to its decomposition products, which contain highly active nitrogen oxide radicals that promote the cleavage of nitrate ester groups in NC and accelerate its degradation [10—13]. What's more,external substances,such as oxygen,water,acids,alkalis,and chemicals containing transition elements, can also speed up the degradation process[14—17].Therefore,to improve the stability of NC-base propellants, stabilizer that can absorb nitrogen oxide radicals was usually added to inhibit the autocatalytic decomposition of NC,thereby improving its thermal stability [18—20].
The most commonly used NC-based propellant stabilizers are arylamine derivatives, such asp-nitro-N-ethylaniline, 2-nitrodiphenylamine, and diphenylamine (DPA), which have a strong absorption capacity for acidic nitrogen oxides.However,the secondary amine group in the structure of this type of stabilizers has strong alkalinity, which has a strong saponification effect,promotes the decomposition of nitrate esters and is not conducive to the stabilization of NC.Researchers introduced a strong electronwithdrawing carbonyl group on the secondary amine group of these stabilizers to reduce their alkalinity and their saponification effect on NC. Such as aromatic urea derivative stabilizers,N,N′-dimethyl-N,N′-diphenylurea (C2) andN,N′-diethyl-N,N′-diphenylurea [21—24], have been reported to reduce the saponification of aromatic amine stabilizers. Usually, phenylurea stabilizer is employed in higher fractions than diphenylamine to take advantage of its plasticizing characteristics.Moreover,the formation ofNnitrous, a known carcinogen, is a great disadvantage of stabilizers containing amine and amide groups, and hence the degradation products will have CMR properties[25].Dejeaifve et al.reveal that nitrate esters can be stabilized using stabilizers such α-ionone and α-tocopherol [26,27]. The heat flow of double base powders containing α-ionone is demonstrated to be stable over time, with autocatalysis occurring 1.5—4 times slower than powders with DPA as a stabilizer [28]. It is an environmental-friendly stabilizer that produces no mutagenic or carcinogenic daughter products when used to stabilize a nitrate ester-based propellant. Unfortunately,none of them can effectively eliminate nitroxide radicals.Therefore,developing a new type of stabilizer is an urgent and meaningful work to improve the stabilization of NC.
Since the first discovery and macroscopic production of fullerenes, fullerenes represented by C60have played an irreplaceable role in materials science, medicinal chemistry, and biotechnology due to their special structure and their chemical and physical properties. Fullerene has attracted wide attention because of its excellent ability to clear free radicals. It is called a free radicalabsorbing sponge and has been widely used in medical aesthetic products[29—37].The instability of NC is attributed to the catalytic decomposition of NC by a large number of nitrogen oxide radicals,and fullerene has an excellent ability to scavenge free radicals;therefore, fullerene can eliminate the nitrogen oxide free radicals produced by NC degradation and inhibit the autocatalytic decomposition of NC [38—42]. Recently, we synthesized a series of fullerene derivatives and studied their stabilizing effects on NC.The results showed that fullerene derivatives with different structures have remarkably different stabilizing effect on NC.In this research,we designed and synthesized a series of novel fullerene anisole derivative stabilizers using C60Cl6and benzyl alcohol as raw materials to continue our research on high-performance fullerenebased stabilizers.The law of their influence on the thermal stability of NC was studied in detail. We found a considerable difference in the thermal stability of NC with and without fullerene-based stabilizers. In addition, the stabilizing mechanism of this kind of fullerene-based stabilizer on NC was also studied in detail.
2. Experimental
2.1. Synthesis of fullerene anisole derivatives
C60Cl6(93 mg, 0.1 mmol) and dry toluene (100 mL) were sequentially introduced into a 250 mL round-bottom single-necked flask under constant stirring in a nitrogen environment.The system was degassed and filled with nitrogen, and then the mixture was agitated at room temperature for 1.5 h until completely soluble of the hexachlorofullerene. Then 84 mg of 3,5-dimethoxybenzyl alcohol (1b, 2 mmol) was added to the reaction system, followed by 24 mg NaH as the inorganic base. The reaction mixtures were agitated at 25◦C for 1.5 h under continuous stirring in a nitrogen atmosphere up to thin layer chromatography (TLC) exhibited the complete disappearance of pristine C60Cl6.The raw products eluted by toluene-hexane(3:1 vol/vol)produced a bright orange solution,which was concentrated using a rotary evaporator to obtain a pure monoadduct benzopyran (2b)and 1,4-adducts (3b)via the large steric effect. The reaction activity decreased when the reaction temperature decreased to 15◦C.A slow temperature rises increased the activity of the reaction.The product yield was the highest when the synthetic temperature reached 25◦C; the yields of2band3bwere 21% and 26%, respectively (Table S1, Entry 2). The further temperature rise in synthesis temperature did not remarkably increase the product yield but produced a larger amount of unidentified by-products. The 3-methoxy group was used in place of the 3,5-dimethoxy group to research the universal applicability of this reaction to other substrates. The reaction of the 3-methoxy group with C60Cl6can also give a fullerene derivative (2a)with a similar structure. The electron-supplying ability of the 3-methoxy group is weaker than that of the 3,5-dimethoxy group; thus, the chemical reaction activity of 3-methoxybenzyl alcohol (1a)is weaker than that of1b. When C60Cl6withp-alkoxybenzyl alcohol(4-methoxybenzyl alcohol1cor 4-ethoxybenzyl alcohol1d) and NaH was introduced to toluene and then heated to 25◦C for 1.5 h,the corresponding fullerene anisole derivatives,2cand2dwere acquired in 17% and 25% yield, respectively. The reaction route is shown in Fig.1.
