Mitochondrial dysfunction in a rat model and the related risk of metabolic disorders
2023-02-15LIHanHUANGXiaominCAIHaiyangHEROKGeorgeHEJingSUYixunLIWeihongYIChenjuOLIVERBrianCHENHui
LI Han,HUANG Xiaomin,CAI Haiyang,HEROK George,HE Jing,SU Yixun,LI Weihong,YI Chenju,OLIVER Brian G,CHEN Hui
LI Han,CAI Haiyang,HE Jing,LI Weihong,Faculty of Basic Medical Sciences,Chengdu University of Traditional Chinese Medicine,Chengdu 611137,China
HUANG Xiaomin,SU Yixun,YI Chenju,Research Centre,the Seventh Affiliated Hospital of Sun Yat-Sen University,Shenzhen 518107,China
HEROK George,OLIVER Brian G,CHEN Hui,School of Life Sciences,University of Technology Sydney,Sydney,New South Wales 2059,Australia
Abstract OBJECTIVE: To explore whether kidney Yang deficiency(KYD) is prone to metabolic disorders may be linked to impaired mitochondrial function in thermogenesis and metabolic tissues.METHODS: A rat model of KYD was used,which was established using Sprague Dawley rat dams with warm preference subjected to herbal treatment that can improve kidney Yang.The human relevance was confirmed by reduced serum corticosterone levels,and increased preference for warm location.RESULTS: KYD Rats were underdeveloped.Adenosinetriphosphate (ATP) production was reduced in the brown fat,but increased in the muscle.However,oxidative phosphorylated complexes to generate ATP and mitochondrial biogenesis marker were reduced in both tissues.When the second insult of high-fat diet (HFD)was introduced,KYD rats gained less weight yet developed more severe lipid and glucose metabolic disorders.This may be driven by disregulated liver gluconeogenesis marker forkhead box protein O1 and lipid metabolic regulator cholesterol 7 alpha-hydroxylase.CONCLUSION: KYD rats exhibited reduced mitochondrial function in the brown fat,but were partially compensated by skeletal muscle,associated with the phenotype of warm preference and metabolic disorder,which was further exacerbated by additional HFD consumption.Future studies can focus on treatment targetting mitochondria function to reverse this phenotype.
Keywords: kidney Yang deficiency;DNA,mitochondrial;adenosine triphosphate;thermogenesis;peroxisome proliferator-activated receptor gamma coactivator 1-alpha
1.INTRODUCTION
The mitochondrion is the cellular ‘powerhouse’,which is essential to generate the energy substance ATP to support all physiological functions.The heat generated during this process also contributes to the maintenance of core body temperature.As such,mitochondrial density is higher in energy demanding and heat-generating organs,such as the skeletal muscle and brown adipose tissue (BAT).1The production of ATP through oxidative phosphorylation (OXPHOS) takes place at the cristae of the mitochondrion and is facilitated by OXPHOS complexes I-V.OXPHOS Complex (C) I is an L-shaped protein that acts as the first entry point of electrons in the respiratory chain.OXPHOS CII is the second entry for electrons to the respiratory chain.OXPHOS CIII facilitates electron transfer to OXPHOS CIV.The generated protons from OXPHOS CI to CIV drive ATP synthase in OXPHOS CV.2Mitochondrial dysfunction has been found to contribute to various disease conditions,including cognitive disorder,metabolic disorders,and kidney disease.3-6Severe cases result in a rare inheritable disease called mitochondrial disease,in which patients suffer from fatigue,weakness,and various organ dysfunctions,such as diabetes mellitus and impairment of growth.
In Chinese Medicine,the kidney is not the same solid organ as the kidney in human anatomy,but is considered the ‘powerhouse’ for the whole body regulating multiple functions,including reproduction and development.Interestingly,a condition in Chinese Medicine called‘kidneyYangdeficiency’ (KYD) displays some similar symptoms to mitochondrial disease,including tiredness,fatigue,labor intolerance,cold hypersensitivity(especially lower limbs),and reduced libido and fertility,7however not as severe as mitochondrial disease.The phenotype of KYD also tends to have a familiar heritage,although no specific gene mutation has been discovered to date.Therefore,it is reasonable to propose that mitochondrial dysfunction is the key to the development of KYD,which is supported by the fact that mitochondrial DNA (mitoDNA) is inherited from mothers,and thus mitochondrial functional disorders in the offspring may be also similar to the mothers,8,9It is believed that prolonged exposure to a cold environment or regular consumption of frozen food (eg.icecreams)can lead to KYD.This may be due to reduced mitochondrial activities in response to hypothermia.10,11Previous studies employing the western type of medical examinations found that patients with KYD commonly have reduced urine levels of 17-hydroxycorticosteroids(17-OHCS,a metabolic product of cortisol) with or without reduced serum triiodothyronine (T3) level.12-16In the clinic,reduced cortisol levels can also contribute to weakness,fatigue,weight loss,and gastrointestinal problems.17,18Low serum T3 levels can lead to symptoms like cold sensitivity,oedema,weight gain,and muscle weakness.19However,the clinical features of patients with KYD only have selected symptoms mentioned above,and the hormone levels do not warrant replacement therapies,suggesting neither cortisol nor T3 disorders are the primary cause of symptoms in patients with KYD.Other mechanisms are underlying the pathological process of this condition,which are unclear.Understanding such mechanisms can better guide the use of complementary treatment strategies.
