Oxidative Stress and Alleviating Effect of Natural Antioxidants on Alzheimer's Disease*
2023-05-16ZHAOBaoLu
ZHAO Bao-Lu
(Institute of Biophysics, Chinese Academy of Sciences, Beijing 100101, China)
Abstract Aging is a major risk factor for neurodegenerative diseases such as Alzheimer's disease (AD). Oxidative stress and free radicals have important biological functions. However, redox imbalance results in oxidative stress that has been implicated in the pathology of many human diseases including AD. The author reviews the involvement of reactive oxygen species (ROS) in the pathogenesis of neurodegenerative diseases, particularly, the interaction of oxidative stress with other critical mechanisms of AD,especially summarized the results about protect effects of tea polyphenols, L-theanine, astaxanthin, EGb761,soy isoflavones and nicotine on AD in cell, animal models and clinical treatments. Hopefully, this review can provide insights into novel preventive and therapeutic strategies for AD.
Key words oxidative stress, free radicals, natural antioxidants, tea polyphenols, L-theanine, astaxanthin, EGb761, soy isoflavones, nicotine
The latest census shows that there are 130 million elderly people over the age of 60 in China,which will reach 439 million by 2050. At present,there are about 16 million Alzheimer's disease (AD)patients in China. China has entered an aging society ahead of time. The incidence rate of AD among people over 65 years old is 4.8%, 11.5% over 75 years old, and 30% above 85 years old. According to this speed of development, AD will become the biggest health risk for the elderly in China and even the world. However, the pathogenesis of AD is not completely clear, and there is no effective drug.Neurofilament winding, senile plaques and β amyloid(Aβ) precipitation generally appear in the brain of patients with AD[1]. At present, neurotoxicity related to Aβ and tau protein, changes in cholinergic neurotransmission, oxidative stress and changes in calcium homeostasis are considered to be the key factors of AD. Studying the pathogenesis of AD and finding effective methods to treat AD have attracted extensive attention, and it is also the bounden task of scientific researchers and medical workers. There is evidence that oxidative stress is closely related to the pathogenesis of AD. Research shows that natural antioxidants have great benefits for AD. The prevention and treatment of AD by natural antioxidants have a certain role but only play a regulatory role in this process. This paper discusses the research progress and problems about oxidative stress and therapeutic effect of natural antioxidants on AD.
1 Oxidative stress and AD
The brain is more susceptible to oxidative stress than other organs, and most of the components of neurons can be oxidized in AD due to mitochondrial dysfunction, elevated metal levels, inflammation, and Aβ peptides. Oxidative stress is involved in the development of AD by promoting Aβ deposition, tau hyper-phosphorylation, and subsequent loss of synapses and neurons. The relationship between oxidative stress and AD suggests that oxidative stress is an important part of the pathological process. Many studies have shown that oxidative stress plays an important role in Aβ-induced cytotoxicity. Aβ induces neuronal cell degradation of mitochondrial function and leads to apoptosis of nerve cells[2]. Oxidative stress promotes the production of amyloid precursor protein (APP). The expression of the β-site APPcleaving enzyme 1 (BACE1) and gama secretase accelerates the generation of Aβ from its precursor APP and forms a vicious circle[3].
Undoubtedly, extra free radical results injury in biological body. Free radicals peroxidize membrane lipids and oxidize proteins, resulting in damage of the plasma membrane and crosslinking of cytoeskeletal proteins. In the brain, the high metabolic rate, the low concentration of glutathione and antioxidant enzyme catalase, and the high proportion of polyunsaturated fatty acids make the brain tissue particularly susceptible to oxidative damage[2]. Oxidative stress,an imbalance toward the pro-oxidant side of the prooxidant/antioxidant homeostasis, protein aggregation occurs in several brain neurodegenerative disorders.
1.1 Reactive oxygen and reactive nitrogen free radicals caused oxidative stress in the brain of AD
Oxidative stress is a state caused by an imbalance between oxidant production and the endogenous antioxidant defense system when oxidant production exceeds the clearance capacity of the antioxidant defense system in the AD brain. During oxidative stress, reactive oxygen species (ROS) and reactive nitrogen species (RNS) react with proteins and lipids, disrupting their function, leading to progressive neuronal cell damage and ultimately brain cell death. The deletion of endothelial ROS and RNS and oxidative stress in the human cerebrovascular endothelium increases the expression of APP and enhances the production of Aβ peptide, indicating that the deletion of endothelial nitric oxide (NO)contributes to AD pathology.
The increase in oxidative stress and ROS and RNS production in AD is based on the fact that,protein oxidation, manifested by increased levels of protein carbonyl and 3-nitrotyrosine, as well as markers of oxidative damage to DNA and RNA, such as 8-OHdG and 8-hydroxyguanosine are prominent in AD brains[4]. The expression of superoxide dismutase(SOD) is abnormally increased in brain neuropathy in AD patients, which may be an adaptive response to increased oxidative damage in these regions. In contrast, most studies have shown reduced activity of antioxidant enzymes in the brain of AD patients[5].Differences in antioxidant enzyme expression and activity may reflect redistribution of antioxidant enzymes in neuropathy or enzyme inactivation due to oxidation.