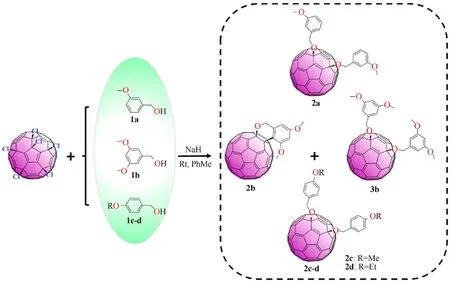
Fig.1. Synthesis of fullerene anisole derivatives.
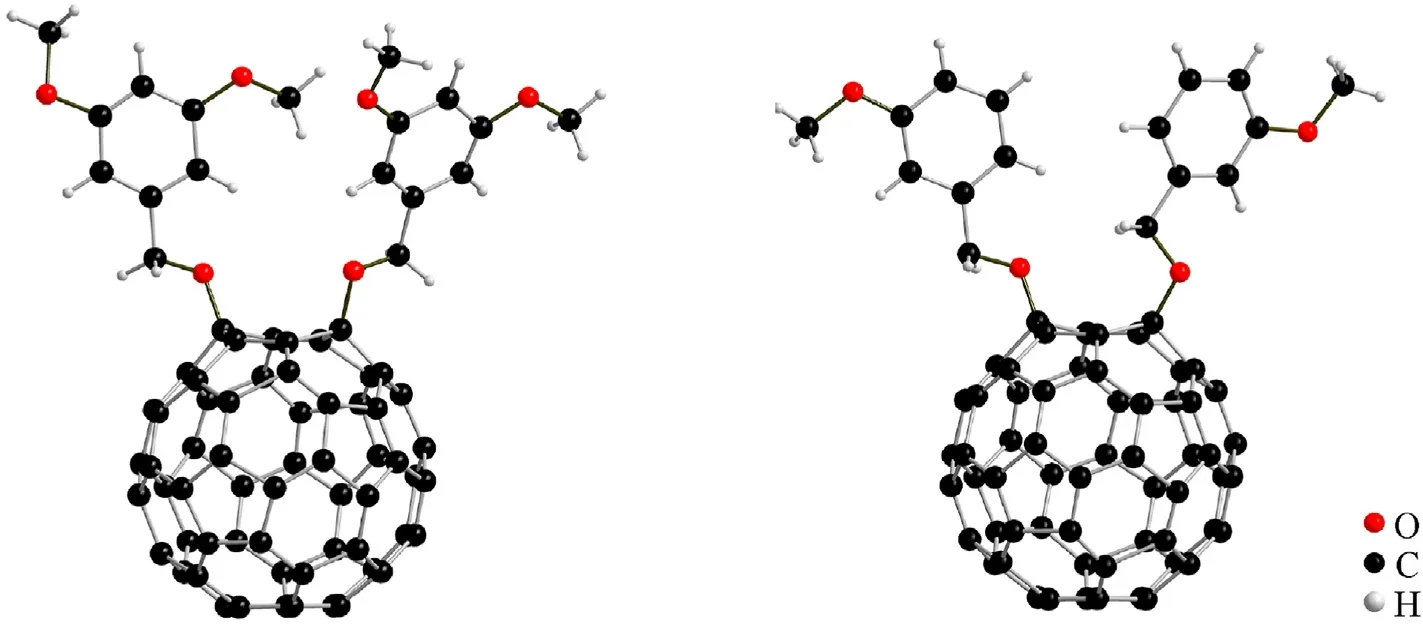
Fig. 2. Single-crystal structures of 2a (right) and 3b (left).
2.2. Methods of performance evaluation
Differential scanning calorimetry (DSC) test, methyl-violet test,vacuum stability test (VST), thermogravimetric analysis (TG),adiabatic accelerated calorimetry (ARC) test, and electron spin resonance (ESR) test were used to compare and evaluate the stabilizing effects of the novel fullerene anisole derivatives,2a,2b,2c,2d(2a-2d)and3b,on NC.The formula of the NC/stabilizers samples is shown in Table 1 and the preparation method of mixed samples is depicted in ESI.
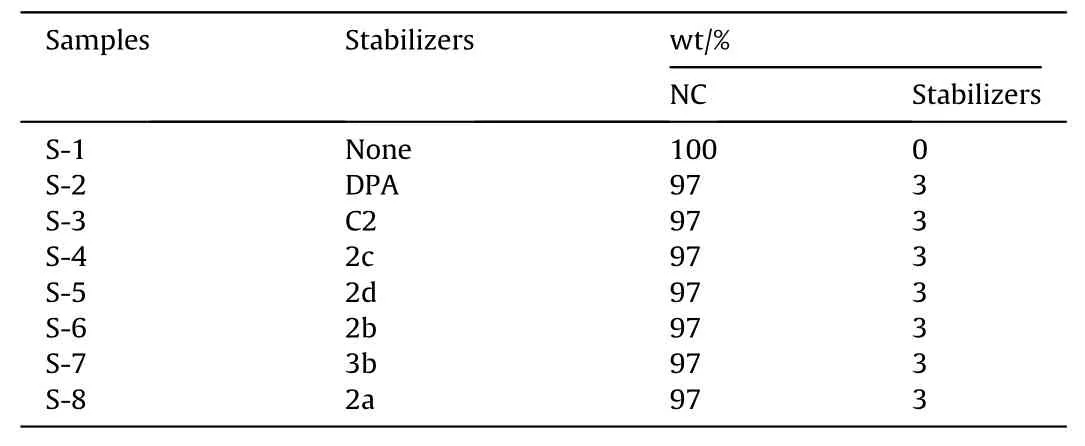
Table 1The formula of the NC/stabilizers samples.