The heat generated during ATP synthesis in the mitochondria contributes to core body temperature,and muscle shivering is an essential mechanism with exposure to a cold environment.20Mitochondrial number insufficiency and/or dysfunction most likely play a major role in determining the phenotype of KYD,particularly in thermogenesis tissue BAT and skeletal muscle.Therefore,we hypothesized that the mechanisms underlying the pathophysiology of KYD are due to the dysfunction of mitochondria or a reduced mitochondrial number in the BAT and skeletal muscles.We aimed to investigate the changes in body temperature,cold preferences,mitochondrial functional markers,and DNA copy numbers in a rat model of KYD.As mitochondria play a key role in metabolic homeostasis,we also examined lipid and glucose metabolic profiles in KYD rats.In addition,mitochondrial function is vital for nutrient metabolism.11,21,22Mitochondrial dysfunction is well known to cause metabolic disorders,such as glucose intolerance (high risk of type 2 diabetes) and dyslipidemia (risk for cardiovascular diseases).21,23-26Therefore,we further hypothesized that KYD phenotype has a higher risk of developing metabolic disorders if a second insult is implemented.To address this hypothesis,we fed the rats a high fat diet from weaning for 10 weeks and measured their glucose and lipid profiles.
2.METHODS
2.1.Animal study
The study was approved by the Animal Ethics Committee of the Chengdu Dossy Experimental Animal Co.,Ltd.[SCXK (Chuan) 2015-030].A KYD deficiency mo-del was established in both male and female Sprague-Dawley rats (10 weeks,Chengdu Dossy Experimental Animal Co.Ltd.) according to Chinese Medicine theory.The sires and dams were first screened by Hot Plate Test.Briefly,rats were allowed to choose the plate ends of 25 ℃ or 40 ℃ for 10 min.The percentage of time spent on the 40 ℃ end was reflected in the preference for the hot end by the rats.Those prone to the hot plate (> 20%) were selected,indicating warm preference.Then KYD phenotype was further reinforced by cold exposure (ambient temperature 5-8 ℃,2 h/d) and the supplement of Huangbai (Cortex Phellodendri Amurensis) in the diet (0.108 g/100 g chow) in the dams during pregnancy,as previously published.27This dose is calculated based on the dose (12 g raw herb/d) for human adults according to Chinese Pharmacopoeia and the ratio of body surface area of humans and rats (0.018).Living and working in icy climates can allow the cold to enter the body to quench the ‘fire’ energy causingYangdeficiency in the kidney,the body’s powerhouse.28Huangbai (CortexPhellodendriAmurensis) is commonly used in Chinese medicine to correct excess kidneyYangenergy and overdose can cause the deficiency ofYangenergy,such as KYD.Therefore,both were used in the dams to reinforce the KYD phenotype.Offspring with positive KYD phenotypes of two consecutive generations were kept as KYD rat strain.To validate the relevance of the rat phenotype to human KYD,the offspring were subjected to the Hot Plate Test at 12 weeks of age for warm preference.
At 13 weeks,male offsprings were fasted overnight,and after anesthesia (2% Pentobarbital sodium 40 mg/kg),the rectal temperature was measured,followed by cardiac puncture for blood.Blood glucose was measured using a glucose meter (Accu-Check®,Roche Diagnostics,Basel,Switzerland).The plasma was kept at -20 ℃ for hormone and lipid analysis.Brown fat,retroperitoneal white fat,kidney,liver,and skeletal muscle were collected and weighed.Brown fat and skeletal muscle were snap frozen and kept at -80 ℃.
Considering the vital role of mitochondria in nutrient metabolism,we further examined if the phenotype will increase the risk of metabolic disorders.In a different cohort (n=6),weaning male rat offspring (3 weeks)were fed a pellet HFD (43% fat,20 kJ/g,Xutong Biological Ltd.Jiangsu,China,following the recipe from our previous studies29-32) for 10 weeks.Their littermates were fed the standard rodent chow (14% fat,12 kJ/g).This yielded 4 groups,Control-chow,KYD-chow,Control-HFD,and KYD-HFD.Body weight and food intake were measured every fortnight.After 9 weeks of HFD feeding,a glucose tolerance test was carried out on all rats using published methods.After 9 weeks of the diet,the rats were fasted for 5 h before an intraperitoneal glucose tolerance test was performed as previously described.29-32All mice were administered with Dglucose (2 g/kg,ip).Tail tip blood was collected at time 0 prior to glucose injection (baseline),then at 15,30,60,and 90 min after glucose administration.Blood glucose levels were measured using a glucose meter (Accu-Check®,Roche Diagnostics,Basel,Switzerland).The trapezoid method was used to calculate the area under the curve (AUC) corresponding to the blood glucose levels over the monitoring period obtained for each animal.At 13 weeks,samples were collected following the same protocol mentioned above.
2.2.Bioassays
Blood corticosterone and T3 levels were measured in the serum by commercial kist (Rat corticosterone enzymelinked immunosorbent assay (ELISA) kit,Shanghai Westang Biotechnology Co.,Ltd.,Shanghai,China;Total T3 ELISA kit,IBL International,Hamburg,Germany).Plasma low-density lipoprotein (LDL) and Cholesterols were measured by commercial kits (Abcam,Cambridge,United Kingdom).Plasma insulin was measured using a commercial kit (Crystal Chem,Elk Grove Village,IL,USA).
