Study on tongue coating microbes of bitter taste,sticky and greasy taste in chronic atrophic gastritis
2023-02-15CAOYangGAIXiaoZHENGYixinGuoBenqiongNIUJingbinQIANPengLINGJianghongLIUGuopingZHENGYu
CAO Yang,GAI Xiao,ZHENG Yixin,Guo Benqiong,NIU Jingbin,QIAN Peng,LING Jianghong,LIU Guoping,ZHENG Yu
CAO Yang,GAI Xiao,ZHENG Yixin,Guo Benqiong,NIU Jingbin,QIAN Peng,LIU Guoping,Shanghai Key Laboratory of Health Identification and Assessment,School of Basic Medical Sciences,Shanghai University of Traditional Chinese Medicine,Shanghai 201203,China
ZHENG Yu,Department II of Digestive Disease,Longhua Hospital,Shanghai University of Traditional Chinese Medicine,Shanghai 200032,China
LING Jianghong,Department of Digestive Disease,Shuguang Hospital,Shanghai University of Traditional Chinese Medicine,Shanghai 201203,China
Abstract OBJECTIVE: To objectively reveal the relationship between tongue coating microbes and bitter taste,sticky and greasy taste in chronic atrophic gastritis (CAG)patients.METHODS: 16S rRNA high-throughput sequencing was used to detect bacterial diversity and community composition of tongue coating microbes from samples of CAG patients.LEfSe algorithm was used for discovering the different tongue coating microbes in CAG patients with or without bitter taste,also that in CAG patients with or without sticky and greasy taste.RESULTS: We respectively compared the features of tongue coating microbes in bitter taste,sticky and greasy taste of CAG patients.At the genus level,25 tongue coating microbes were significantly different in CAG patients with bitter taste or without bitter taste;17 tongue coating microbes were significantly different in CAG patients with sticky and greasy taste or without sticky and greasy taste.Campylobacter and Rothia were closely related to CAG patients with bitter taste.Enterococcus,Serratia,Leptotrichia and Selenomonas were closely related to CAG patients with stick and greasy taste.CONCLUSION: Campylobacter and Rothia possibly contribute to bitter taste of CAG patients,and Enterococcus,Serratia,Leptotrichia and Selenomonas contribute to stick and greasy taste of CAG patients,which is potential for the diagnosis and treatment of CAG.
Keywords: gastritis,atrophic;taste;Campylobacter;Enterococcus;Serratia;Leptotrichia;Selenomonas
1.INTRODUCTION
Chronic atrophic gastritis (CAG) is considered as a precancerous lesion of GC.1The incidence of CAG in china is about 25.8%.2
The tongue manifestations are a unique diagnostic method in Traditional Chinese Medicine (TCM),and are considered as an external feature of the spleen and stomach.3Moreover,our previous study4confirmed that tongue manifestations could classify different TCM subtypes in CAG patients.Recently,more and more researches have investigated the relationship between tongue coating microbes and diseases,such as gastritis,5CAG,6GC,7pancreatic head cancer,8etc.Therefore,tongue coating microbes play a critical role in human diseases.Furthermore,tongue coating microbes can reflect the mechanism of symptom development.Bitter taste,sticky and greasy taste are common symptoms of CAG patients in the oral cavity.The two symptoms sometimes appear alone or together.However,the detailed mechanism on the tongue coating microbes contributing symptoms such as bitter taste,sticky and greasy taste remains unclear.Whether the two symptoms are caused by the same or different tongue coating microbes remains unclear.
We collected the tongue coating samples of 34 CAG patients.16S rRNA analysis have shown that tongue coating microbes could be objectively revealed the production mechanisms of bitter taste,sticky and greasy taste in CAG.