2.2.1. Differential scanning calorimetry test
Compatibility test were performed according to STANAG 4147 using differential scanning calorimetry(DSC).NC(0.75 mg)and NC/stabilizers (1.5 mg) were placed in an aluminum crucible and heated from room temperature to 300◦C at a heating rate of 10◦C/min in a nitrogen flow atmosphere (50 mL/min), and their DSC curves were obtained respectively.
2.2.2. Methyl violet test
Methyl violet tests subjected to GJB 770B-2005 were performed to estimate the stabilizing effect. In the presence of methyl violet paper, the sample was heated at 134.5◦C in a test tube with a sample mass of 2.5 g,the test procedure was described inESI.The nitrogen oxides from NC degradation will transform the color of the methyl violet paper from purple to orange, time was recorded in minutes. The longer the time demanded for discoloration, the better the stability of the stabilizer.
2.2.3. Vacuum stability test
Vacuum stability test was conducted by the national military standard method GJB-772A-97. A certain quality sample was put into a specific container with a consistent capacity,vacuumed,and then keep it at 100◦C for 48 h, and the pressure change in the container was recorded.
2.2.4. Thermogravimetric test
The GJB 772A-97 method was used for the constant temperature thermogravimetric experiment to test the sample's thermal stability. The sample was held at 134.5◦C for 360 min in a nitrogen flow atmosphere(40 mL/min),and the curve of the sample weight loss rate versus time was recorded.
2.2.5. Adiabatic accelerated calorimetry test
Hastelloy bomb with a thermocouple clip at the bottom of the bomb was used. The test mode was heating-waiting-search, and the heating parameters were as follow.The initial temperature was 140◦C (the sample does not undergo self-accelerating decomposition at temperatures below 140◦C),the steps of temperature rise were set to 2◦C,the time of waiting was set to 20 min,the time of search detection was set to 10 min, the end temperature was 300◦C,the rate of temperature rise was set to 0.2◦C/min,and the threshold of heat release was set to 0.02◦C/min. The related parameters were recorded simultaneously.
2.2.6. Electron spin resonance test
The test parameters included: sample g factor, 2.0400; central magnetic field, 3373.05 G; and scanning width, 150.0 G. Diethyldithiocarbamate (40 mM/L), FeSO4∙7H2O (20 mM/L), sodium nitroprusside (2 mM/L), and fullerene derivatives with different concentrations (3.2 mM/L, 1.6 mM/L, 0.8 mM/L, 0.4 mM/L, and 0.2 mM/L) were fully mixed and oscillated. Then, an appropriate amount of the mixture was placed into a glass capillary.The signal intensity of free radical was recorded.
3. Results and discussion
3.1. Characterization
The structures of products2a-2dand3bwere preliminarily determined by structural analysis methods, namely, highresolution mass spectrometry (HRMS), nuclear magnetic resonance (NMR), Fourier transform infrared (FT-IR) spectroscopy, and UV—Vis spectroscopy. The structures of2aand3bwere further identified by single-crystal X-ray diffractometry(XRD).Supporting information provides the spectra of (Fig. S1- Fig. S25) and crystallographic data (Table S2 and Table S3) of all the fullerene derivatives. The crystal stacking diagram of2aand3bis shown in Fig.S26.The HRMS of2a-2dand3bproduced corresponding[M]+ion peaks,which were consistent with their molecular masses.The benzyl hydrogen atom of2ccorresponds to a set of double-double peaks at δ 5.58 ppm(dd,J1=10.50 Hz,J2=22.14 Hz,4H)in the1H NMR spectra,two sets of double peaks on the benzene ring at δ 7.54(d,J= 8.52 Hz, 4H) and 6.94 ppm (d,J= 8.52 Hz, 4H), and parasubstituted alkyl hydrogen at δ 3.85 ppm (s, 6H). In addition, the following five sets of peak signals are exhibited in the1H NMR spectrum of2b: hydrogen protons on hexahydropyran ring in δ 5.69 ppm (s, 2H), two sets of non-equivalent methyl protons at δ 3.98 (s, 3H) and 3.75 ppm (s, 3H), and two protons on benzene ring long-coupled to each other at δ 6.74 (d,J= 2.58 Hz,1H) and 6.56 ppm(d,J=2.52 Hz,1H).Similarly,the characteristic peaks also appeared in the1H NMR spectra of2aand3b; benzyl hydrogen atoms are present at δ 5.65(dd,J1=11.04 Hz,J2=22.50 Hz,4H)and 5.59 ppm (dd,J1=11.16 Hz,J2= 22.32 Hz, 4H), and benzene ring hydrogen and meta-substituted methyl hydrogen signals are exhibited at the expected positions. In the13C NMR spectra of all compounds,alkyl carbons appeared in the high-field area,and sp3-carbons were observed at 60—80 ppm.Notably,25—28 signals were revealed in the range of 140—156 ppm, which proves that the compounds have theCs-symmetry mode.