2.3.Mitochondrial number
The mitochondrial number was measured using the mitochondrial DNA copy number.Genomic DNA was extracted from tissues using the DNeasy Blood and Tissue kit (Qiagen,Hilden,Germany).The content of mtDNA was calculated using real-time quantitative polymerase chain reaction (PCR) by measuring the threshold cycle ratio (cCt) of a mitochondrial-encoded gene (COX1,forward 5'-ACTATACTACTACTAACAGACCG-3',reverse 5'-GGTTCTTTTTTTCCGGAGTA-3') versus a nuclear-encoded gene (cyclophilin A,forward 5'-ACACGCCATAATGGCACTGG-3',reverse 5'-CAGTCTTGGCAGTGCAGAT-3').
2.4.Western blotting
OXPHOS complexes,antioxidant manganese superoxide dismutase (MnSOD),Glutathione peroxidase(GPx),phosphorylated (p)-and total insulin receptor substrate 1 (IRS1) and its downstream protein kinase B(Akt) were measured by Western Blotting.Total and mitochondrial protein was extracted from brown adipose tissue and skeletal muscle using our published method.33Proteins of the whole tissue lysate and mitochondrial fraction were extracted by the differential speed extraction method.The tissues were homogenized in 200µL of lysis buffer (Shanghai West Tang Biotechonology Ltd.,Shanghai,China).Whole cellular proteins and mitochondria proteins were quantified using Bio-Rad DC protein assay (Bio-Rad Laboratories,Hercules,CA,USA) according to the manufacturer’s instructions.Proteins samples (2 µg/µL) were loaded into each well of NuPAGE® Novex 4-12% Bis-Tris protein gels(Thermo Fisher Scientific,Waltham,MA,USA) and separated on the gel.The separated proteins were then transferred to PVDF membranes using either semi-dried or wet transfer methods where applies (Thermo Fisher Scientific,Waltham,MA,USA).The PVDF membrane was then blocked with 5% skim milk for one hour at room temperature.Primary antibodies (OXPHOS complexes 1∶2500,MnSOD 1∶5000,GPx-3 1∶1000,p-IRS1 1∶1000,IRS1 1∶1000,p-Akt 1∶500,Akt 1∶1000,GAPDH 1 ∶1000,Abcam,Cambridge,UK;voltage-dependent anion channel (VDAC) 1 ∶1000,Thermo Fisher Scientific,Waltham,MA,USA) were incubated with the PVDF membrane at 4 ℃ overnight,followed by secondary antibodies (Peroxidaseconjugated AffiniPure Goat Anti-Rabbit IgG and Peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG,1 ∶10000,Jackson Immuno Research Laboratories,West Grove,PA,USA) and SuperSignalTM West Pico Chemiluminescent Substrate (Thermo Fisher Scientific,Waltham,MA,USA).The bands on the membrane were detected with LAS-3000 Imaging system (Fujifilm,Tokyo,Japan).The results are expressed as a ratio of the intensity of the protein of interest relative to the band intensity of GAPDH or VDAC.
2.5.Real-time PCR
mRNA expression of thermoregulator uncoupling protein (UCP)1,mitochondrial biogenesis marker peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PCG1α) and insulin sensing marker Peroxisome proliferator-activated receptor gamma(PPARγ) was measured in brown and skeletal muscle using real-time PCR where applies.Lipid metabolic marker Cholesterol 7 alpha-hydroxylase (CYP7A1) and gluconeogenesis marker Forkhead box protein O (FOXO)1 were measured in the liver.The tissues (10-100 mg)were extracted using mirVana™ miRNA Isolation Kit(Thermo Fisher Scientific,Waltham,MA,USA)following the manufacturer’s instructions.
Quantification was performed with a two-step reaction process: reverse transcription (RT) and PCR.cDNA was synthesied using HiScript II Q RT SuperMix IIa(Vazyme Biotech Co.,Ltd.,Jiangsu,China) in a GeneAmp® PCR System 9700 (Thermo Fisher Scientific,Waltham,MA,USA).The genes of interest was measured by SYBR green primers using LightCycler® 480 Ⅱ real-time PCR Instrument (Roche Diagnostic,Basel,Switzerland).The primer sequences were designed in the laboratory and synthesized by Generay Biotech [GenerayBiotech (Shanghai) Co.,Ltd.,Shanghai,China] based on the mRNA sequences obtained from the NCBI database (CYP7A1 forward 5'-CCTGCCGGTACTAGACAGC -3',reverse 5'-AGGGTCTGGGTAGATTTCAGGA -3';FOXO1 forward 5'-TAGGAGTTAGTGAGCAGGCAAC -3',reverse 5'-TGCTGCCAAGTCTGACGAAA -3' PPARγ forward 5'-ATCAAGAAGACGGAGACAGATA-3',reverse 5'-GAAGGAACACTTTGTCAGCGA-3';PGC1-α forward 5'-GGATATACTTTACGCAGGTCG -3',reverse 5'-ATCGTCTGAGTTTGAATCTAGG-3';UCP1 forward 5'-TCCGGGCTTAAAGAGCGA -3',reverse 5'-TGGGTACCGAACTCTCAAC-3';18s forward 5'-CGGCTACCACATCCAAGGAA-3',reverse 5'-GCTGGAATTACCGCGGCT -3').