2.MATERIALS AND METHODS
2.1.Sample collection
Thirty-four CAG patients who received an endoscopic examination in Shanghai University of TCM affiliated Longhua Hospital and Shuguang Hospital were recruited.The clinical symptoms were recorded using the questionnaire of spleen (stomach) disease.The histological assessment was done by the experienced pathologists following clinical guidelines according to“the updated Sydney System”.9The inclusion criteria were a confirmed diagnosis of CAG according to pathological examination.This study was approved by the Medical Ethical Committee (2020-834-41-01).All participants signed the informed consent.
2.2.Tongue coating collection
Before collecting,the participants rinsed mouth with saline 3 times.Tongue-coating swabs were used to collect tongue coating of participants.Tongue coating was scraped with swabs from the root to the tip 3 times,the swabs were put into a 2 mL centrifuge tube,then all the tubes were stored at -80 ℃ until analysis.
2.3.DNA extraction and PCR amplification
Microbial community genomic DNA was extracted from 34 samples using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek,Norcross,GA,USA) according to manufacturer’s instructions.The DNA extract was checked on 2% agarose gel,and DNA concentration and purity were determined with NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific,Wilmington,DE,USA).The hypervariable region V3-V4 of the bacterial 16S rRNA gene were amplified with primer pairs 338 F(5'-ACTCCTACGGGAGGCAGCAG-3') and 806 R (5'-GGACTACHVGGGTWTCTAAT-3') by an ABI GeneAmp® 9700 PCR thermocycler (ABI,Madison,WI,USA).The PCR amplification of 16S rRNA gene was performed.PCR reactions were performed in triplicate.The PCR product was extracted from 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit(Axygen Biosciences,Union City,CA,USA) according to manufacturer’s instructions and quantified using Quantus™ Fluorometer (Promega,Waltham,MA,USA).
2.4.Illumina MiSeq sequencing
Purified amplicons were pooled in equimolar and pairedend sequenced on an Illumina MiSeq PE300 platform(Illumina,San Diego,CA,USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co.Ltd.(Shanghai,China).The raw reads were deposited into the NCBI Sequence Read Archive (SRA)database (Accession Number:PRJNA764325-SRP337893).
2.5.Processing of sequencing data
The raw 16S rRNA gene sequencing reads were demultiplexed,quality-filtered by fastp version 0.20.010and merged by FLASH version 1.2.7.11
Operational taxonomic units (OTUs) with 97% similarity cutoff12,13were clustered using UPARSE version 7.1,12and chimeric sequences were identified and removed.The taxonomy of each OTU representative sequence was analyzed by RDP Classifier version 2.214against the 16S rRNA database (eg.Silva v138) using confidence threshold of 0.7.
2.6.Data processing and statistical analysis
The data were analyzed on the online platform of Majorbio Cloud Platform (www.majorbio.com).LEfSe algorithm was used for discovering significantly different tongue coating microbes from the phylum to the genus level;Spearman analysis was used to calculate the correlation between the symptoms of bitter taste,sticky and greasy taste and tongue coating microbes.The functional prediction of tongue coating microbes was performed using the PICRUSt software package.P<0.05 was considered statistically significant.
3.RESULTS
3.1.Characteristics of patients with CAG
The average age of 34 patients with CAG was (58 ± 10)years old (age range 36-74),including 14 males and 20 females,male: female=1:1.43.The average age of male was (57 ± 9) years old and BMI was 22.9 ± 1.3.The average age of female was (58 ± 10) years old and BMI was 20.8± 2.9.3 CAG patients with Helicobacter pylori infection were diagnosed according to pathological examination.
The clinical symptoms of 34 CAG patients were counted,and the order of frequency was stomach distention (26 cases,76.47%),bitter taste,(25 cases,73.53%),belching(22 cases,64.71%),ungratifying defecation (16 cases,47.06%),pantothenic acid (12 cases,35.29%),sticky and greasy taste (10 cases,29.41%),heavy limbs (10 cases,29.41%),yellow urine (10 cases,29.41%),stomach pain(9 cases,26.47%),dry stool (6 cases,17.65%).