Oxidative stress has become one of the important factors in the pathogenesis of AD, the mechanism of the redox balance change and the source of ROS and RNS may be related with mitochondria.Mitochondrial dysfunction Aβ-mediated processes and microglial activation are thought to play important roles in redox imbalance[6]. Data suggest that oxidative damage occurs in the AD brain. Aβ peptides have been shown to produce H2O2through metal ion reduction with the release of thiobarbituric acid reactant substrance (TBARS). The oxidation of peptide is mainly mediated by ·OH. This leads to the formation of alkoxy radicals and hydroxylation of the peptide backbone. The oxidation of amino acid depends largely on their structure. An important oxidation process with structural consequences involves irreversible nitration of tyrosine residues by ONOO-[7]. Understanding these mechanisms related with ROS and RNS free radicals may provide new therapeutic targets for the prevention and treatment of AD.
1.2 Metal ion metabolic homeostasis and oxidative stress in AD
Many studies have shown that there is a close relationship between the destruction of metal stasis and AD. One mechanism of Aβ accumulation may be due to the disorder of metal homeostasis in AD brain.Aβ contains a copper binding domain. We have studied the steady state failure of iron and copper,oxidative stress Aβ, APP, iron regulatory protein (IRP)and divalent metal transporter 1 (DMT1). It is found that iron and copper overload may be closely related to oxidative stress injury in the later stage of AD, and iron and copper deficiency may be closely related to the early onset of AD. Natural antioxidants can protect AD by regulating iron and copper homeostasis. The destruction of metal homeostasis direct cause oxidative stress[8].
In theC. elegansAβ-expressing strain CL2006 and SH-SY5Y cells, Aβ was observed to increase iron content and oxidative stress levels in the Swedish mutant form of human β amyloid precursor protein(APPsw). Intracellular iron and calcium levels and production of ROS and nitric oxide in APPsw cells were significantly increased compared to control cells. In addition, iron accumulation increased in AβexpressedC. elegans[9]. We have also found that the overexpression of wild-type human AβPP695 decreased the iron content and increased the oxidative stress in neuroblastoma SH-SY5Y cells. The mitochondrial membrane potential of AβPP695 cells was significantly lower than that of the control cells.Moreover, iron treatment decreased ROS and calcium levels and increased cell viability of AβPP695 cells.The iron deficiency in AβPP695 cells may contribute to the pathogenesis of AD. Cu2+acts on β peptides as a cofactor to promote the process of oxidative stress.Double electrons can be transferred to oxygen (O2),resulting in β-bound copper and reduction of Cu2+to Cu+. Then, Cu+reacts with H2O2to generate hydroxyl radicals[10]. These ROS directly induce oxidative damage in AD brain.
We studied the effects of nicotine on metal homeostasis in hippocampus and cortex of APPV717I transgenic mice. After nicotine treatment, the metal contents of copper in senile plaques and nerve fibers decreased significantly. After nicotine treatment, the distribution density of copper in hippocampal CA1 region was also reduced[11]. Nicotine decreases by regulating metal homeostasis β-amyloidosis. These data suggest that nicotine reduces the role of βamyloidosis is partly mediated by regulating the balance of metal internal environment[12]. We found that two subtypes of DMT1, DMT1-IRE and DMT1-nonIRE, associate AD with Aβ in postmortem cerebral plaques. Co-location and overexpression of APPsw resulted in increased expression levels of DMT1-ire and DMT1 nonire in SH-SY5Y cells. Interestingly,endogenous DMT1 is silenced by RNA interference,thereby reducing divalent ion influx, resulting in APP expression and Aβ decreased secretion. These findings suggest that DMT1 plays a key role in ion mediated neuropathy of AD[13]. We investigated the interaction and toxicity of Aβ1-42 and copper in the Aβ1-42 transgenicC. elegansCL2006. Our data show that the paralysis behavior of CL2006 worms significantly deteriorated after exposure to copper.The ROS generation induced by Aβ and copper appear to be throughsod-1,prdx-2,skn-1andhsp-16.2genes[14].Our data suggest that the accumulation of ROS was responsible for the paralysis induced by Aβ and copper in CL2006.
Understanding these mechanisms related with toxicity of metals to brain nerves may provide new therapeutic targets for the prevention and intervention of AD.
1.3 Mitochondrial dysfunction and oxidative stress in AD
Oxidative modification of cellular components disrupts membrane integrity and alters the function of essential proteins, leading to mitochondrial dysfunction, and ultimately activation of apoptotic and neuronal cell death. The mitochondrial respiratory chain is the main site in cells where ROS and RNS are produced. Data from transgenic mice suggest that Aβ in mitochondria is associated with impaired mitochondrial metabolism and increased mitochondrial ROS and RNS. In addition, oxidative stress mediated by Aβ accumulation can lead to modification and damage of cellular components,including enzymes essential for antioxidant defense.Decreased activity of antioxidant defense enzymes may further increase ROS levels and impair mitochondrial function, leading to loss of mitochondrial membrane potential and ultimately caspase activation and apoptosis[15].
Aβ has been shown to alter the protective mechanisms of other cells against oxidative stress.Uncoupling proteins (UCPs) are a family of mitochondrial “anion-ionomer” proteins located on the inner mitochondrial membrane and have a variety of physiological functions. The study found that UCP2 and UCP4-dependent upregulation of mitochondrial free calcium after superoxide treatment was attenuated in cells overexpressing APP or APP mutants, suggesting that Aβ accumulation may be related to the dysfunction of mitochondria as an intracellular calcium reserve pool, leading to increased cellular sensitivity to calcium homeostasis loss[16]. We found that nicotine inhibits MPP+and calcium-induced high-amplitude mitochondrial swelling and cytochrome c release of intact mitochondria[17].
