Effectiveness and safety of Jinshuibao capsules (金水宝胶囊) in treatment of residual cardiopulmonary symptoms in convalescent patients of coronavirus disease 2019: a pilot randomized,double-blind,placebo-controlled clinical trial
2023-02-15ZHANGYuehongDONGDandanYANYouqinZHANGHaoWANGGuangliZHOUWeiLIWeiQIULiLITingmingLIUQuanXIAPingMAOLinaYANGDanlinYANGLuLIANFengmeiTONGXiaolinBAYuanming
ZHANG Yuehong,DONG Dandan,YAN Youqin,ZHANG Hao,WANG Guangli,ZHOU Wei,LI Wei,QIU Li,LI Tingming,LIU Quan,XIA Ping,MAO Lina,YANG Danlin,YANG Lu,LIAN Fengmei,TONG Xiaolin,BA Yuanming
ZHANG Yuehong,LIAN Fengmei,TONG Xiaolin,Department of Endocrinology,Guang’anmen Hospital,China Academy of Chinese Medical Sciences,Beijing 100053,China
DONG Dandan,YAN Youqin,WANG Guangli,ZHOU Wei,LI Wei,QIU Li,LI Tingming,YANG Danlin,Department of Infectious Diseases,Wuhan No.7 Hospital,Wuhan 430071,China
ZHANG Hao,LIU Quan,YANG Lu,BA Yuanming,Department of Electrocardiograms,Hubei Provincial Hospital of Traditional Chinese Medicine,Wuhan 430061,China;Department of Respiratory,Hubei Provincial Hospital of Traditional Chinese Medicine,Wuhan 430061,China;Department of Nephrology,Hubei Provincial Hospital of Traditional Chinese Medicine,Wuhan 430061,China
XIA Ping,MAO Lina,Department of Orthopaedics,Wuhan No.1 Hospital,Wuhan 430061,China;Department of Respiratory,Wuhan No.1 Hospital,Wuhan 430061,China
Abstract OBJECTIVE: To evaluate the effectiveness and safety of Jinshuibao capsules (金水宝胶囊),a Traditional Chinese Medicine (TCM),in the treatment of residual cardiopulmonary symptoms in convalescent corona virus disease 2019 (COVID-19) patients.METHODS: A total of 200 participants with COVID-19 in convalescence phase were randomly assigned into two groups at a 1:1 ratio in this multicenter randomized,double-blind,placebo-controlled trial.One group received Jinshuibao capsules,and the other received placebo.The patients were followed up at one and two weeks of treatment.Five symptoms (dry cough,shortness of breath,sweating,chest tightness and palpitation)improvement rates and full recovery rates were compared.RESULTS: All baseline characteristics were comparable between the two groups.After two weeks of treatment,symptom improvement rates for dry cough (74.00% vs 50.00%,P =0.015),shortness of breath (78.95% vs 46.15%,P < 0.001),sweating (80.00% vs 57.75%,P=0.004),chest tightness (87.06% vs 60.47%,P < 0.001)and palpitation (82.50% vs 64.56%, P=0.010) were significantly higher in the Jinshuibao group compared with the control group.Meanwhile,Jinshuibao capsules treatment also displayed more satisfactory full recovery rates of all five symptoms (dry cough 58.00% vs 19.57%,shortness of breath 18.95% vs 7.69%,sweating 36.00%vs 19.72%,chest tightness 32.94% vs 13.95%,and palpitation 48.75% vs 29.11%) in participants with COVID-19 in convalescence phase compared with the control group (P < 0.05).No severe adverse events were reported in either group.CONCLUSIONS: Jinshuibao capsules have the potential to improve residual cardiopulmonary symptoms in convalescent COVID-19 patients,with few adverse events.