3.2.Analysis of bitter taste and tongue coating microbes
3.2.1.Comparison on tongue coating microbes in CAG patients with or without bitter taste
We compared the tongue coating microbes between 25 CAG patients with bitter taste and 9 CAG patients without bitter taste.At the genus level,as shown in Figure 1,there were 11 tongue coating microbes relatively enriched in CAG patients with bitter taste,includingCampylobacter,Alloprevotella,Selenomonas,F0058,norank_f__norank_o__Clostridia_UCG-014,Catonella,Lachnoanaerobaculum,Megasphaera,unclassified_f__Veillonellaceae,Candidatus_Saccharimonas,Treponema.There were 14 tongue coating microbes relatively enriched in CAG patients without bitter taste,includingActinomyces,Parascardovia,Rothia,Peptostreptococcus,Micro-bacterium,Propionibacterium,Streptomyces,DNF-00809,Desulfobulbus,Paenibacillus,Blautia,Pajaro-ellobacter,Reyranella,Marinobacteras demonstrated in Figure 1B.
3.2.2.Correlation between tongue coating microbes and bitter taste
We carried out spearman correlation analysis between the symptom of bitter taste and 287 tongue coating microbes at the genus level.
As shown in Figure 2,11 genera were positively correlated with bitter taste,includingCampylobacter(P< 0.001,R=0.615),Alloprevotella(P=0.001,R=0.561),Selenomonas(P=0.001,R=0.544),unclassified_ f_ Veillonellaceae(P=0.004,R=0.483),Megasphaera(P=0.006,R=0.459),Lachnoanaerobaculum(P=0.018,R=0.404),norank_f_norank_o_Clostridia_ UCG-014(P=0.028,R=0.377),F0058(P=0.030,R=0.373),Candidatus_Saccharimonas(P=0.034,R=0.364),Treponema(P=0.037,R=0.359),Catonella(P=0.039,R=0.356);15 genera were negatively correlated with bitter taste,includingParascardovia(P=0.002,R=-0.518),Rothia(P=0.01,R=-0.432),Peptostreptococcus(P=0.012,R=-0.425),Marinobacter(P=0.014,R=-0.417),Reyranella(P=0.014,R=-0.417),Blautia(P=0.014,R=-0.416),DNF00809(P=0.014,R=-0.416),Microbacterium(P=0.014,R=-0.416),Paenibacillus(P=0.014,R=-0.416),Pajaroe-llobacter(P=0.014,R=-0.416),Actinomyces(P=0.016,R=-0.411),Streptomyces(P=0.017,R=-0.407),Propi-onibacterium(P=0.040,R=-0.355),Bacteroides(P=0.041,R=-0.452),Desulfobulbus(P=0.048,R=-0.342).
3.2.3.Functional prediction of tongue coating microbes related to bitter taste
At the genus level,Kyoto Encyclopedia of Genes and Genomes (KEGG) functional prediction was performed on the tongue coating microbes enriched in the group with bitter taste.At the same time,42 KEGG pathways(level 2) and abundance value were obtained.In descending order of abundance value,the top 10 pathways were Global and overview maps,Carbohydrate metabolism,Amino acid metabolism,Metabolism of cofactors and vitamins,Energy metabolism,Translation,Replication and repair,Nucleotide metabolism,Membrane transport,Glycan biosynthesis and metabolism (Figure S1A).