At the end of the PCR cycles,the melting curve analysis was performed to validate the specific generation of the expected PCR product.mRNA expression was calculated using 2-ΔΔCtmethods using 18 s as the housekeeping gene.The Control group was assigned the calibrator against which all other results were expressed as fold changes.
2.6.Statistical method
The results are expressed as mean ± standard error of the mean.The data between 2 groups were analyzed by unpairedt-test.The data between 4 groups were analyzed by two-way analysis of variance followed by post hoc Fisher’s least significant difference tests (Graphpad Prism 9,San Diego,CA,USA).P <0.05 was considered statistically significant.
3.RESULTS
3.1.Baseline assessment in the KYD rats
3.1.1.Biometric parameters
Before pregnancy,KYD dams were 9% smaller than the controls (P <0.05),consistent with weight loss in patients with KYD.28The litter size of KYD mothers(6.33 ± 0.88) was also half of the Control group (13.00 ±1.16,P <0.05).KYD dams seem to produce fewer male offspring in each litter,reflected by the sex ratio (male to female: KYD 1.57 ± 0.81,Control 2.37 ± 1.20).However,it did not have statistical significance.Thus,KYD dams showed issues of reproduction,similar to human patients with KYD.34
Male offspring from KYD dams had significantly smaller birth weight (P <0.01vsControl,Table 1),which was maintained in adulthood (P <0.05vsControl,Table 1).KYD offspring also had smaller kidney mass(P <0.05vsControl,Table 1) and white fat mass (P <0.01vsControl,Table 1),however,bigger muscle mass at 13 weeks (P <0.05vsControl,Table 1).After standardizing for body weight,KDY rats had a bigger percentage of liver and muscle weights (bothP <0.05vsControl,Table 1),and a smaller percentage of white fat mass (P <0.01,Table 1).
3.1.2.Serum metabolic markers
To further confirm the human relevance of this rat model,we measured serum corticosterone and T3 levels.In KYD offspring,serum corticosterone levels were significantly lower than in the Control group (P <0.05,Table 1),while T3 levels were only marginally reduced(Table 1),similar to the clinical manifestation in humans with KYD.12-16
We also measured blood glucose and lipid levels.The blood glucose level in the KYD offspring was similar to the Control group (Table 1),whereas serum LDL and cholesterol levels were significantly increased in KYD offspring (P <0.05 and 0.01vsControl,respectively,Table 1),suggesting lipid metabolic disorders,which is also consistent with the risk in human patients.
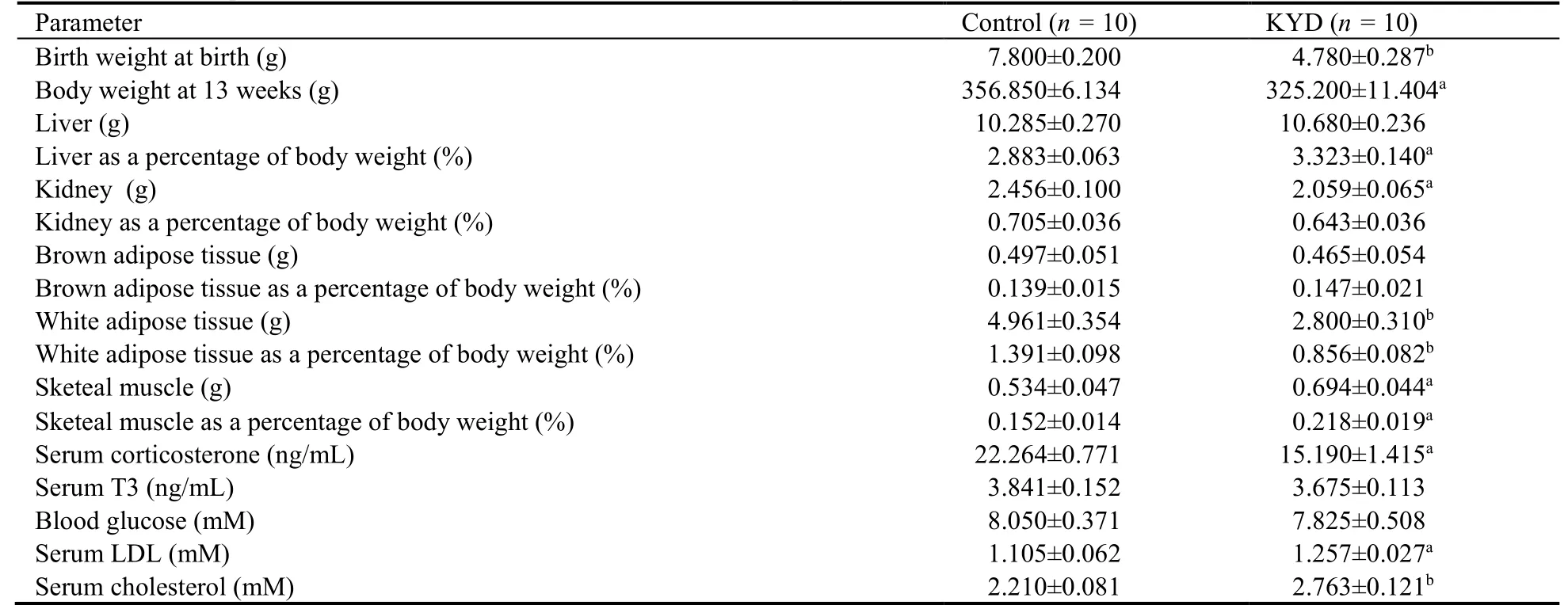
Table 1 Biometric parameters and blood metabolic markers in male offspring at 13 weeks
3.1.3.Body temperature and warm/cold preference