Keywords: COVID-19;convalescence;signs and symptoms;randomized controlled trial;Jinshuibao capsules
1.INTRODUCTION
Residual cardiopulmonary symptoms are common after corona virus disease 2019 (COVID-19) recovery,with most patients reportedly displaying impaired lung function.1,2Detailed observation of the first COVID-19 patients in Hubei province with long follow-up revealed that symptoms persisted even after recovery,including cough,fatigue,dry mouth and throat,loss of appetite,wheezing and chest tightness.3Unfortunately,despite data from clinical trials being continuously generated,the effectiveness of COVID-19 symptomatic treatment and recommendations for prescription remain controversial.4,5
It is recommended to apply predictive,preventive and personalized strategies in COVID-19 treatment.6Traditional Chinese medicine (TCM) is an important part of individualized management,and plays a vital role in the prevention and treatment of COVID-19.7,8The main ingredient of Jinshuibao capsules (金水宝胶囊) is fermented Dongchongxiacao (Cordyceps)(CS-4)isolated from fresh Chinese caterpillar fungus,9which previously demonstrated various beneficial properties such as anti-cancer,anti-inflammatory,antioxidant,antifibrotic,anti-arteriosclerosis and immunomodulatory effects.10These properties are related to cytokine and nitric oxide production,phagocytosis stimulation of immune cells,and stimulation of inflammatory responseviathe mitogen-activated protein kinase pathway.11,12Jinshuibao capsules have been used as a TCM preparation for a long time and are effective in chronic obstructive pulmonary disease,chronic bronchial asthma and other lung diseases.13
Up to now,there is a lack of consensus on the optimal therapeutic modality for the symptomatic treatment of COVID-19 during the recovery phase.Although the number of studies dedicated to the application of TCM in COVID-19 is high and constantly growing,studies assessing the efficacy of TCM for the treatment of residual cardiopulmonary symptoms in the recovery phase are rare.Therefore,we performed this important controlled clinical trial to evaluate the efficacy and safety of Jinshuibao capsules in the treatment of residual cardiopulmonary symptoms in convalescent COVID-19 patients,providing a therapeutic basis for TCM in clinical COVID-19.
2.METHODS
2.1.Study design
This was a multicenter,randomized,double-blind,placebo-controlled clinical trial in patients during recovery period of COVID-19 treated with Jinshuibao capsules.This study was registered with the Chinese Clinical Trial Registry (ChiCTR2100050587).The study protocol was approved by the Ethics Committee of Hubei provincial Hospital of TCM (HBZY2020-C27-01).All patients provided written informed consent before enrollment.
2.2.Patients
From April 27,2020 to May 15,2020,COVID-19 patients in the recovery phase with decreased pulmonary and cardiac functions were enrolled at the outpatient clinic of Hubei provincial Hospital of Traditional Chinese Medicine,Wuhan No.7 Hospital and Wuhan No.1 Hospital.
Inclusion criteria: (a) 18-70 years of age;(b) recovery after COVID-19 and discharge 2-4 weeks prior to enrollment;(c) with at least three of the following five symptoms,i.e.,cough,chest tightness,shortness of breath,sweating and palpitation,or any one of these symptoms with a VAS scores > 4 points.According to the 7th edition of the National COVID-19 Diagnosis and Treatment Guidelines released on March 3,2020,14discharge criteria: (a) normal body temperature for ≥ 3 d;(b) significant improvement in respiratory symptoms;(c) significant improvement of acute exudative lesions in chest imaging;(d) two consecutive negative nucleic acid tests of sputum or nasopharyngeal swabs (at least 24 h interval between sampling).
Exclusion criteria: (a) difficulty in taking drugs orally;(b)allergic constitution or allergy to Jinshuibao capsules;(c)mental illness or cognitive disorder;(d) clinically significant comorbidities not well controlled;(e) no suitability for enrollment for other reasons judged by the investigator.
2.3.Randomization and blinding
The patients were randomly divided into two groups by the central random system at a 1:1 ratio.Both the investigators,patients and statistics were blinded to grouping,only if emergency occurred.