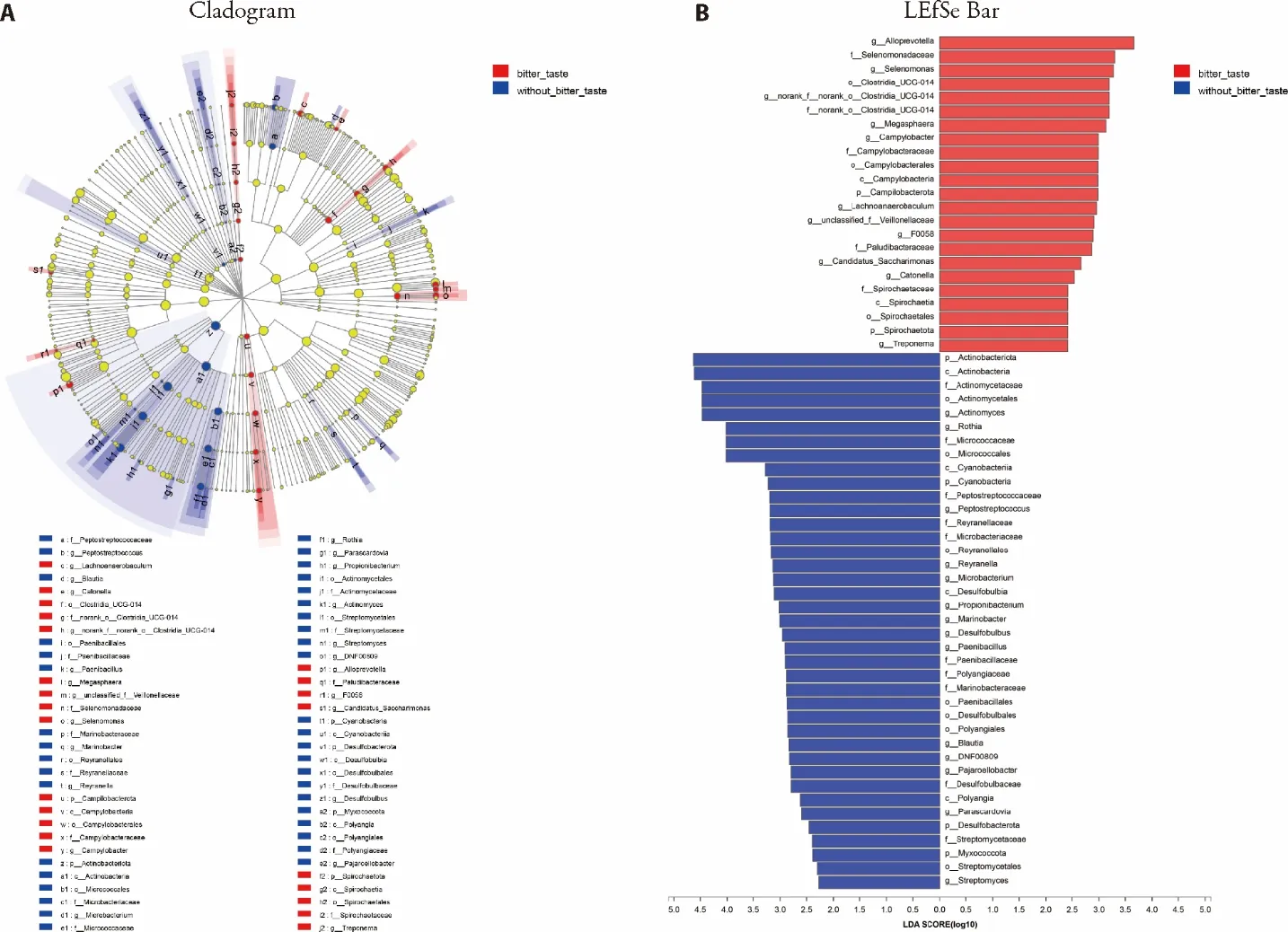
Figure 1 Comparison on tongue coating microbes in CAG patients with or without bitter taste
Furthermore,at the genus level,the KEGG functional prediction was performed on the 14 tongue coating microbes enriched in the group without bitter taste,and a total of 44 KEGG pathways (level 2) and abundance value were obtained.In descending order of abundance,the top 10 pathways were Global and overview maps,Carbohydrate metabolism,Amino acid metabolism,Energy metabolism,Metabolism of cofactors and vitamins,Translation,Replication and repair,Nucleotide metabolism,Membrane transport,Lipid metabolism(Figure S1B).