Taken together, Aβ toxicity, oxidative stress and mitochondria dysfunction appear to be interlinked in the pathogenesis of AD. These results show that interaction of oxidative stress generated from mitochondrial respiratory chain together with antioxidant effects should be considered in the neuroprotective diseases.
1. 4 Aβ toxicity and oxidative stress induce AD
Numerous studies have been conducted on oxidative stress and Aβ-induced neurotoxicity. The experiments in cell models have shown that Aβ treatment increased levels of H2O2and lipid peroxides. In hippocampal neuronal cell, soluble Aβ oligomers induce ROS-induced activation of NMDA receptors and are associated with a rapid increase in neuronal calcium, suggesting that soluble Aβ oligomers may be involved as proximal neurotoxins as well as oxidative stress in soluble Aβ oligomerinduced synaptic damage and neuronal loss. At the same time, Aβ is found to be localized to mitochondria of AD patients and transgenic mouse and neuroblastoma cells that stably express human mutant APP[17]. The promoter and 5' untranslated region ofBACE1gene contain binding sites for multiple transcription factors including the redoxsensitive activator protein1 and nuclear factor-κB. As JNK has also been implicated in Aβ-induced neuronal apoptosis, pharmacological inhibition of the redoxsensitive signaling pathways may reduce Aβ accumulation and inhibit neuronal apoptosis[18-19].
These evidences suggest that oxidative stress enhancing Aβ formation is important for the development and progression of AD. Increased oxidative stress in the AD brain may trigger activation of a series of redox-sensitive cell signaling pathways,including JNK, that promote the expression of BACE1 and PS1, ultimately enhancing Aβ production and deterioration of cognitive function.
1.5 Tau protein, neurofibrillary tangles and oxidative stress
The most common feature of AD is neurofibrillary tangles (NFTs) composed of the tau protein. The pathological features of AD are senile plaques, NFTs, and neuronal degeneration, which are associated with increased oxidative stress. There is indeed a mechanistic link between ribosylation and tau hyper-phosphorylation. Targeting ribosylation by inhibiting advanded glycated end-product (AGE)formation may be a promising therapeutic strategy to prevent AD-like tau hyper-phosphorylation and diabetic encephalopathies[20-21]. A study found that the number of activated microglia containing ferritin in senile tree shrew increased, and microglia of malnutrition phenotype were more abundant in elderly individuals. RNA oxidative damage (8-OHG)increases significantly with age in all hippocampal regions, while tau hyper-phosphorylation is enhanced in old age. Phagocytosis inclusion bodies of 8-OHGdamaged cells are observed in M2 microglia activated in aged and elderly animals[22].
The above evidence suggests that the accumulation of Aβ and NFTs composed of tau protein promotes oxidative stress, thereby inducing neurotoxic Aβ and NFTs, and further enhances Aβ and NFTs produced by Tau protein, forming a vicious cycle in the pathogenesis of AD. Oxidative stress is an important factor in the development of AD. Removing ROS or preventing its formation may inhibit the progression of AD.
2 Preventive and therapeutic effects of natural antioxidants on AD
Unless effective preventive and/or therapeutic strategies are formulated, the incidence rate of AD will continue to rise by an increase of over 100%.Many drugs have been approved for the treatment of AD, however, they have little benefit and cause a variety of side effects[23]. Cumulative evidence suggests that natural antioxidants are associated with a lower risk of AD. Natural antioxidant drugs not only cure diseases but also boost the patient's immunity,which leads to better health, and they often contain several compounds or mixtures. For AD, natural antioxidant drugs are suitable drugs because the pathogenesis of AD is complex, with many targets and pathways[24-26]. Natural antioxidants can reduce oxidative stress, prevent apoptosis, promote neurogenesis, inhibit the accumulation of Aβ, restore calcium homeostasis, reduce transition metal overload in the brain, and have anti-inflammatory properties.Natural antioxidants can also reduce high serum cholesterol and glucose tolerance[27].