2.4.Interventions
Patients in the Jinshuibao group received oral administration of Jinshuibao capsules (Cat.No.CYZB1208841,0.42 g × 4) three times a day.Those in the control group received oral administration of Jinshuibao analogues (0.42 g × 4) three times a day.Jinshuibao capsules and analogues were provided by Jiangxi Jinshuibao Pharmaceutical Co.,Ltd.(Jiangxi,China),and the treatment lasted for two weeks.
2.5.Endpoints
The symptom (dry cough,shortness of breath,sweating,chest tightness and palpitation) improvement and full recovery rates were observed.The visual analogue scale(VAS) was used to assess the severity of symptoms in both groups at baseline,one week and two weeks after treatment.Patients marked their symptom scores from 0-10 points on a calliper;the higher the score,the more severe the symptom.Symptom improvement was defined as a VAS score reduction by ≥ 1 point in patients with baseline VAS score ≥ 1 point.Full recovery was defined as the VAS score decreasing to 0 in patients with baseline VAS score ≥ 1 point.
Any treatment emergent adverse events (TEAEs) and TEAEs leading to drug withdrawal were recorded,as well as whether symptoms recovered after medication discontinuation.
2.6.Statistical analysis
As this was a pilot study,a sample size of 200 was considered sufficient to explore the effect on symptom relief.The per-protocol set (PPS) was used for efficacy analysis.The PPS included patients who had completed prescribed treatments in the whole trial period without severe protocol violation.
Data analysis was performed with SPSS (ver.25.0,IBM Corp.,Armonk,NY,USA).Continuous variables conforming to normal distribution are expressed as mean± standard deviation,and the Student’sttest was performed to compare the two groups.Those not conforming to normal distribution are expressed as median (25th,75th percentiles),and compared by the Mann-WhitneyUtest.Categorical variables are expressed as number and frequency,and compared by the χ2test or Fisher's exact test,as appropriate.Statistical significance was defined as a two-sidedPvalue < 0.05.
3.RESULTS
A total of 200 patients were enrolled and randomly divided into the Jinshuibao group (n=100) and the control group (n=100).In the Jinshuibao group,97 patients were included in the PPS analysis.In the control group,95 patients were included in the PPS analysis(Figure 1).

Figure 1 Flow diagram of participant screening,randomization,and treatment
3.1.Baseline characteristics
The comparisons of baseline data were made between the two groups.The values showed no differences between groups.Detailed baseline characteristics of the patients are shown in Table 1.
3.2.Symptom scores
The five symptom scores are shown in Table 2 and the improvement value of symptom scores are shown in Table 3.There were no significant differences in VAS scores of the five symptoms at baseline and after one week of treatment between the two groups (allP> 0.05),except for VAS score of sweating at baseline.After two weeks of treatment,VAS scores for dry cough,shortness of breath,sweating and chest tightness were statistically significant compared between Jinshuibao group and the control group (P< 0.05).However,VAS scores for palpitation were similar between the two groups.Notably,after two weeks of treatment,statistical difference with the improvement value of the five symptom scores was found between the two treatment groups (allP< 0.05).
3.3.Symptom improvement and full recovery rates
Symptom improvement and full recovery rates are summarized in Table 4.Of all five symptoms,only chest tightness showed a statistically significant difference in symptom improvement rate between the Jinshuibao and control groups after one week of treatment (41.18%vs25.58%,P=0.031).There were no differences in symptom improvement rates for dry cough,shortness of breath,sweating and palpitation between the two groups(allP> 0.05).After two weeks of treatment,symptom improvement rates for dry cough (74.00%vs50.00%,P=0.015),shortness of breath (78.95%vs46.15%,P<0.001),sweating (80.00%vs57.75%,P=0.004),chest tightness (87.06%vs60.47%,P< 0.001) and palpitation(82.50%vs64.56%,P=0.010) were significantly higher in the Jinshuibao group compared with the control group.Regarding full recovery rates,there were no statistically significant differences between the two groups after one week of treatment (P> 0.05 for all).After two weeks of treatment,full recovery rates for dry cough (58.00%vs19.57%,P< 0.001),shortness of breath (18.95%vs7.69%,P=0.024),sweating (36.00%vs19.72%,P=0.029),chest tightness (32.94%vs13.95%,P=0.003)and palpitation (48.75%vs29.11%,P=0.011) were significantly higher in the Jinshuibao group compared with control group.