The hot plate test allows the rats to choose the preferred temperature on two plates,25 ℃ as cool and 40 ℃ as warm.In the literature,the preferred temperature for the rat is 24-27 ℃.35KYD rats showed a preference for warm locations,which was reflected in the rats spending twice as long as the Control rats on the warm plate (P <0.05vsControl,Figure 1A),which is similar to the behavior of humans with KYD.28
To investigate whether such preference was due to low core body temperature,we measured rectal temperature and mRNA expression of uncoupling proteins (UCP)1 in BAT and muscle.The major function of brown fat is nonshivering thermogenesis against the cold,36mainly relying on UCPs,especially UCP1.37Mice lacking UCPs were cold intolerant.38Muscle contraction contributes to shivering thermogenesis.However,there was no difference in body temperature between the KDY offspring and Control offspring (Figure 1B).Not surprisingly,the regulator of thermogenesis UCP1 expression was not significantly changed in both BAT and skeletal muscle (Figure 1C,1D).
3.2.Mitochondrial metabolic markers in the BAT and muscle
In BAT,ATP production was significantly lower in the KYD rats (P <0.01vsControl,Figure 2A).Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC1a) is a multiple-functional molecule,which also regulates mitochondrial biogenesis.PCG1α in BAT was reduced in KYD rats (P <0.05,vsControl,Figure 2B),suggesting an impaired ability to replenish mitochondrial shortage.This is associated with reduced mitoDNA copy number (P <0.001vsControl,Figure2C).Results show that OXPHOS CI was nearly diminished in KYD rats,and OXPHOS CV was also significantly lower in KYD rats (bothP <0.05vsControl,Figure 2D,2E).Endogenous antioxidants MnSOD and GPx were not different between the groups (data not shown),suggesting oxidative stress is not involved.
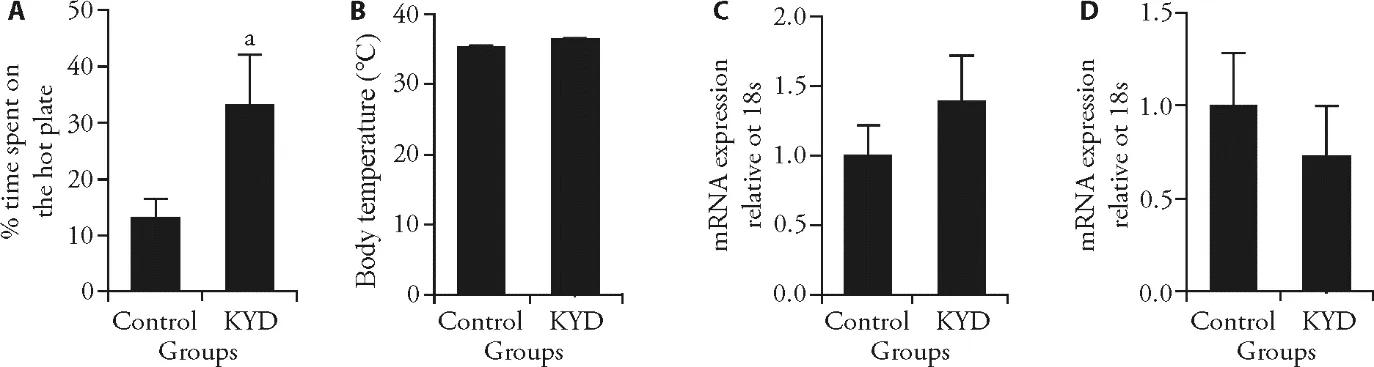
Figure 1 Markers involved in temperature and thermogenesis
In the skeletal muscle,ATP levels were significantly higher in the KYD rats (P <0.05vsControl,Figure 3A).Mitochondrial biogenesis and metabolic regulator PCG1α was lower in the KYD rats (Figure 3B),however mitochondrial DNA copy number was not different between the groups (Figure 3C),suggesting impaired metabolic capacity in the muscle.OXPHOS CI was not different between the groups (Figure 3D),however,OXPHOS CV was nearly depleted in the skeletal muscle(P <0.01,Figure 3E).Similarly,in BAT,mitochondrial endogenous antioxidant MnSOD and GPx did not show any difference between the Control and KYD groups (not shown).Therefore,the difference between mitochondrial biogenesis and functional units might not result from oxidative stress.