Table 1 A list about the effects of natural antioxidants on AD in cells, animals, epidemiological survey or clinical trials
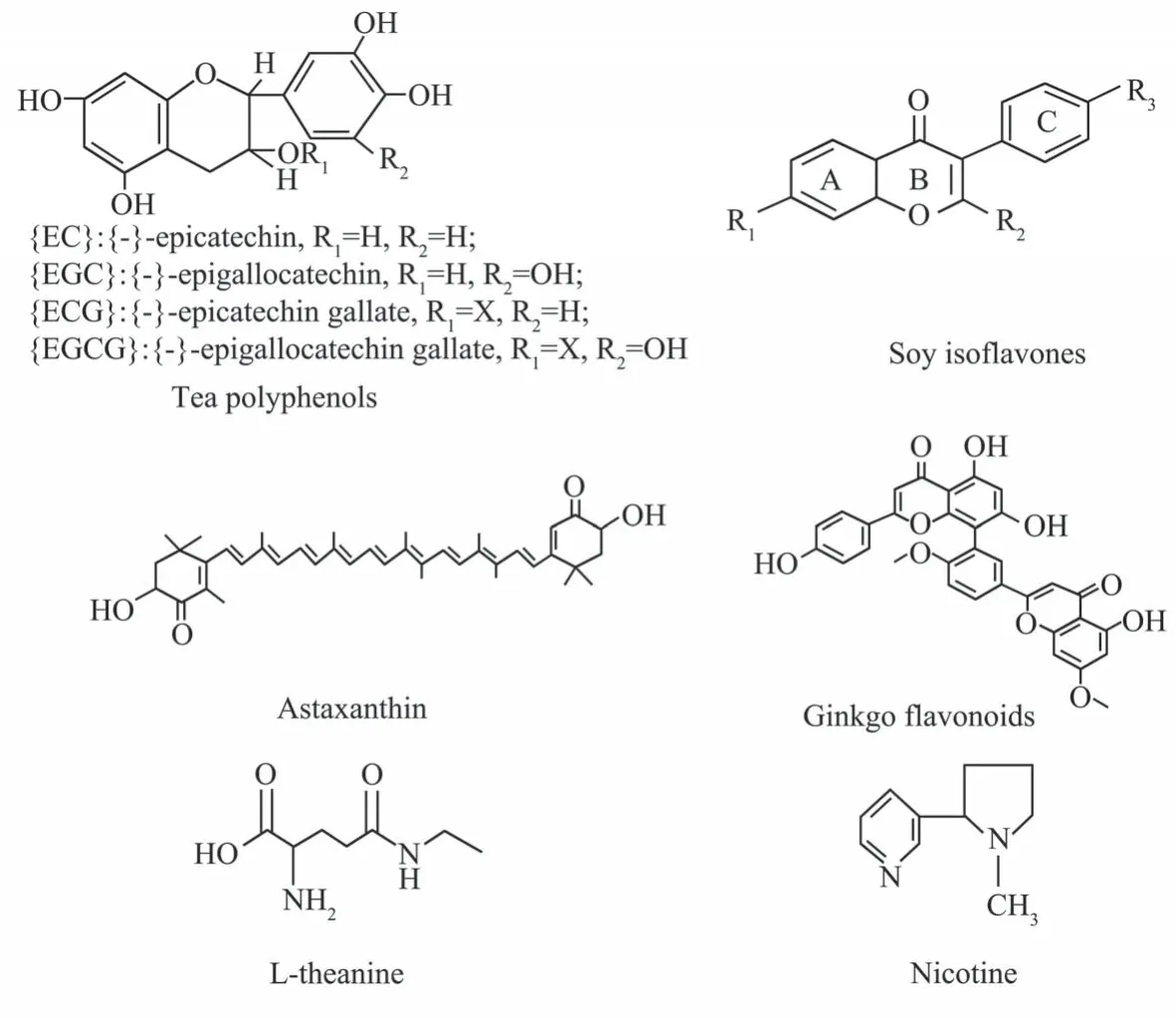
Fig. 1 Structure of natural antioxidant green tea polyphenols, astaxanthin, soy isoflavones, ginkgo flavonoids and nicotine
2.1 Preventive and therapeutic effects of tea polyphenols on AD
Epidemiological investigation shown prevention and treatment effects of taking tea on AD. Many human epidemiological and animal studies have shown that green tea polyphenols can promote health,reduce the occurrence of disease, and possibly prevent AD. The incidence of AD in Asia is only about 5%-10% of that in Western countries, which may be related to green tea consumption in Asian countries[11].A cohort study was conducted in older adults and an epidemiological analysis was performed on the correlation between green tea consumption and cognitive function. In 1 003 Japanese over 70 years old, the prevalence of cognitive impairment was significantly reduced in those who drank more green tea. Green tea consumption is inversely correlated with the prevalence of cognitive impairment[28]. A study conducted in the United States showed that 1 438 participants had cognitive function assessed for 1-2 years. Higher levels of tea consumption were significantly associated with a lower prevalence of cognitive decline.Tea consumption has been found to be significantly negatively correlated with the risk of cognitive impairment, and a linear relationship has been observed[29-30].
Green tea polyphenol EGCG plays a beneficial role in reducing brain Aβ levels, thereby alleviating cerebral amyloidosis in AD mice. EGCG can regulate APP processing, thereby enhancing cleavage of the APP's α-COOH-end fragment (α-CTF), and increased“CTF” cleavage is associated with elevated sApp-α[31]. One month after oral administration of 10 mg·kg-1·d-1EGCG, cognitive impairment assessed by the water maze was reversed with significant reductions in ROS levels and NO production[32]. Oral combined treatment of fish oil and EGCG had a synergistic effect on inhibition of brain Aβ deposits in Tg2576 mice. Effective doses of EGCG in humans may exceed clinical convenience and/or safety, and this study provides a solution through co-treatment of EGCG and fish oil to improve the bioavailability and safety of EGCG, allowing supplementation in moderate doses to achieve a significant therapeutic effect[33].The effects of green tea extract on learning,memory, behavior, and acetylcholinesterase (AchE)activity in young and elderly male rats showed that the learning and memory abilities of aged Wistar rats treated with green tea extract were significantly improved compared with control young rats, and AchE activity in the brains of treated elderly rats was reduced[34]. Mice were given 0.2% tea polyphenols dietally, and scopolamine-induced amnesia was reversed. Tea polyphenols also significantly inhibit AchE activity[35]. In addition to p53, Bax, and caspase-3 expression, green tea extract dose-dependently attenuates Aβ-induced cell death, intracellular ROS levels, and 8-oxoG formation, but upregulates Bcl-2.Green tea extract inhibits the activation of NF-κB and ERK/p38 MAP kinase pathways through antioxidant mechanisms, inhibiting β-amyloid-induced PC12 cell death[36].