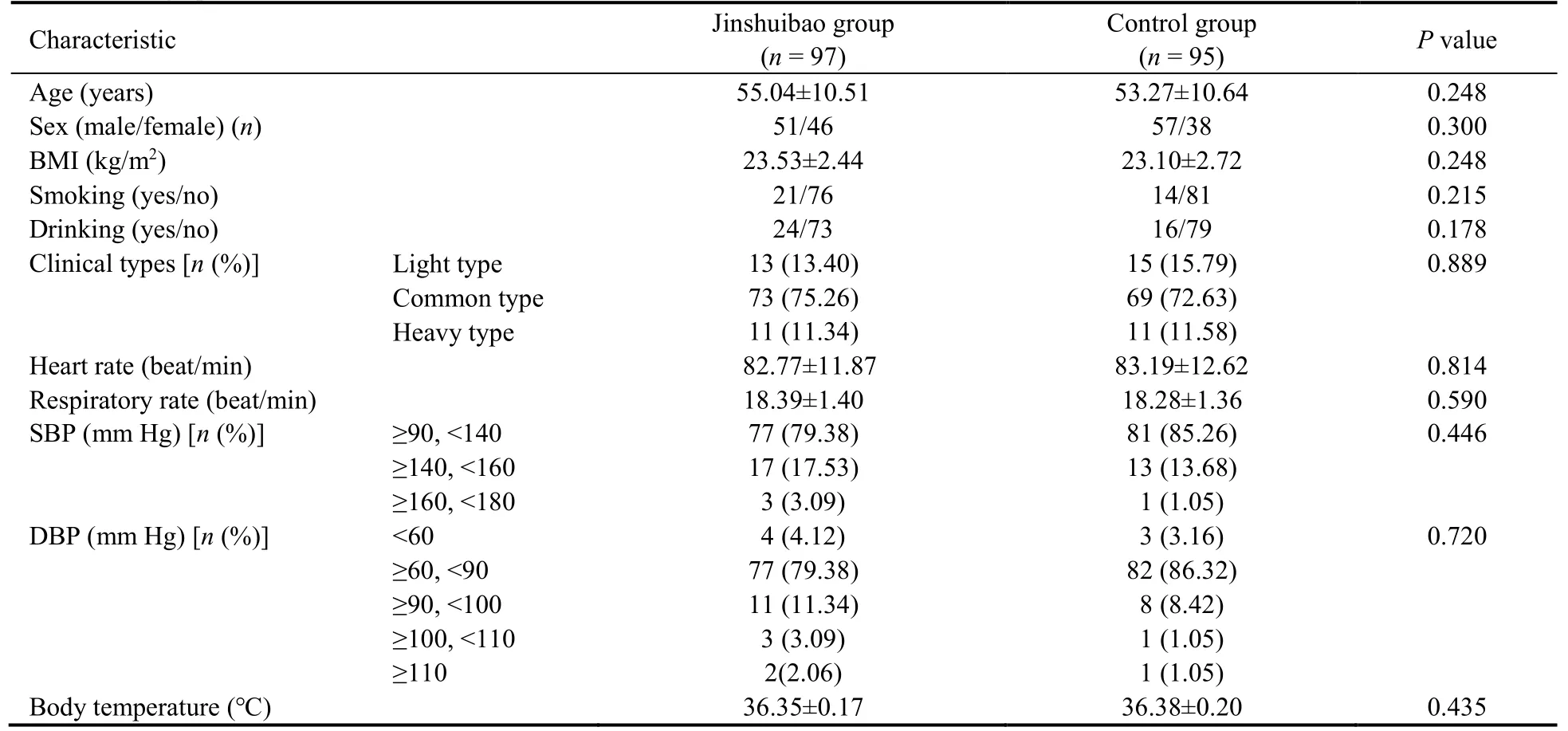
Table 1 Demographic and clinical characteristics

Table 2 Symptom scores at baseline and after one and two weeks of treatment
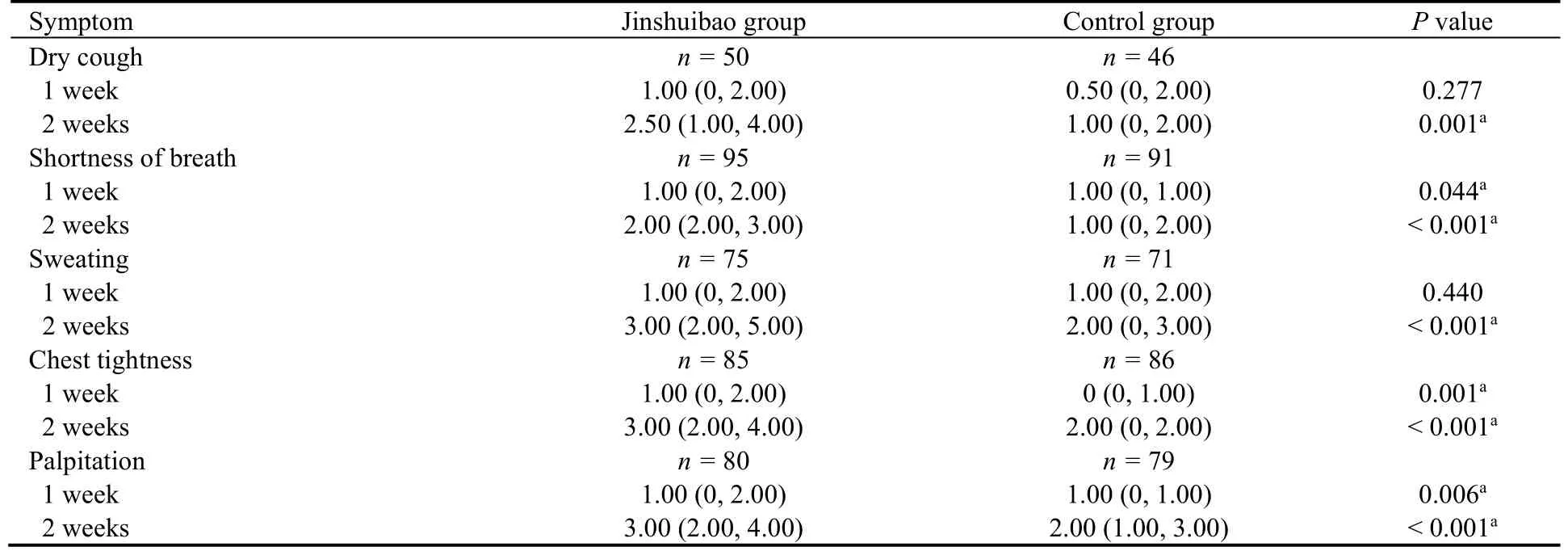
Table 3 Improvement value of symptom scores after one and two weeks of treatment
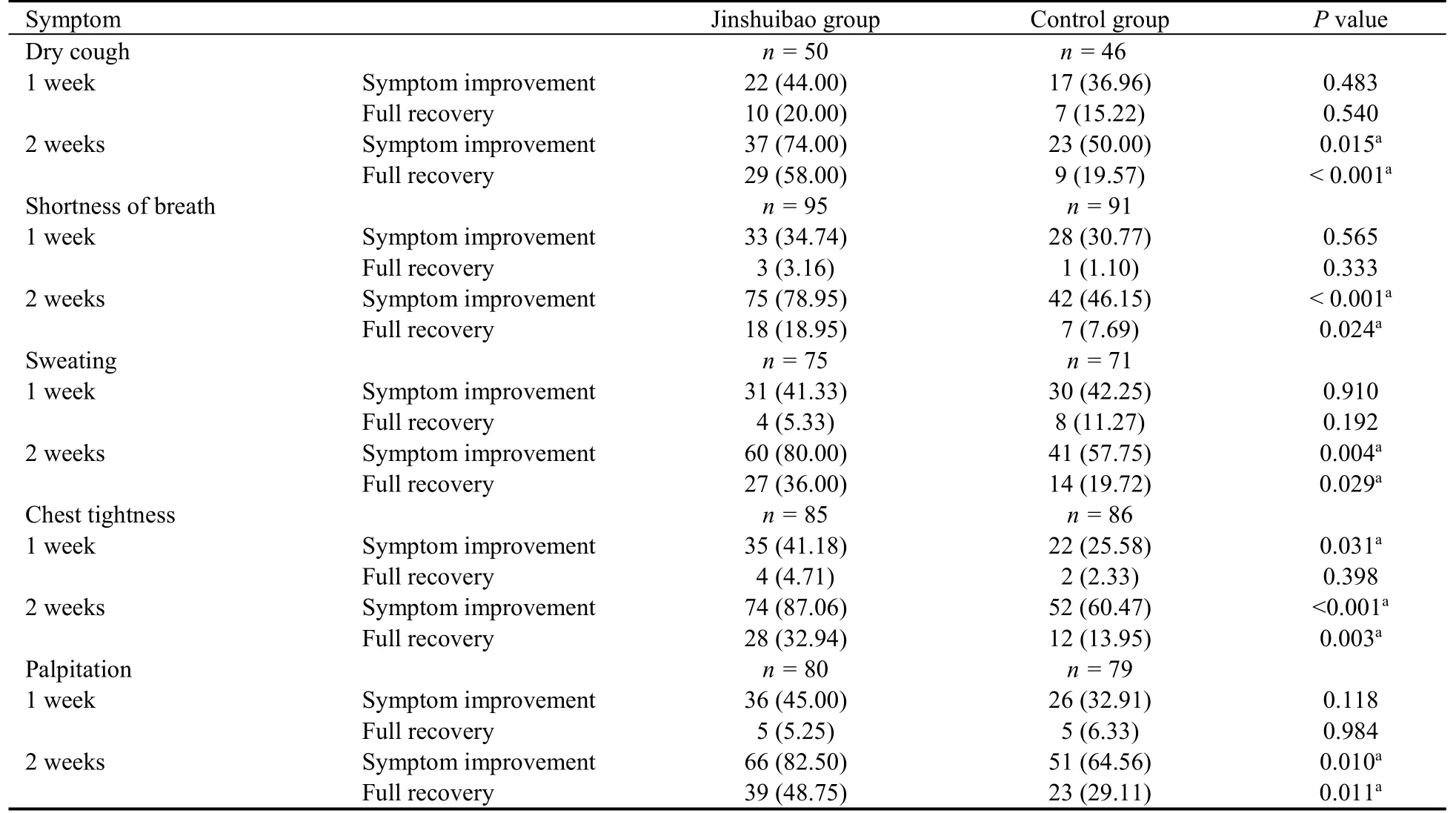
Table 4 Symptom improvement and full recovery rates [n (%)]
3.4.Safety evaluation
A total of three TEAEs were documented in the study.In the Jinshuibao group,there were two cases of dizziness with slightly elevated blood pressure.The investigators decided not to withdraw the drug,and these patients successfully recovered without additional treatment.In the control group,there was one case of dizziness with slightly elevated blood pressure,which also did not lead to drug withdrawal,and the patient successfully recovered without additional treatment.
4.DISCUSSION
The present study aimed to evaluate the efficacy and safety of Jinshuibao capsules in the treatment of residual cardiopulmonary symptoms including shortness of breath,sweating,dry cough,chest tightness and palpitation in convalescent COVID-19 patients,to provide a therapeutic basis for TCM application for the treatment of COVID-19.