The molecular structures of2aand3bwere further verified by single-crystal XRD.As shown in Fig.2,the single crystal XRD results show that we obtained 1,4-addition fullerene derivatives that2apossess a monoclinic system with a P21/c space group and3bpossess an orthorhombic system with a Pca21space group. The above evidence is consistent with the HRMS and NMR characterization results, which indicate that fullerene anisole derivatives were synthesized successfully.
3.2. Application of potential NC-based propellants stabilizers
3.2.1. Compatibility assessment
Compatibility between energetic materials and stabilizers is an important index used to evaluate the safety of explosives. A majority of the criteria devoted to assessing the compatibility are subject to the North Atlantic Treaty Organization standardization agreement, STANAG 4147, which indicates that NC and mixed samples have excellent compatibility if the difference value between the exothermic peak temperatures of the mixed samples and pure NC is less than 4◦C in differential scanning calorimetry(DSC)test[43,44].Therefore,the DSC test was applied to assess the compatibility of fullerene anisole stabilizers and NC.As exhibited in Fig. 3, the decomposition peak temperature of NC is 195.8◦C,whereas the exothermic peak temperatures of the mixture of NC with2a,2b,2c,2d,and3bare 197.6◦C,196.9◦C,197.3◦C,197.8◦C,and 198.1◦C, respectively. Compared with the exothermic peak temperature of pure NC, the peak temperature difference of the mixture is between 0.9◦C and 2.3◦C, which is less than 4◦C. This value indicates that the synthesized fullerene anisole stabilizers have good compatibility with NC.
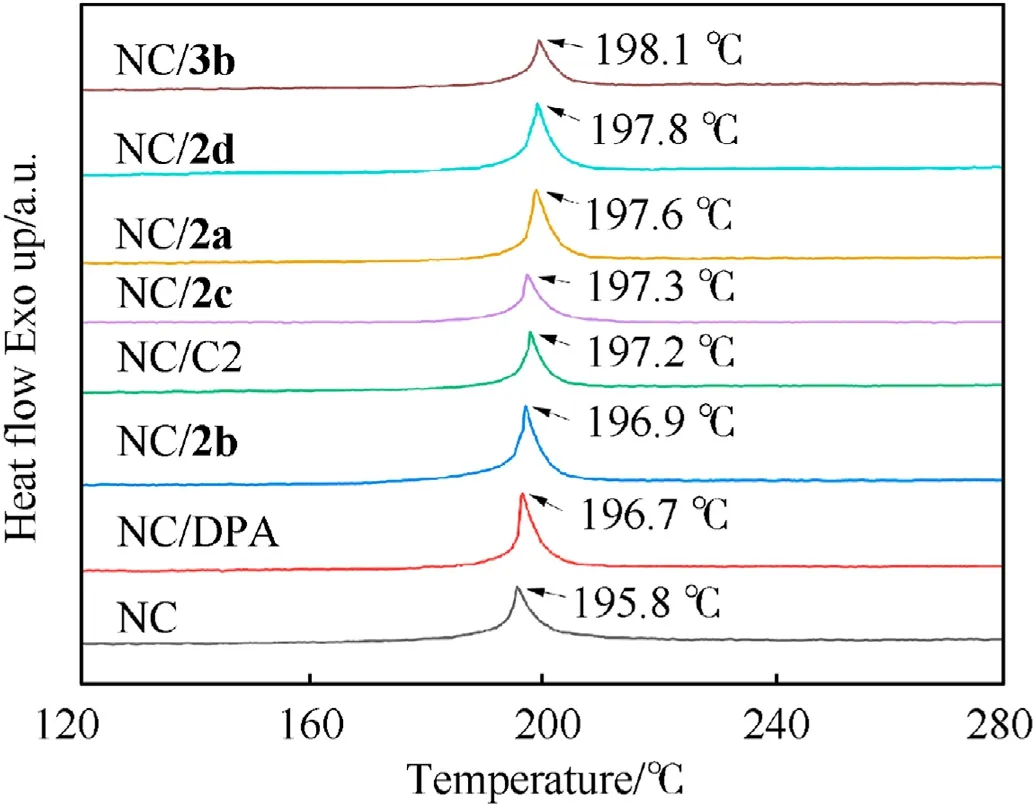
Fig. 3. DSC curves of mixtures and pure NC.
3.2.2. Methyl violet test
The methyl violet test show that the fullerene derivatives can clear away nitrogen oxides by chemical reaction, which restrains the accelerated autocatalytic decomposition of NC to lengthen the discoloration period. As depicted in Fig. 4 and Fig. S29, the discoloration time of S-1 to S-8 were 56 min, 67 min, 73 min, 78 min,96 min,71 min,98 min,and 85 min,respectively.The color change time were in the sequence:S-7>S-5>S-8>S-4>S-3>S-6>S-2>S-1.Consequently,the stability was ranked as:3b>2d>2a>2c>C2>2b> DPA > NC. The results uncovered that the new anisole derivatives had better performance than DPA and C2.

Fig. 4. Discoloration time of methyl violet paper.
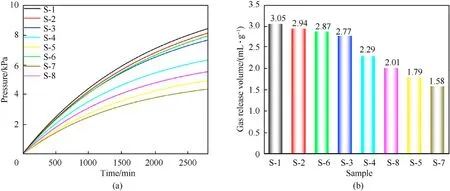
Fig. 5. (a) Pressure curves and (b) gas volume under standard conditions of S-1 to S-8.
3.2.3. Stability test under vacuum conditions
The samples were thermally decomposed under vacuum conditions, and gas was released to increase the pressure in the volume,which was converted to the gas volume in the standard state.The stability of the mixed sample was indirectly determined by the released amount of gas.The greater the amount of gas released,the worse the stability of the stabilizer, and vice versa.