AD with special reference to clinical trials indicated that nutraceuticals like EGCG, showed better neuroprotective activity against various neurodegenerative diseases in human clinical trial.
The epidemiological investigation suggests the prevention and treatment effects of tea polyphenols on AD. There are some different results, which may be caused by the different consumed amount of tea[37].EGCG showed a positive effect in AD with a shorter escape latency. Studies have also shown that EGCG reduces Aβ1-42 levels, participates in the regulation of α, β, γ secretase activity, inhibition of tau phosphorylation, antioxidant, anti-inflammatory, antiapoptosis and neuroprotective mechanisms that inhibit AchE activity. Although more than 100 clinical trials have been registered in ClinicalTrials.gov, only one clinical trial has been conducted to test the therapeutic efficacy of EGCG on AD progression and cognitive performance[38].So more studiesneed to be conducted to confirm these results.
2.2 Preventive and therapeutic effects of L-theanine on AD
L-theanine is an important component of tea and a very good natural antioxidant. Research have found L-theanine is known as “natural sedative”. L-theanine is also known as N-ethyl-γ-glutamine, which is a unique amino acid in tea. Its content is positively correlated with the quality of tea, with a correlation coefficient of 0.787-0.876. It is one of the important indexes to evaluate the quality of green tea[39].
Excitatory glutamatergic neurotransmission of N-methyl-D-aspartate receptor (NMDAR) is essential for synaptic plasticity and survival of neurons. Studies have shown that different outcomes of NMDARmediated responses are induced by regionalized receptor activity, followed by different downstream signaling pathways that activate initiating plasticity and stimulate cell survival. Activation of extrasynaptic NMDAR also promotes cell death,thereby contributing to the etiology of AD[40]. We found that L-theanine, like NMDA receptor inhibitor and NO synthase inhibitor, attenuated the decrease of cell viability and apoptosis of APPsw cells induced by glutamate. L-theanine pretreatment significantly inhibited the increase of calcium level in APPsw cells.After L-theanine pretreatment, decreased the content of Aβ in APPsw cells and increased the amount of a secreted by APPsw cells into the culture medium Aβ1-40. It can also significantly reduce the content of ROS and internal calcium, increase the protein expression of neuronal nitric oxide synthase (nNOS)and inducible nitric oxide synthase (iNOS),significantly inhibit the upregulation of p-JNK and Caspase-3 expression in cells caused by glutamate,improve the mitochondrial membrane potential and reduce the apoptosis of APPsw cells. Study of the neuroprotective effects of L-theanine using AD cell model overexpression of APPsw support this hypothesis[41]. We have found that mitochondrial impairment is a very early event in AD pathogenesis and abnormal expression of Mfn1 and Mfn2 caused by excessive intracellular Aβ is the possible molecular mechanism. Interestingly, L-theanine has significant effects on regulating mitochondrial fusion proteins in APPsw cells. Overall, our results not only suggest a new early mechanism of AD pathogenesis but also propose a preventive candidate, L-theanine, for the treatment of AD[42].
In particular, the current literature supports the use of L-theanine, which have a positive impact on cognitive impairment used alone or in combination with other drugs.The use of some botanicals seems very promising to delay the onset and progression of neurodegenerative diseases[43]. Further well-designed clinical research is certainly needed to finally confirm the efficacy and safety profile of botanicals such as L-theanine.
Directly she was missed there was a great hue20 and cry, and every corner, possible and impossible, was searched. Then the king sent out parties along all the roads, but the fairy threw her invisible mantle21 over the girl when they approached, and none of them could see her.
These results suggest that the glutamate receptor NMDA isotype-related pathway is a key point for L-theanine to inhibit AD neuroprotection, and L-theanine may provide effective preventive and therapeutic effects for AD. However, rigorous clinical trial validation is required.
2.3 Preventive and therapeutic effects of astaxanthin on AD
Studies have shown that AST has a good antioxidant effects. Studies have confirmed that AST can cross the barrier from the blood to the brain, so AST can play a protective role in the brain, central nervous system and the whole body, and has a variety of biological functions.AST's antioxidant potential is largely due to its interaction with cell membrane lipids. The inhibitory effect of AST on lipid peroxidation is related to its ability to capture ROS within and on both sides of the membrane.There is growing evidence that AST improves mitochondrial function by reducing the production of mitochondrial reactive oxygen species (mtROS), increasing ATP production, mitochondrial content, and the activity of respiratory chain complexes.Studies have proven that AST protects nerves and prevents neurodegenerative diseases such as AD. A study shown that mice treated with AST showed slower memory decline and reduced deposition of Aβ and tau proteins. The neuroprotective potential of these supplements has only been examined separately in studies[44]. One study found that AST partially restored spinal loss in hippocampal CA1 pyramidal neurons and improved behavioral deficits in AD-like rats, so AST could be a potential option for slowing AD progression[45].
This study investigates the therapeutic effects of different doses of AST on the cerebral cortex and hippocampus of AD-like rats. The results showed that AST significantly and dose-dependently improved the performance of AD-like rats treated with AST during MWM and inhibited the accumulation of Aβ1-42 and malondialdehyde. In addition, AST significantly inhibits AchE and monoamine oxidase activity and the expression of BACE1. ATX also significantly increased levels of acetylcholine, serotonin and nuclear factor erythrocyte-2-related factor 2 and miRNA-124 expression. AST may be a promising therapeutic agent for AD by targeting different pathogenic pathways. The experimental results showed that the proportion of nematodes cultured on different concentrations of AST medium was reduced compared with that of nematodes cultured on solvent control medium[46].