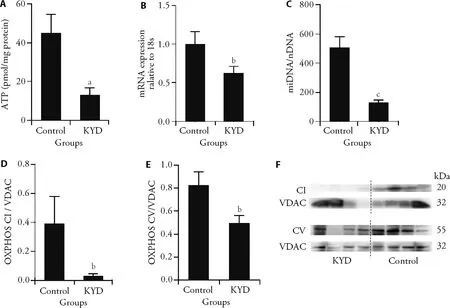
Figure 2 Mitochondrial metabolic markers in the BAT
3.3.Effect of HFD consumption on metabolic profiles
At weaning,the body weights were not significantly different among the groups (Table 2).At 13 weeks,KYD-chow rats were smaller than the Control-chow rats(P <0.05,Table 2),consistent with the first cohort.Interestingly,KYD-HFD rats gained less body weight by HFD consumption compared with the Control rats (P <0.01 KYD-HFDvsControl-HFD,Table 2),whose body weight was similar to the Control-chow rats,although KYD rats had similar daily caloric intake as the Control regardless of the diet (Table 2).HFD consumption significantly increased white fat mass in both Control-HFD and KYD-HFD rats (P <0.05,Table 2),but only increased liver mass in the KYD-HFD rats (P <0.01vsKYD-chow,Table 2) both as net weight and percentage of body weight.
During the glucose tolerance test,it was expected that Control-HFD rats developed glucose intolerance reflected by a grater AUC value (P <0.01vsControlchow,Table 2);however,KYD-HFD rats showed more severe glucose intolerance than Control HFD rats (P <0.01 KYD-HFDvsKYD-chow and Control-HFD,Table 2) albeit smaller body weight and fat mass.This may be due to insulin insufficiency,as serum insulin level (P <0.01 Control-HFDvsControl-chow and KYD-HFD) was significantly increased in the Control-HFD group,but remained unchanged in the KYD-HFD rats.Interestingly,hyperlipidemia was worsened in KYD rats after HFD consumption,especially the LDL,which was increased in the KYD-HFD group (P <0.01 KYD-HFDvsKYDchow and Control-HFD,Table 2),not the Control-HFD group.Cholesterol was increased in Control-HFD group(P <0.05vsControl-chow Table 2),which was further increased in the KYD-HFD group (P <0.05vsControl-HFD,P <0.01vsKYD-chow group,Table 2).

Table 2 Biometric parameters and blood metabolic markers in male offspring fed a HFD
To further investigate the molecular pathway,we found in the muscle,the energy regulator PGC-1α was significantly downregulated in the KYD-chow rats (P <0.05vsControl-chow,Figure 4A),which was less reduced in the Control-HFD and KYD-HFD rats,suggesting diet and phenotype may independently affect this marker.The insulin sensing marker PPARγ expression was only reduced by HFD consumption (P <0.01 Control-HFDvsControl-chow,KYD-HFDvsKYD-chow,Figure 4B).The ratio between p-IRS1 and total IRS1 was reduced in KYD-chow rats and by HFD consumption (bothP <0.01,Figure 4C).However,there was some increase in p-IRS1/IRS1 in KYD-HFD rats compared with their chow-fed littermates (P <0.01).The downstream signaling p-Akt/Akt ratio was only reduced in KYD rats independent of the diet (P <0.01vsControl rats fed the same diet,Figure 4D).In the liver,FOXO1 expression was suppressed in the KYD-chow rats (P <0.01vsControl-chow,Figure 4E),and to a less extent in the Control-HFD-rats (P <0.01vsControl-chow);however,FOXO1 was significantly upregulated by HFD feeding in KYD rats (P <0.05 KYD-HFDvsKYD-chow,Figure 4E).The lipid metabolic marker CYP7A1 was more than halved in the liver of the KYD-rats albeit without statistical significance (Figure 4F).CYP7A1 mRNA was however increased in Control-HFD (P <0.01vsControl-chow),which was at a normal level in KYD-HFD rats (P <0.01vsControl-HFD,Figure 4F).Overall,the mechanisms discovered in the rat model of KYD are summarised in Appendix 1.