We found that Jinshuibao capsules have the potential to improve cardiopulmonary symptoms in convalescent COVID-19 patients.At the end of the follow-up period,symptom improvement and full recovery rates for all five symptoms were significantly higher in the Jinshuibao group compared with the control group without the aggravation of adverse effects.In addition,chesttightness achieved a statistically significant difference in symptom improvement rate between the two groups after one week of treatment (P< 0.05).
The symptom pattern based theory of TCM provides some insights into the pathogenesis of cardiopulmonary symptoms during COVID-19 recovery.15According to TCM theory,the disease mechanism is closely related to the changes in symptoms at the early stage,manifesting as deficiency,heat,dryness,cold,dampness,blood stasis and toxin with Qi deficiency and lingering maliciousQias the core.16The clinical manifestations of those syndromes include dry cough,shortness of breath,fatigue,sweating,heart palpitation and other symptoms related to cardiopulmonary functions.
Jinshuibao capsule consists of fermented Dongchongxiacao (Cordyceps) powder (CS-4),which has a positive effect on deficiency of lung and kidney,deficiency of vital energy,chronic cough,chronic bronchitis and chronic renal insufficiency.17Preclinical pharmacodynamics demonstrated that Jinshuibao capsules can effectively inhibit the production of endothelin 1 (ET-1)and impact the activity of nitric oxide,to restore the normal state of relaxation and contraction of pulmonary vascular and bronchial smooth muscles,and control chronic pulmonary inflammation,thereby improving airway remodeling and airway inflammation.18Other than that,Jinshuibao capsules can significantly enhance phagocytosis in macrophages in mice with low immune function,so that immunity can be restored to the normal level.Apart from the above,toxicological studies showed that Jinshuibao capsules had no obvious side effects.19
Results of this study are in line with previous clinical studies,which showed that Jinshuibao capsules can significantly improve the symptoms of chronic bronchial asthma,20suggesting that Jinshuibao capsules have the potential to alleviate cardiopulmonary symptoms such as cough,phlegm and wheezing.Other clinical studies have shown that combining Jinshuibao capsules therapy with conventional treatment can regulate immunity in chronic obstructive pulmonary disease (COPD),improve pulmonary ventilation function,reduce acute attacks and delay the progression of COPD.21In the present study,after two weeks of treatment with Jinshuibao capsules,symptom improvement and full recovery rates for all five symptoms were higher than those of the control group.These results are consistent with previous studies of chronic bronchitis and bronchial asthma described above,while providing some evidence for Jinshuibao capsules application during COVID-19 recovery.
Nevertheless,this study also has some limitations.Firstly,the follow-up period was short,and the effect of longterm use deserves further investigation.Ongoing studies in our group will extend the observation period to one year.Secondly,symptoms in this study were evaluated by the self-assessment method,rather than using objective indicators or physician-evaluated results,which may lead to some bias.Finally,this was only a pilot study without sample size calculation.Still,we consider the sample size of 100 patients in each group to be sufficient for exploring the effect on symptoms relief,and the present results have certain reference value in designing future prospective studies.
In conclusion,Jinshuibao capsules are effective in improving residual cardiopulmonary symptoms in convalescent COVID-19 patients,which include dry cough,shortness of breath,sweating,chest tightness and palpitation.In parallel to this,Jinshuibao capsules are well tolerated,with few adverse events.
猜你喜欢
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Effects of the Huangkui capsule (黄葵胶囊) on chronic kidney disease: a systematic review and Meta-analysis
- Effectiveness of moxibustion alone on lumbar disc herniation: a Meta-analysis of randomized controlled trials
- Efficacy of acupuncture therapy for post-stroke fatigue: a systematic review and Meta-analysis
- Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism
- Tilianin extracted from Xiangqinglan (Herba Dracocephali Moldovicae) inhibits apoptosis induced by mitochondrial pathway and endoplasmic reticulum stress in H9c2 cells after oxygenglucose deprivation/reoxygenation
- Comparing the effects of three decoctions for coronavirus disease 2019 on severe acute respiratory syndrome coronavirus 2-related tolllike receptors-mediated inflammations