These results indicate that the neuroprotective effect of AST on AD, and supports the notion that AST may provide effective prophylaxis and treatment for AD. AST could be a promising therapeutic antiinflammatory agent for the treatment of AD, autism,etc. Effective AST delivery systems should be developed and further tested with appropriate clinical trials[47].
2.4 Preventive and therapeutic effects of Ginkgo biloba extracton on AD
Ginkgo bilobaextract 761(EGb761) is a patented and well-defined blend of active compounds extracted fromGinkgo biloba. This extract contains two main classes of active compounds, flavonoids (24%) and terpenoids (6%). We investigated the protective effect of EGb761 on dissociated cortical neurons using spin labeling techniques. The results showed that the membrane fluidity of free radical attack was lower than that of the control. With an increase in the concentration of EGb761, the membrane fluidity increases in a dose-dependent manner. EGb761 also protected the change of the protein conformation on the membrane caused by free radical. EGb761 was also found to have protective effects on the cells attacked by free radicals. The protective effect of EGb761 and its active ingredients on apoptosis was investigated, and the results showed that the apoptosis induced by fenyl hydroxyl radicals was associated with decreased levels of Bcl-2, mRNA and elevated protein levels, causing changes in sulfhydryl binding sites on membrane proteins. EGb761 protects cerebellar granule cells from hydroxyl radical-induced oxidative damage and apoptosis. The total terpene of EGb761 does not prevent apoptosis. Flavonoids and terpenes have shown synergistic effects[48-50]. In a randomized, double-blind, multicenter study with a large number of participants and adequate follow-up,the once-daily formulation of EGb761® was safe for the treatment of dementia in patients with neuropsychiatric features and was superior to placebo in this population. Another multicenter, double-blind,randomized, placebo-controlled trial demonstrated the efficacy and safety of EGb761® extract in patients with mild to moderate dementia associated with neuropsychiatric symptoms[51].
These results indicate that the protective effect of EGb761 on AD, and supports the notion that EGb761 may provide effective prophylaxis and treatment for AD.
2.5 Preventive and therapeutic effects of soy isoflavone on AD
Soy isoflavones have protective effects against several chronic diseases, such as atherosclerosis, a condition associated with postmenopausal estrogen deficiency. In the nervous system, isoflavones have been reported to inhibit Aβ25-35-induced ROS production in isolated rat brain synaptosomes.Epidemiological studies have shown that estrogen replacement therapy is associated with a lower risk of developing AD, but is associated with a higher risk of breast cancer and certain cardiovascular diseases. This effect promotes the potential therapeutic effects of the soybean-derived estrogenic compound soy isoflavones[52].
The neuroprotective effects of genistein on Aβ25-35-induced apoptosis in cultured hippocampal neurons were studied[53]. When co-treated with 40 μmol/L genistein and aging Aβ25-35, Aβ25-35-induced ROS production was reduced by approximately 63%, while genistein 0.1 μmol/L reduced Aβ25-35-induced ROS production by approximately 18%. Genistein at concentrations of 0.1 and 40 μmol/L to save age Aβ25-35 induced a 7.2%and 13.9% reduction in survival, respectively, which indicates Aβ25-35-induced apoptosis. The study also found thatthe three compounds tested, only glycitein alleviated Aβ expression-induced paralysis in the transgenicC. elegans. This activity of daidzein is associated with a decrease in hydrogen peroxide in the transgenicC.elegans. Thein vitroscavenging effect of daidzein on three reactive oxygen species confirmed its antioxidant properties. In addition, transgenicC. elegansfed with daidzein showed reduced Aβ formation. These findings suggest that specific soy isoflavones daidzein can inhibit Aβ toxicity by combining antioxidant activity and inhibition of Aβ deposition, which may have therapeutic potential for the prevention of Aβ-related neurodegenerative diseases[54].Soy isoflavones supplementation appears to have a positive effect on improving summary cognitive function and visual memory in postmenopausal women. Postmenopausal women may have a critical window of opportunity to start soy isoflavones at an earlier age, and geography and duration of treatment appear to be factors influencing the effectiveness of soy isoflavones supplementation.All individuals in the included study should be followed up to observe the incidence of AD and dementia, and adverse effects of soy isoflavones should be reported[55].
These results suggest the protective effect of isoflavones on AD, and soy isoflavones may provide effective drugs for the prevention and treatment of AD, but more rigorous clinical trials are needed. In addition, how to overcome soy isoflavones addiction is also a problem that must be solved.