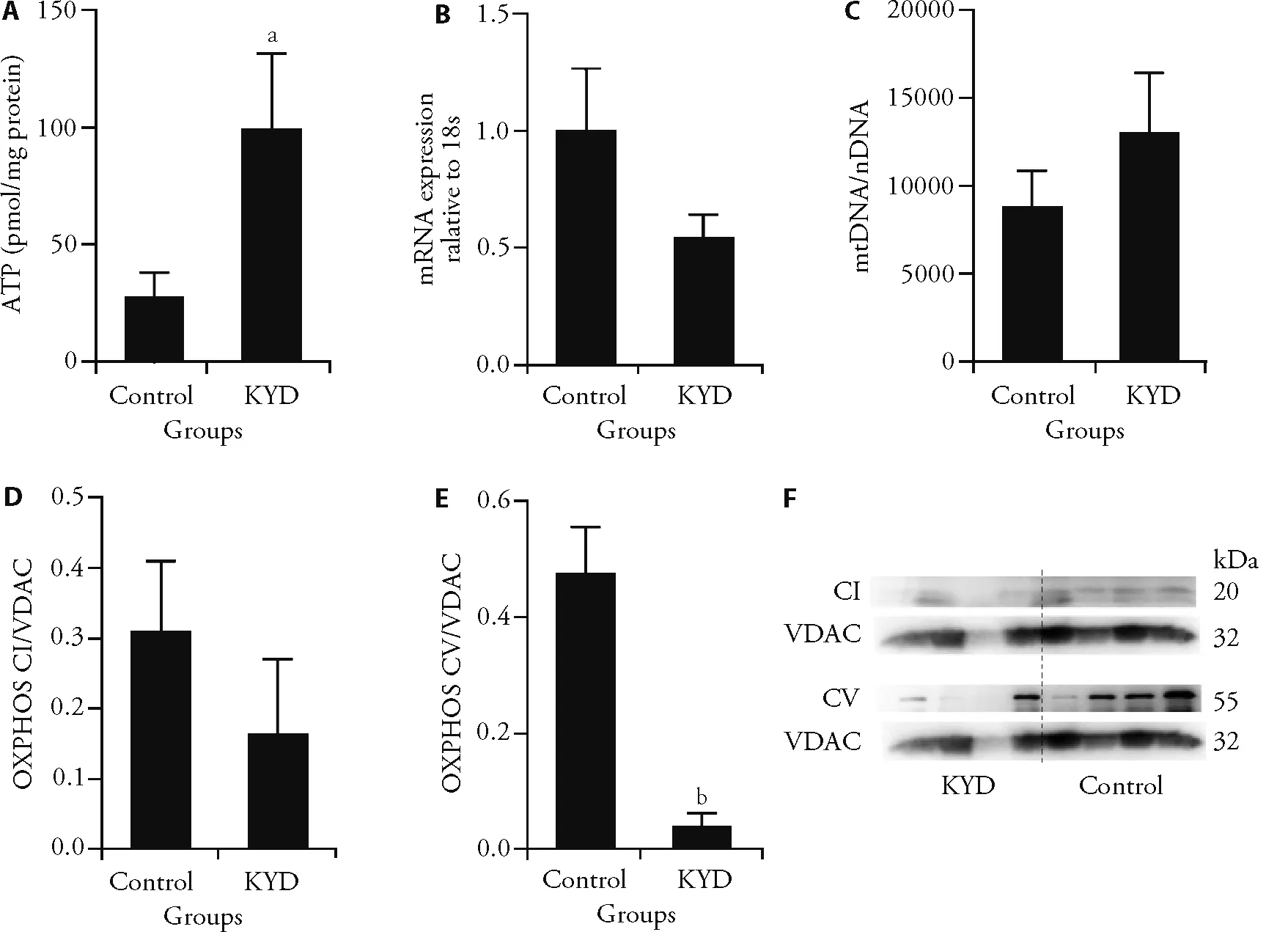
Figure 3 Mitochondrial metabolic markers in the muscle
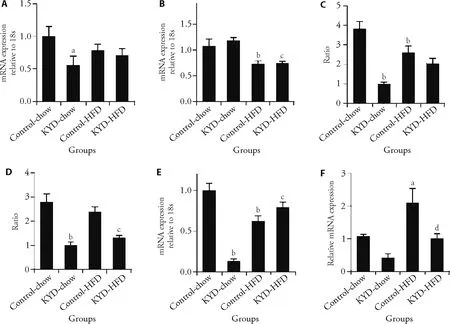
Figure 4 Energy metabolic regulators in the muscle and liver
4.DISCUSSION
As the kidney in Chinese Medicine is considered the‘powerhouse’,the condition of KYD is mostly caused by mitochondrial dysfunction.In this study,we used a clinically relevant rat model to demonstrate that in KYD,markers of the mitochondrial number,biogenesis,and function are differentially regulated in different key heat production organs,which may contribute to the warm preference but unchanged core body temperature.Reduced metabolic main regulator PCG1α may contribute to blood hyperlipidemia.The KYD rats showed a similar phenotype to humans,including warm preference,small birth weight,slow postnatal growth,impaired fertility function,hyperlipidemia,and reduced corticosterone levels,suggesting its clinical relevance.According to the theory of Chinese medicine,KYD is closely related to lifestyle or environment,such as living and working in icy climates.28However,the dietary supplement Huangbai(Cortex Phellodendri Amurensis) is not known to suppress T3,rather,its anti-inflammatory and gonadotropin-releasing hormone suppressing effects.39-41This is may explain reduced corticosterone levels,which is also an anti-inflammatory hormone.However,reduced T3 levels in some patients with KYD may be secondary to reduced energy supply,which is not seen in this rat model.
As the cellular powerhouse in modern science,electron respiration within the mitochondrial OXPHOS complex generate both ATP to fuel the cells,and heat to warm the body.In small rodents and infants,BAT is a discrete organ for heat production;whereas in adult humans,brown adipocytes diffuse into white fat tissue,skeletal muscles,and around major arteries to maintain core temperature.42Here in the KYD rats,ATP production is significantly reduced in BAT,and somehow it was partially compensated by muscle ATP production.In Chinese medicine,Kidney-Yangis the source of energy to promote blood movement.Therefore KYD can affect microcirculation in the limbs resulting in cold extremities.43In humans,cold intolerance in KYD patients mainly occurs in the low limbs and not in the whole body,which may explain why KYD rats prefer warm places,although the core body temperature did not change.