3.3.Analysis of sticky and greasy taste and tongue coating microbes
3.3.1.Comparison on tongue coating microbes in CAG patients with or without sticky and greasy taste
We compared the tongue coating microbes between 10 CAG patients with sticky and greasy taste and 24 CAG patients without sticky and greasy taste.At the genus level,as shown in Figure 3A,there were 4 tongue coating microbes relatively enriched in CAG patients with sticky and greasy taste,includingSerratia,Enterococcu,DNF00809,Enhydrobacter.There were 13 tongue coating microbes relatively enriched in CAG patients without sticky and greasy taste,includingLeptotrichia,Fusobacterium,Selenomonas,Eubacterium_saphenum_group,Aggregatibacter,Mycoplasma,Comamonas,Catonella,Lautropia,Treponema,Fretibacterium,norank_f_norank_o_norank_c_Gracilibacteria,Lachnoanaerobaculum,as shown in Figure 3B.
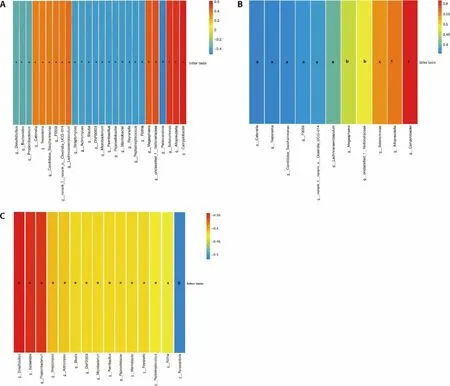
Figure 2 Heatmap of spearman correlation between tongue coating microbes and bitter taste
3.3.2.Correlation between tongue coating microbes and sticky and greasy taste
We carried out spearman correlation analysis between the symptom of sticky and greasy taste and 287 tongue coating microbes at the genus level.At the same time,we drew the correlation heatmap.
As shown in Figure 4,4 genera were positively correlated with sticky and greasy taste,includingEnterococcus(P=0.024,R=0.387),Enhydrobacter(P=0.024,R=0.387),DNF00809(P=0.024,R=0.387),Serratia(P=0.028,R=0.376).13 genera were negatively correlated with sticky and greasy taste,includingAggregatibacter(P=0.001,R=-0.528),Fretibacterium(P=0.002,R=-0.517),Lachnoanaerobaculum(P=0.004,R=-0.484),Catonella(P=0.008,R=-0.448),norank_f_norank_o_norank_c_Gracilibacteria(P=0.014,R=-0.417),Selenomonas(P=0.023,R=-0.388),Eubacterium_saphenum_group(P=0.026,R=-0.382),Fusobacterium(P=0.030,R=-0.372),Leptotrichia(P=0.032,R=-0.369),Mycoplasma(P=0.040,R=-0.354),Treponema(P=0.044,-0.348),Comamonas(P=0.046,R=-0.345),Lautropia(P=0.049,R=-0.341).
3.3.3.functional prediction of tongue coating microbes related to sticky and greasy taste
At the genus level,KEGG functional prediction was performed on the tongue coating microbes enriched in the group of sticky and greasy taste.At the same time,a total of 42 KEGG pathways (Level 2) and abundance value were obtained.In descending order of abundance value,the top 10 pathways were Global and overview maps,Carbohydrate metabolism,Amino acid metabolism,Membrane transport,Metabolism of cofactors and vitamins,Energy metabolism,Signal transduction,Cellular community-prokaryotes,Nucleotide metabolism,Xenobiotics biodegradation and metabolism (Figure S2A).
At the genus level,the KEGG functional prediction was performed on the 13 tongue coating microbes enriched in the group without sticky and greasy taste,and 42 KEGG pathways (Level 2) and abundance values were obtained.In descending order of abundance,the top 10 pathways were Global and overview maps,Carbohydrate metabolism,Amino acid metabolism,Membrane transport,Metabolism of cofactors and vitamins,Energy metabolism,Translation,Replication and repair,Nucleotide metabolism,Cellular communityprokaryotes (Figure S2B).