The pressure curves of S-1 to S-8 are presented in Fig.5(a).Gas volume under standard conditions was calculated by the transformation equation (seeESI). The gas volumes released per unit mass of samples S-1 to S-8 heated at 100◦C for 48 h were 3.05 mL/g,2.94 mL/g, 2.87, mL/g 2.77 mL/g, 2.29 mL/g, 2.01 mL/g, 1.79 and 1.58 mL/g, respectively. The thermal stability order of the samples was in the order: S-1> S-2> S-6> S-3> S-4> S-8> S-5> S-7.Therefore,the stabilizing effects of the fullerene anisole stabilizers on NC was in the order:3b>2d>2a>2c>C2>2b>DPA>NC.The experimental data show that the fullerene anisole derivatives have a remarkable stabilizing performance on NC, and their stabilizing effect is better than those of traditional stabilizers (C2 and DPA).
3.2.4. Stability test under isothermal conditions
The samples were decomposed by heat and gas was released under isothermal conditions, which resulted in a decrease in sample mass. A higher weight loss indicates that more gas was released during the decomposition, which further indicates that the sample has poorer stability. The thermal weight loss analysis method was used.
As shown in Fig. 6, the weight-loss rates of samples S-1 to S-8 were 17.14%,12.15%,11.63%,10.16%, 7.17%,11.91%, 6.05%, and 8.31%,respectively.Therefore,the thermal stability of samples was in the order: S-7> S-5> S-8> S-4> S-3> S-6> S-2> S-1. Moreover, the stabilizing performance of the fullerene anisole derivatives on NC was in the order:3b>2d>2a>2c> C2>2b> DPA > NC. The stabilizing effect of fullerene anisole derivatives on NC is be superior to those of C2 and DPA.
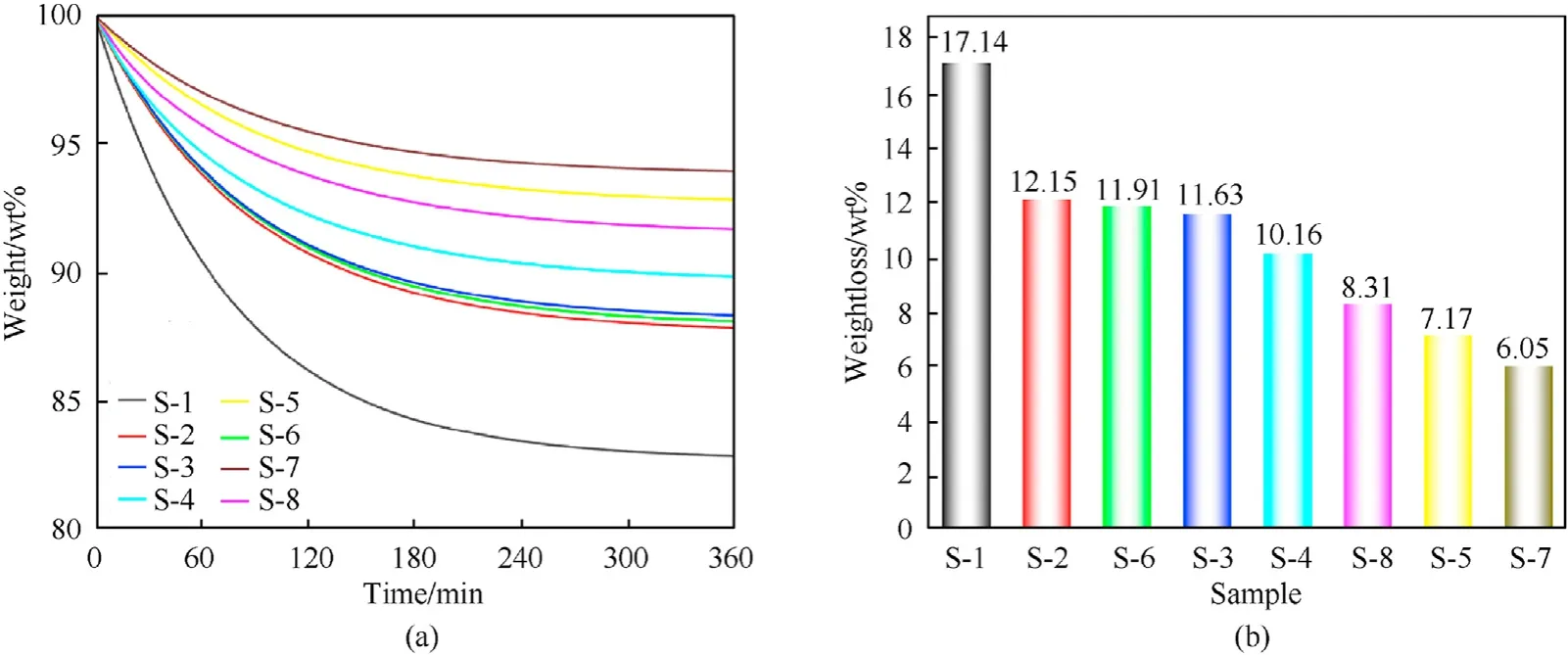
Fig. 6. (a) TG curves and (b) weight-loss rates of S-1 to S-8.