2.6 Preventive and therapeutic effects of nicotine on AD
Nicotine, as the main component of cigarette smoke, is thought to have a protective effect on neurons. Our study shows that nicotine protects cultured hippocampal neurons from Aβ-induced apoptosis. Nicotine potently inhibits apoptosis in hippocampal cultures caused by Aβ25-35 or Aβ1-40 treatment. Measurements of cellular oxidation and intracellular free Ca2+showed that nicotine inhibited Aβ-induced free radical accumulation and intracellular free Ca2+increase. The protective part of nicotine is through nicotinic receptors. Our findings suggest that nicotine may help delay neurodegenerative diseases such as AD[56]. Our study explored the effect of nicotine on metal homeostasis in the hippocampus and cortex of APP transgenic mice. Significantly reduced metal content of copper and zinc in senile plaques and neurooccipital plaques was found after nicotine treatment. After nicotine treatment, the density of copper and zinc in the CA1 subfield of the hippocampus also decreased. We further investigated the mechanism of nicotinemediated metal homeostasis using SH-SY5Y cells overexpressing APPsw. Nicotine treatment reduces intracellular copper concentration and attenuates Aβ-mediated neurotoxicity promoted by the addition of copper, and nicotine's effect on reducing β-amyloidosis is mediated in part by modulating metal homeostasis[11]. In this study, we found that nicotine reduced the accumulation of Aβ in the APP(V717I) transgenic mouse cortex and hippocampus.Nicotine prevents the activation of NF-κB and c-Myc by inhibiting the activation of MAP kinases(MAPKs). It can also reduce the activity of NOS and the production of NO. The nicotine-mediated process described above requires α7 nAChR. Nicotine lowers Aβ by activating α7nAChRviathe MAPK, NF-κB,and c-myc pathways. Nicotine was also able to inhibit apoptosis and cell cycle progression in this mouse line. Nicotine provides a mechanistic basis for the potential development of drug targets for neuroprotective therapy for AD[12].
One study showed that nicotine significantly improved attention performance as measured by the Conners Continuous Performance Test (CPT). Missed errors on CPT were significantly reduced, and such errors persisted throughout long-term nicotine administration. Chronic nicotine also significantly reduces the variability of hit response time on CPT[57].These results indicate that the neuroprotective effect of nicotine on AD, and supports that nicotine may provide effective prophylaxis and treatment for AD.But nicotine can cause addiction and nicotine dependence in patients. It would be of great significance if there is a substance that could suppress nicotine addiction without affecting its preventive and therapeutic effects on AD. We found that tea and L-theanine can inhibit nicotine addiction and also have beneficial preventive and therapeutic effects on AD, and if the two substances are combined, it will have great significance for the prevention and treatment of AD[58-59].
3 Molecular mechanism of natural antioxidants in prevention and treatment of AD
There are many reports about the mechanism of antioxidants can prevent AD, most of which focus on scavenging free radicals, iron chelating characteristics, inhibition of inflammation, regulation of cell survival/death genes and induction of neuronal activity by mitochondrial function, Aβ deposits and signal transduction pathway. Many studies on the molecular mechanism of the effect of natural antioxidants on AD have been carried outin vivoandin vitro[60].
3.1 Preventive effects of natural antioxidant on AD by scavenging free radicals
We studied the pathogenic mechanism of iron in AD and the regulatory effect of natural antioxidants on ROS and RNS free radicals. The results show that natural antioxidants reduced the content of Aβ in APPsw cells, ROS and intracellular calcium, and improve the mitochondrial membrane potential.Natural antioxidants may have a preventive and therapeutic effect on AD by scavenging ROS in APPsw cells[8-9]. In fact, lipid peroxide, protein and oxidized DNA are increased in AD patients, and the antioxidant effects of natural antioxidants may help prevent AD[61-62]. Natural antioxidants may have a preventive and therapeutic effect on AD by their scavenging ROS and antioxidant effects.
3.2 The regulatory effect of natural antioxidants on iron imbalance in AD
The imbalance of iron in the body is closely related to the occurrence of AD. The important thing is that iron overload leads to nerve cell damage and ad related diseases. In addition, if iron deficiency leads to anemia and hypoxia, it is also related to AD. The chelating properties of natural antioxidants for metal iron may contribute to these antioxidant effects. Metal ions such as copper (II) and iron (III) can be chelated by natural antioxidants, and iron chelation reduces the production of ROS by inhibiting the Fenton reaction[63]. We studied the pathogenic mechanism of iron in AD and the regulatory effect of natural antioxidants on iron imbalance. The results show that natural antioxidants EGCG can reduce the oxidative damage of AD cells by complexing too much iron, so as to protect AD cells. APPsw of transferred Aβ into SH-SY5Y cells were treated with different concentrations of natural antioxidants EGCG for 48 h and measured its iron content in the cell iron pool.After treatment with EGCG, the iron content in the cell iron pool was significantly reduced. EGCG may have a preventive and therapeutic effect on AD by chelating excessive iron in the iron pool of APPsw cells[9,64].
These studies show that natural antioxidants may have a preventive and therapeutic effect on AD by chelating excessive iron in the iron pool and reduce oxidative stress in peripheral and brain tissues and may inhibit behavioral changes associated with cognitive impairment.
3.3 Inhibition effect of natural antioxidants on Aβ accumulation in brain
Studies have also shown that natural antioxidants can prevent Aβ plaque formation, enhances cognitive function and may therefore be helpful in the treatment of patients with AD. It was reported that long-term administration of natural antioxidants could prevent Aβ-induced cognitive impairment in rats. In addition to prevent cognitive impairment, lipid peroxides and ROS in hippocampus and plasma were more 20%lower than those in the control group[60]. We treated SH-SY5Y cells transferred into Aβ with different concentrations of EGCG for 48 h and it was found that Aβ1-42 content in APPsw cell culture medium significantly reduced[65-66]. These data raise the possibility that inhibition effect of tea polyphenols on Aβ accumulation in brain, natural antioxidants may provide effective prophylaxis for AD.