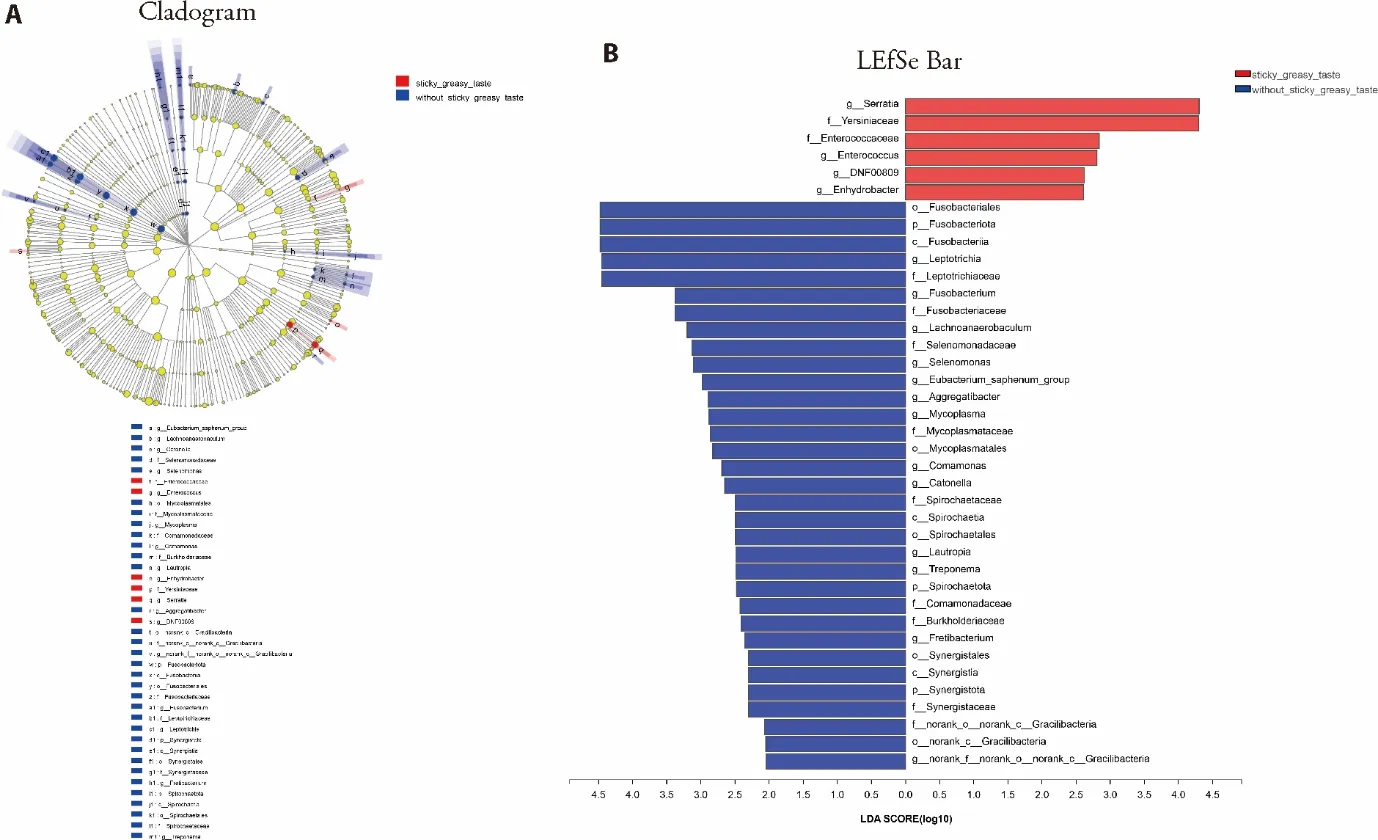
Figure 3 Comparison on tongue coating microbes in CAG patients with or without sticky and greasy taste
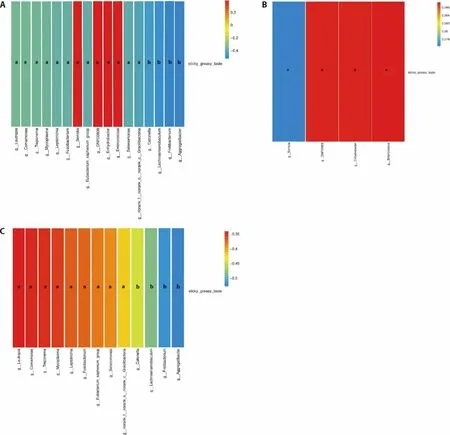
Figure 4 Heatmap of the spearman correlation between tongue coating microbes and sticky greasy taste
4.DISCUSSION
In this study,16S rRNA high-throughput sequencing was used to investigate the variation of tongue coating microbes in CAG patients with bitter taste,sticky and greasy taste.This study provided reference for clarifying the production mechanism of bitter taste,sticky and greasy taste of CAG patients on tongue coating microbes.The tongue manifestations are the external signs of internal organs according to the theory of TCM,which like a mirror to show changes in internal organs ofZangandFu.However,the tongue coating is the external signs most closely associated with the Spleen and Stomach in all organs of Zang and Fu.3Several studies have proved the relationship between tongue coating microbes and the occurrence of gastritis.There were 123 and 258 specieslevel OTUs enriched in the tongue coating of gastritis with Cold Syndrome,or with Hot Syndrome,respectively.5Bacilluswas present only in the yellow tongue coating of patients with chronic erosive gastritis.15Microbial components of tongue coating in CAG patients were significantly different from chronic non-atrophic gastritis patients and healthy people.6In CAG patients,there were 9 tongue coating microbes in genus namelyStreptococcus,Veillonella,Leptotrichia,Prevotella,Rothia,Stomatobaculum,Lachnoanaerobaculum,Solobacterium,Unidentifiedetc,16significantly different from healthy people.Contents of dominant microbes(Streptococcus,Prevotella-7,Moraxell) changed in CAG patients with greasy tongue coating group.17
Campylobacter,belonging to Gram-negative bacteria,is one of the main factors inducing human bacterial gastroenteritis.18