3.2.5. Stability test under adiabatic conditions
ARC test was applied to assess the thermal safety of NC and NC/fullerene anisole derivative mixtures. The temperatures and pressure changes of samples S-1 to S-8 in the self-accelerating decomposition stage under adiabatic conditions are shown in Fig.S27.The temperature data collected by ARC are not the practical temperature of the sample but that of the closed bomb system and the sample.The sample was heated by taking full advantage of the heat released from the reaction under ideal adiabatic conditions;therefore,thermal inertia coefficient φ(seeESI)was introduced to correct the experimental data and make the measurement data closer to reality[45].
The ARC test results present us with a variety of crucial parameters, namely, self-heating temperature(T0), temperature at maximum heating rate (Tm), and the time required to reach the maximum heating rate(Qm),which can reflect the thermal stability of the sample[46](Fig.7(a)).The sample with higherT0,Tm,andQmvalues possesses more superior stability.S-1 to S-8 hadT0value of 152.46◦C, 153.86◦C, 156.52◦C, 157.34◦C, 162.19◦C, 154.45◦C,166.09◦C, and 159.12◦C, respectively;Tmvalue of 161.14◦C,161.16◦C,163.62◦C,166.29◦C,170.19◦C,162.09◦C,180.25◦C, and 168.20◦C, respectively; andQmvalues of 33.12 min, 42.31 min,50.86 min, 60.07 min, 72.38 min, 43.25 min, 111.37 min, and 64.29 min,respectively.Therefore,the thermal stability of S-1 to S-8 were in the order:S-7>S-5>S-8>S-4>S-3>S-6>S-2>S-1.Moreover, the stability capacity of the stabilizers was ranked as:3b>2d>2a>2c> C2 >2b> DPA > NC. Time to maximum rate(TMR) [47] is important to predict the time for the fastest selfaccelerating decomposition of propellant and the safe storage period under different temperatures in the actual use of propellant and explosives. The fitting curves of the TMR shown in Fig. 7(b)appear to provide further evidence of the order of thermal stability.
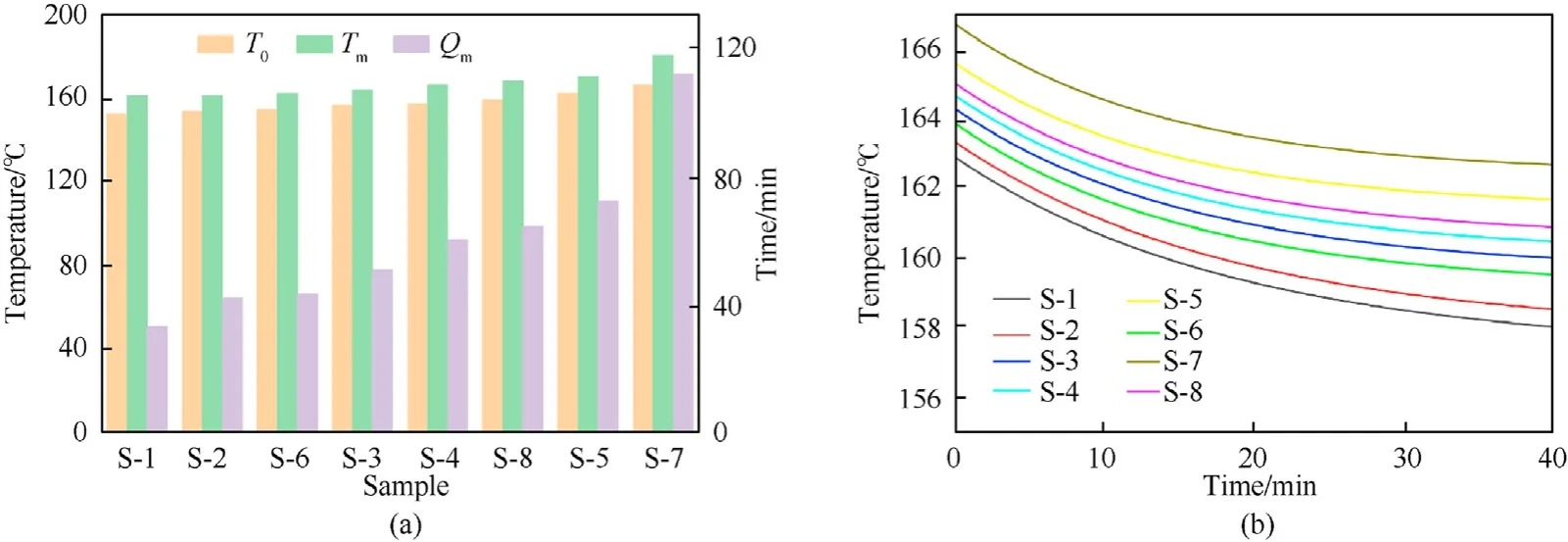
Fig. 7. (a) Detailed values of T0, Tm, and Qm; (b) TMR fitting curves of samples.
3.3. Stability mechanism of the novel fullerene anisole derivatives
The ability of fullerene anisole derivatives to clear nitro radicals was studied by electron spin resonance (ESR) spectroscopy.