ATP reduction in BAT may be directly attributed to reduced mitochondrial number and biogenesis,reflected by mitoDNA number and PCG1α expression,respectively.In addition,OXPHOS complexes,where electron enters the inner membrane OXPHOS CI and ATP is produced in OXPHOS CV,were both reduced,contributing to reduced ATP production.In the muscle,we observed significantly increased ATP production.However,although the mitochondrial number and OXPHOS CI seem to be unchanged,OXPHOS CV was depressed in the muscle.It is unclear whether this is an outcome of the overproduction of ATP,or it is an outcome of KYD,which requires further investigation.Reactive oxygen species are released as a by-product during ATP production,which is normally consumed by endogenous antioxidants,such as MnSOD and GPx.Thus if MnSOD and GPx are overconsumed,increased oxidative stress can damage the mitochondria and cells.Mitochondrial antioxidant MnSOD and GPx did not show any difference between the Control and KYD groups in both BAT and muscle.Therefore,the differences between mitochondrial biogenesis and functional units are unlikely due to oxidative stress.
Mitochondria have been implicated as key factors regulating reproductive processes.44,45The downregulation of mitochondrial biogenesis marker PGC1α and OXPHOS CV may play some role in reduced litter size in KYD dams.In addition,the healthy mitochondrial function also plays a key role in maintaining nutrient homeostasis,and is the main regulator for both glucose and lipid metabolism.46,47However,reduced PGC1α seems to only affect the blood lipid profile,maybe because of the need for glucose being used to produce ATP in the muscle.Therefore,we postulate that mitochondrial insufficiency and dysfunction is the underlying mechanism of hyperlipidemia observed in patients with KYD.48,49
The highlight of this study is the introduction of a second insult,ad libitumHFD feeding,which confirmed our hypothesis that KYD rats are prone to glucose and lipid metabolic disorders when the obesogenic environment is present.However,KYD rats did not gain as much weight as the Control rats,yet exhibited more severe glucose and lipid disorders,suggesting underlying dysfunction in tissue nutrient metabolism.During the glucose tolerance test,the unchanged serum insulin in the KYD-HFD group suggests the inability of the pancreas to produce more insulin in response to increased energy influx,eg.rising blood glucose levels after meals.The muscle is a major organ for insulin-stimulated glucose deposition.Therefore,we investigated markers related to glucose metabolism in the muscle.Previously,we have found that in rats with glucose intolerance,PGC1a is the main switch for energy sensing and metabolism.Its expression is significantly reduced in skeletal muscle,50,51which was only displayed in KYD-chow rats,whereas there was only a marginal reduction in Control-HFD rats.The change in KYD-chow rats may already determine their poor metabolic capability.Insulin sensing marker PPARγ was suppressed by HFD consumption in both HFD and KYD-HFD rats,which can only explain increased insulin resistance by HFD consumption,but cannot explain why KYD-HFD had more severe glucose intolerance.Then,we further investigated the liver,which plays a key role in both glucose homeostasis and lipid metabolism (especially in the synthesis of cholesterol and major lipoproteins,eg.LDL).What needs to be noted here is the increased gluconeogenesis marker FOXO1 in KYD-HFD rats compared with their chow-fed littermates.Although the level is comparable to a normal level as reflected in the Control-chow rats,such an increase is perhaps sufficient to cause higher blood glucose levels during glucose tolerance test thereafter,more severe glucose intolerance in KYD-HFD rats compared with Control-HFD rats,whose FOXO1 was actually suppressed by HFD feeding.
Another worsened profile in KYD-HFD rats is the blood lipids,ie.LDL and cholesterol.CYP7A1 is an important enzyme to regulate cholesterol metabolism.In humans,CYP7A1 deficiency is directly related to increased circulating cholesterol and LDL levels.52Overexpression of CYP7A1 in the liver can protect against HFD-induced obesity.53Here,we observed a marked increase in this enzyme in HFD-fed mice,which may be an adaptive change to prevent further increases in circulating cholesterol and LDL levels.Interestingly,such adaptation was lost in KYD rats when they consumed the HFD diet,attributed to its low baseline level in the KYDchow rats.As such,dyslipidemia seems more problematic in KYD-HFD rats.Future studies can follow up on the cardiovascular consequence in these rats.
We need to acknowledge the limitation of using rats to model a condition with no clear origins,which represents a partial match to the phenotype of human patients.However,it may still be useful to determine the risk of other conditions,such as vascular damage,and investigate the efficacy of certain treatments to improve mitochondrial function.
In conclusion,the ‘kidney’ in Chinese Medicine seems to be closely related to mitochondrial function.This study showed that mitochondrial dysfunction exists in both BAT and skeletal muscle.Although the function in the BAT can be partially compensated by the muscle,KYD rats still have abnormal temperature sensations and hyperlipidemia.As such,glucose and lipid metabolic disorders have been exacerbated when HFD was introduced.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Effects of the Huangkui capsule (黄葵胶囊) on chronic kidney disease: a systematic review and Meta-analysis
- Effectiveness of moxibustion alone on lumbar disc herniation: a Meta-analysis of randomized controlled trials
- Efficacy of acupuncture therapy for post-stroke fatigue: a systematic review and Meta-analysis
- Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism
- Tilianin extracted from Xiangqinglan (Herba Dracocephali Moldovicae) inhibits apoptosis induced by mitochondrial pathway and endoplasmic reticulum stress in H9c2 cells after oxygenglucose deprivation/reoxygenation
- Comparing the effects of three decoctions for coronavirus disease 2019 on severe acute respiratory syndrome coronavirus 2-related tolllike receptors-mediated inflammations