Campylobacter concisusandCampylobacter rectusin species were associated with the Hot Syndrome of gastritis in TCM.19In addition,tongue coating microbes ofCampylobacterin genus was potential related with bitter taste in gastritis patients.20Moreover,TNF which can be induced byCampylobacter concisuswas associated with bitter taste.21Our study also foundCampylobacterwas significantly enriched and related in CAG patients with bitter taste.
In TCM,many factors22,23are involved in the pathogenesis of bitter taste,such as liver-gallbladder damp-heat,liver depression transforming into fire,upflaming of gallbladder fire,damp-heat in the stomach and intestines,and heat fire flaming upward.Researchers found that the increased bile acids might be the main theoretical basis of bitter taste.Bitter taste is related to salivary bile acids migrating from the blood.24Firmicutes in the gut could positively regulate bile acid (BA)metabolism.25In our results,several genera of tongue coating such asSelenomonas,norank_f__norank_o__Clostridia_UCG-014,Catonella,Lachnoanaerobaculum,Megasphaera and unclassified_f__Veillonellaceaebelong toFirmicuteswere enriched.Whether tongue coating microbes belong toFirmicutesmediate the bitter taste through modulating BA metabolites and enhancing BAs migrating from blood into saliva of CAG patients,need to be confirmed in our further study.
The abundance ofRothiain the tongue coating of liverfire hyperactivity syndrome in hypertensive patients with bitter taste was significantly higher than that of the healthy people.26However,in our study,Rothiaenriched in CAG patients without bitter taste,indicating that the different tongue coating microbes might mediate bitter taste in different diseases,and possible many factors are involved in the bitter taste.