3.4 Preventive effects of natural antioxidants on AD by inhibition of related diseases
Many studies have looked at the role of dietary patterns on cognition later in life, and increased adherence to Mediterranean dietary patterns is associated with cognitive decline and reduced AD events. Studies have found that obesity,hyperlipidemia, hypertension, cardiovascular disease and stroke, and diabetes are associated with AD risk.Epidemiological studies have shown that regular consumption of foods and beverages rich in natural antioxidants is associated with a reduced risk of a wide range of pathological conditions, from high blood pressure to coronary heart disease, stroke, and AD[67-68].
The natural antioxidants may provide potential benefits for reducing the risk of diabetes and AD by targeting common risk factors, including obesity,hyperlipidemia, hypertension, cardiovascular disease and stroke[69].
3.5 Preventive effects of natural antioxidants on AD by inhibition of inflammation
It has been found that brain nerve injury caused by inflammation is an important factor in AD. The inflammatory process produces a large number of ROS and RNS free radicals, leading to oxidative stress damage. Increased oxidative stress and neuronal inflammation are also associated with neuronal dysfunction and neurodegeneration. Neuronal injury or injury leads to the secretion of pro-inflammatory factors, which triggers neuronal death natural antioxidants also has anti-inflammatory properties,which may also be the basis of its mechanism of action on AD[70]. In a study using mice injected with lipopolysaccharide, it was demonstrated that natural antioxidants were given in advance can prevent lipopolysaccharide induced memory damage and inhibit the increase of cytokines and inflammatory proteins in untreated control group[71-72]. Another studiesin vitrostudy on BV-2 microglia showed that the reactions related to lipopolysaccharide induced inflammation were inhibited by natural antioxidants[73].
Above studies suggest that natural antioxidants may be used for the prevention and treatment of a variety of neurodegenerative diseases by inhibition of inflammation.
3.6 Preventive effects of natural antioxidants on AD through signal transduction pathway
Many factors leading to AD can activate multiple signaling molecules such as PKC, Bax, NF-κB and ERK through multiple signaling pathways. Protein kinase C (PKC) related mechanisms may also contribute to the effect of natural antioxidants on AD.PKC plays an important role in cell survival and soluble non-toxic amyloid β (SAPP) in generation[74].Several isozymes including PKCα and ε which activate α-secretory enzymes directly lead to the cleavage of amyloid APP into non-toxic Aβ. Other possible mechanisms of natural antioxidants have also been reported[34-35].
Above discussion suggests that the preventive and therapeutic effects of natural antioxidants on AD are multi-target, including antioxidant effect, free radical scavenging, iron chelating characteristics,inhibition of hydroxyl-dopamine, signal transduction pathway, regulation of cell survival/death genes and induction of neuronal activity by mitochondrial function. Studies have also shown that natural antioxidants can prevent Aβ generation. The formation of plaque enhances cognitive function, so it may be helpful to treat patients with AD or dementia.Therefore, the use of natural antioxidants as multitarget drugs is of great significance for the prevention and treatment of AD.
The mechanism of natural antioxidants in the prevention and treatment of AD can be summarized in Figure 2.
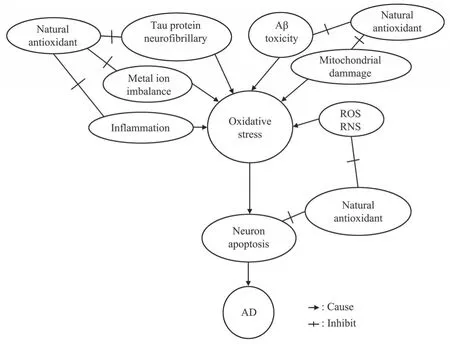
Fig. 2 The pathway and mechanism of natural antioxidants in prevention and treatment of AD
4 Conclusion
From the above discussion, we can see that oxidative stress plays an important role in leading to nerve injury and AD, and the randomized epidemiological study of the population supports habitual drinking tea to reduce the risk of PD and effectively control the disease syndrome. A large number of studies at the cellular and animal levels have shown that natural antioxidant can regulate signal transduction pathways, transcription factors,DNA methylation, mitochondrial function and autophagy through anti-oxidation, anti-apoptosis,inhibition of inflammation, and play many useful biological roles to effectively control AD syndrome.Therefore, it is suggested that natural antioxidant cooperate with established drugs in clinic to maximize its role at a specific level of the typical pathway of disease phenotype induced by AD. In addition, natural antioxidants are readily available in tea, soy milk,fresh fruits and vegetables.
Concerning the use of antioxidant therapies,results from most clinical trials have been disappointing despite the positive effects observed in preclinical studies. The first needs to know that neurodegenerative disease is a complex multifactorial disease. So far there is no drug can achieve satisfactory therapeutic results in the clinic, which includes antioxidants. By the way, to achieve satisfactory therapeutic results in the clinic, the antioxidants need to be considered in the context of species, time, place, level, and target.To achieve this situation requires a very long period of clinical trials,which in turn requires a lot of manpower, material resources and time. Even so, it is worth conducting more rigorous clinical trials in the future to find drugs can satisfactory therapeutic results for neurodegenerative disease, saving more patients for the benefit of humanity.Recently, Professor Chen's team[74-75]proposed the 5R principle of antioxidants,which is worth referring to in future research about antioxidants.