The absorption peak intensities of different samples at the same concentration are an important standard to evaluate the ability of the samples to remove free radicals. A lower absorption intensity demonstrates a better ability to remove free radicals.As depicted in Fig. 8, the ESR signal intensity of the NO∙was weaker after the fullerene anisole derivatives(2a-2d/3b)was loaded.Moreover,the higher the sample concentration,the weaker the ESR signal.Hence,these novel fullerene anisole derivatives held remarkable free radical scavenging ability. The NO∙scavenging rates calculated by the formula(seeESI)of the fullerene anisole derivatives are shown in Fig. 9(b). The results showed that the fullerene anisole derivatives had extraordinaire adsorption capacity for NO∙. The NO∙scavenging rates of3bat 3.2 mM/L could come up to 92%. The fitting curves of the NO∙scavenging rates under the concentration gradient of fullerene anisole derivatives are depicted in Fig.9(c)and were calculated via the transformation formula (seeESI). The fitting parameters are shown in Table S4. The half-maximal inhibitive concentrations (IC50) of the fullerene derivatives were calculated to compare their free radical scavenging activities. The IC50values were acquired by the fitting equation as depicted in Fig.9(d).A smaller IC50value represents a stronger ability to absorb nitro radicals. The IC50values of2a,2b,2c,2d, and3bwere 0.83 mM/L,0.94 mM/L, 0.86 mM/L, 0.75 mM/L and 0.61 mM/L, respectively.Hence,the NO∙scavenging capacity were ranked as:3b>2d>2a>2c>2b> C60, which was consistent with the results above.
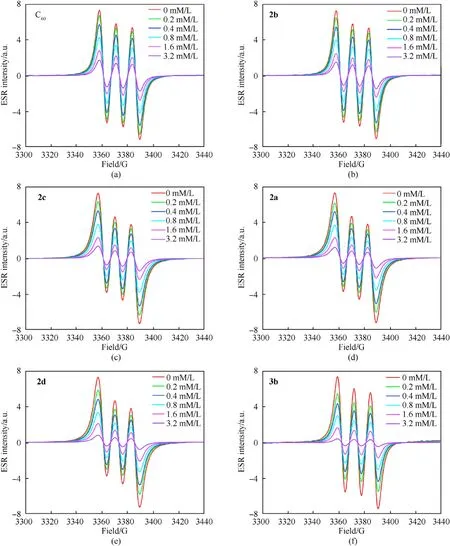
Fig. 8. ESR signals of the NO∙scavenging activities of fullerene derivatives.
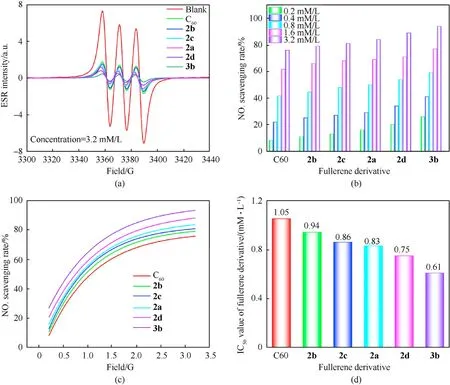
Fig.9. (a)ESR signals of fullerene derivatives at the 3.2 mM/L;(b)NO∙scavenging rates of fullerene derivatives;(c)Fitting curves of the NO∙scavenging rates;(d)IC50 values of the fullerene derivatives.
After2dabsorbed the nitrogen oxide generated by NC thermolysis, the intermediate products were analyzed for the functional groups.The results are shown in Fig.10.The NO2symmetric and asymmetric vibrational peaks are located near 1276 cm-1and 1664 cm-1.Moreover,the peak of the C—N extensional vibration of aromatic nitro compound appeared at 825 cm-1, and the C—N—O bending vibration emerged near 744 cm-1. These phenomena proved that the nitro-substituted fullerene derivatives were produced by the chemical reaction between2dand the nitrogen oxides. Based on the above experimental results, the process of the stability effect of fullerene anisole stabilizer is shown in Fig. 11.Under extreme conditions, NC decomposes to produce nitrous oxide free radicals and nitrous oxide acid gases.Fullerene derivatives can remove these nitrogen oxides in time to inhibit the selfcatalyzed reaction of NC and prolong the service cycle of the propellant.
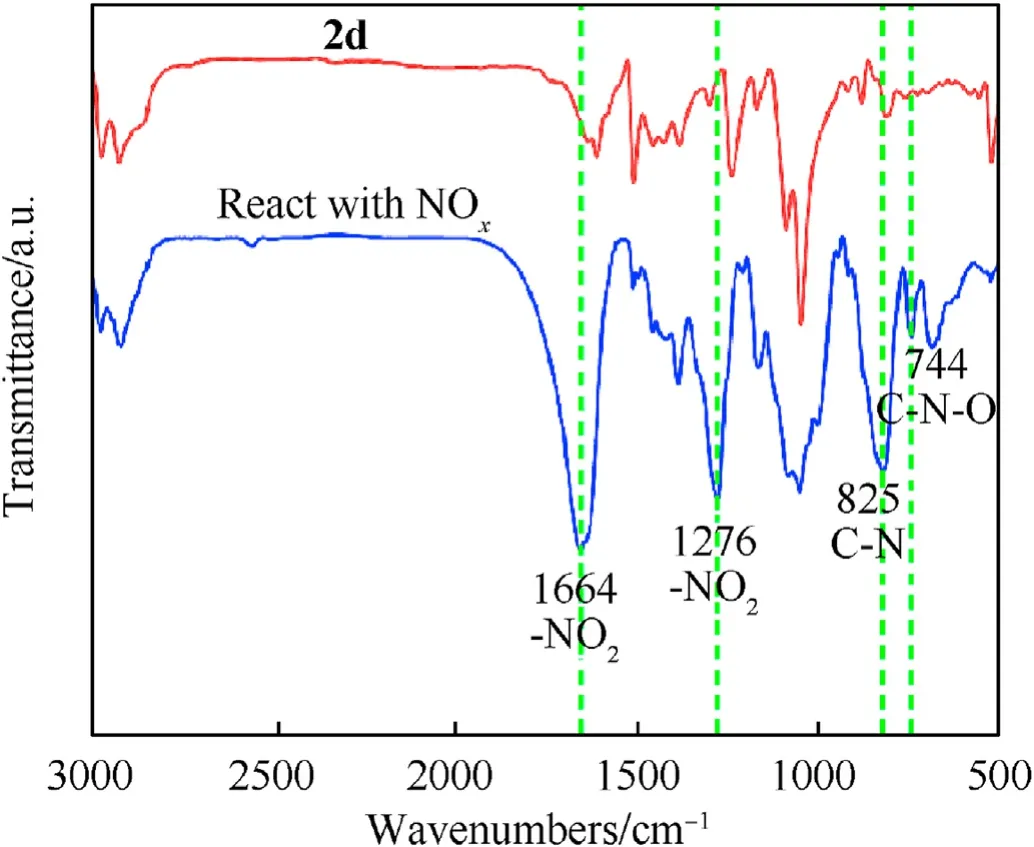
Fig.10. FT-IR spectra of 2d after and before interaction with NC.