At the genus level,we found that 4 tongue coating microbes were relatively enriched and related in CAG patients with sticky and greasy taste,includingSerratia,Enterococcus,DNF00809andEnhydrobacter.TheEnterococcus,27belonging to ubiquitous Gram-positive bacteria,is a common opportunistic pathogen in the oral cavity and gastrointestinal tract,which is also the leading cause of health care-associated infections (HAIs)globally.
Serratia28is an opportunistic pathogen known to cause an array of infectious presentations including pneumonia,wound infections,skin and soft tissue infections.
EnterococcusandSerratiaincrease the inflammatory response of the body,thereby leading to decreased salivary gland function and slow salivary flow.29The saliva viscosity of chronic gastritis patients is significantly higher than that of healthy people,and the saliva flow rate is significantly reduced .30
At the same time,we found that 13 tongue coating microbes were not enriched and related in CAG patients with sticky and greasy taste,such asLeptotrichiaandSelenomonas.Leptotrichia,belonging to Gram-negative bacteria,inhabits the oral cavity,intestines,urinary system,and female genital tract of humans,31and can ferment carbohydrates and produce lactic acid.32Selenomonaswas compatible with periodontal health,33and related to glucose metabolism.34LeptotrichiaandSelenomonascan decompose carbohydrates and glucose of food.So,the digestive function of CAG patients with sticky and greasy taste may be weaken and enhance the retention of food residues in the oral cavity.Thus,our findings indicated that these above bacteria may increase the viscosity of saliva and the retention of food residue in the oral cavity,finally resulted in the symptoms of sticky and greasy taste.
According to the above results,we can see that the tongue coating microbes related to the symptoms of bitter taste,sticky and greasy taste were completely different.However,based on the functional prediction results,it can be seen that the functions of the tongue coating microbes related to the symptoms of bitter taste,sticky and greasy taste were mostly same,and they were all involved in Carbohydrate metabolism,Amino acid metabolism,Energy metabolism.
The reasons of functional prediction may be that 34 patients belong to CAG disease and had the clinical manifestations of yellow and greasy tongue coating.The theory in TCM believes that yellow and greasy tongue coating is the reaction of damp-heat accumulation in the spleen and stomach.Therefore,the results of functional prediction may reflect the characteristics of CAG diseases and syndromes.However,the in-depth mechanism such as bitter taste,sticky and greasy taste needs to be further explored combing with other technologies.
In summary,our findings demonstrated thatCampylobacterandRothiawere related to CAG patients with bitter taste;Enterococcus,Serratia,Leptotrichia and Selenomonaswere related to CAG patients with stick and greasy taste.The results indicated that the mechanisms of bitter taste,stick and greasy taste caused by tongue coating microbes were completely different.Tongue coating microbes might be objective,sensitive and non-invasive methods for CAG diagnosis with different symptoms in clinic,which should be further investigated in more bigger sample set.However,in the present study,the limitation was that the distribution of symptoms of CAG patients is uneven,and the sample set should be expanded for in-depth research in the future study.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Effects of the Huangkui capsule (黄葵胶囊) on chronic kidney disease: a systematic review and Meta-analysis
- Effectiveness of moxibustion alone on lumbar disc herniation: a Meta-analysis of randomized controlled trials
- Efficacy of acupuncture therapy for post-stroke fatigue: a systematic review and Meta-analysis
- Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism
- Tilianin extracted from Xiangqinglan (Herba Dracocephali Moldovicae) inhibits apoptosis induced by mitochondrial pathway and endoplasmic reticulum stress in H9c2 cells after oxygenglucose deprivation/reoxygenation
- Comparing the effects of three decoctions for coronavirus disease 2019 on severe acute respiratory syndrome coronavirus 2-related tolllike receptors-mediated inflammations