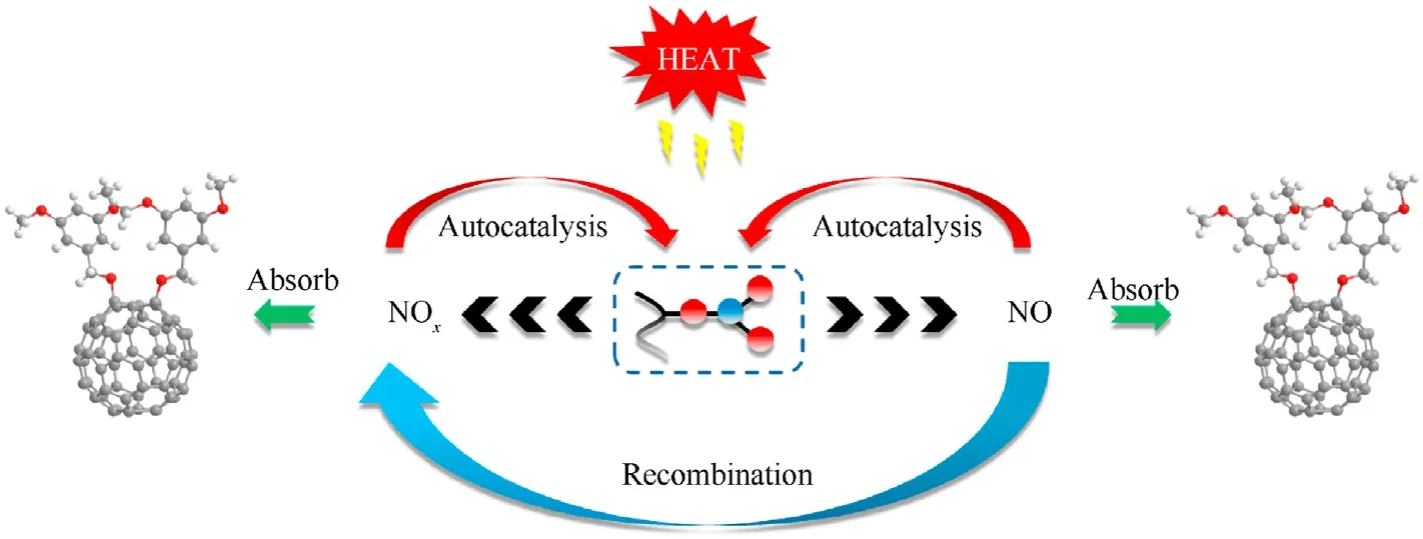
Fig.11. Stability mechanism of fullerene derivatives.
4. Conclusions
In summary,five fullerene anisole derivatives were synthesized by a simple method,and their thermal stabilizing effect on NC was studied by methyl violet test, DTA, TG, ARC test, and VST. The experimental phenomena reveal that the fullerene derivatives have good compatibility with NC,can effectively prolong the storage life of the propellant, improve the safety level during production and use, and have better performance than the traditional stabilizers(C2 and DPA). The stabilization ability of fullerene anisole derivatives is affected by the substituents on the benzene ring, in which extending the length of the carbon chain of theparasubstituted group of the benzene ring and increasing the number of substituents on the benzene ring can improve the stability performance.Considering the potential of fullerene anisole derivatives as alternative stabilizers for NC-based propellants, we will focus on study the possibility of practical application of these fullerenebased stabilizers in propellants in the future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was carried out with the financial support received from the Natural Science Foundation of China (Grant No.51972278), Outstanding Youth Science and Technology Talents Program of Sichuan (Grant No.19JCQN0085), and Open Project of State Key Laboratory of Environment-friendly Energy Materials(Southwest University of Science and Technology, Grant No.20fksy16).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2022.02.006.
杂志排行
Defence Technology的其它文章
- A review on lightweight materials for defence applications: Present and future developments
- Study on the prediction and inverse prediction of detonation properties based on deep learning
- Research of detonation products of RDX/Al from the perspective of composition
- Anti-sintering behavior and combustion process of aluminum nano particles coated with PTFE: A molecular dynamics study
- Microstructural image based convolutional neural networks for efficient prediction of full-field stress maps in short fiber polymer composites
- Modeling the blast load induced by a close-in explosion considering cylindrical charge parameters
