Astragaloside IV plays a role in reducing radiation-induced liver inflammation in mice by inhibiting thioredoxin-interacting protein/nod-like receptor protein 3 signaling pathway
2023-02-15DINGYanpingDONGXiaoqingMAYifanCHENLiliZHOUJieLIXinyanSHAOBaoping
DING Yanping,DONG Xiaoqing,MA Yifan,CHEN Lili,ZHOU Jie,LI Xinyan,SHAO Baoping
DING Yanping,DONG Xiaoqing,MA Yifan,CHEN Lili,ZHOU Jie,LI Xinyan,College of Life Science,Northwest Normal University,Lanzhou 730000,China
SHAO Baoping,College of Life Science,Lanzhou University,Lanzhou 730000,China
Abstract OBJECTIVE: To investigate the efficacy of Astragaloside IV (AS-IV) on radiation-induced liver inflammation in mice.METHODS: The mice were divided into normal group,dimethyl sulfoxide solvent group,irradiation group (IR),irradiation+AS-IV (20 mg/kg) group (IR+AS-20) and irradiation+AS-IV (40 mg/kg) group (IR+AS-40).One month after intraperitoneal injection of AS-IV,the mice were irradiated with 8Gry Co60γ,the blood was collected for biochemical analysis,and the liver was collected for hematoxylin-eosin staining,immunofluorescence and electron microscopic observation,oxidative stress,and Western blot analysis.RESULTS: The AS-IV treatment significantly ameliorated the pathological morphology of liver and reduced the alanine aminotransferase and aspertate aminotransferase levels in serum induced by radiation;AS-IV treatment also significantly reduced the expression of inflammatory factors tumor necrosis factor alpha and interleukin 6 and antagonized malonaldehyde content and superoxide dismutase activity in liver caused by radiation;in addition,AS-IV treatment can significantly inhibited the positive expression of thioredoxin-interacting protein (TXNIP) and nod-like receptor protein 3 (NLRP3)inflammasome in liver tissue after radiation;The expression of TXNIP,NLRP3 inflammasome,apoptosisassociated speck-like protein containing a CARD,cysteinyl aspartate-specific proteinase 1 and interleukin 1beta in the AS-IV prevention group decreased significantly compared to the radiation group.CONCLUSIONS: These findings suggested that Co60γ radiation can cause structural and functional damage to the liver,which may be related to the NLRP3 mediated inflammatory pathway;AS-IV may play a protective role by inhibiting the TXNIP/NLRP3 inflammasome signaling pathway in the radiation-induced liver injury model.
Keywords: radiation,ionizing;astragaloside IV;liver inflammation;NLR family,pyrin domain-containing 3 protein;thioredoxin-interacting protein
1.INTRODUCTION
Radiation can cause systemic inflammation.The liver is one of the main target organs susceptible to radiation.Accumulating evidence shows that inflammation and oxidative stress play an important role in radiationinduced liver toxicity and the activation of inflammatory bodies leads to liver damage,inflammation and fibrosis.1,2Thioredoxin-interacting protein (TXNIP) is an important endogenous regulator of cellular redox balance and plays an important role in the pathogenesis of acute liver failure.Recent studies have shown that TXNIP is a key signaling molecule that links oxidative stress to the activation of nod-like receptor protein 3(NLRP3) inflammasomes,and TXNIP activates NLRP3 inflammatory bodies directly under oxidative stress.3,4
The NLRP3 inflammasome is a multiprotein complex,which consisted of the NLRP3 protein,apoptosisassociated speck-like protein containing a CARD (ASC)and precursor caspase-1.5Interestingly,the combination of these factors can result in the production of inflammation molecules,such as interleukin 1beta (IL-1β) and interleukin-18 (IL-18).4
Recent experiments and studies have shown that upregulation of NLRP3 inflammasome have a big impact on radiation damage,which include radiation-induced oral mucositis,radiation-induced skin reactions,radiation-induced lung damage,radiation-induced intestinal injury and radiation-induced changes in other systems.6Down-regulation of TXNIP can inhibit NLRP3 inflammatory body activation and can prevent radiationmediated liver inflammation,7so targeting TXNIP may be a method of treating liver disease.
AS-IV is one of the important active ingredients in Astragalus,which has the effects of anti-radiation,antioxidation,anti-hypertension,anti-inflammatory and antiapoptosis,etc.8,9AS-IV plays a protective role in a variety of liver injury models: AS-IV can effectively protect cisplatin induced liver injury by inducing autophagy and limiting the expression of NLRP3;10Weiet al11used carbon tetrachloride injection to induce rat liver cirrhosis model,the results showed that AS-IV can inhibit the inflammatory response and reduce the expression of collagen;AS-IV pretreatment can protect the liver failure induced by paracetamol by activating the nuclear factor erythroid 2-related factor 2 antioxidant signaling pathway;12AS-IV attenuates endoplasmic reticulum stress and lipid accumulation induced by free fatty acids in liver cells in an AMPK-dependent manner,suggesting that AS-IV can be an effective method for the treatment of hepatic steatosis;13A large number of documents have shown that AS-IV has anti-radiation effect,14,15but whether AS-IV can protect the radiationinduced liver tissue through the TXNIP/NLRP3 pathway has not been reported so far.This study aimed to investigate the mechanism and protective effect of ASIV on radiation-induced liver injury.
2.METHODS
2.1.Animals and treatments
One hundred 30-day-old male Kunming mice (20-25 g)were purchased from the Medical and Laboratory Animal Center of Lanzhou University and housed in pathogen free conditions at 20-25 ℃,with free access to water and food.All procedures complied with the guiding principles for the care and use of animals and were approved by the Committee on Ethics of Animal Experimentation at Lanzhou University (EA2021037).The 100 mice were randomly divided into into five groups: control group (Con),dimethyl sulfoxide (DMSO)group,irradiation group (IR),irradiation group+ASIV (20 mg/kg) group (IR+AS-20),irradiation+ASIV (40 mg/kg) group (IR+AS-40) group.In the DMSO(D8370,Solarbio,Beijing,China) group,DMSO(40 mg/kg per day) was injected intraperitoneally into the mice daily for 30 days.In the AS-IV (Dalian Meilun Biotechnology Co.,Ltd.,Dalian,China,> 98%,BR,CAS NO.MB1955)+IR group,AS-IV (20 or 40 mg/kg per day) was injected intraperitoneally concomitantly for 30 days.On day 31,all mice except the control group received Co60γ radiation (Lanzhou Weite radiation Co.,Ltd.,Lanzhou,China),which the cumulative radiation dose was 8 Gy.In the Control group,saline was given in the same volume intraperitoneally.On day 38,the mice were killed.16Each group was divided into two parts with 10 mice in each group.10 mice were anaesthetized with 10% chloral hydrate,blood was collect from heart,and then the liver were collect after fixed by 4% Paraformaldehyde perfusion through the heart for hematoxylin-eosin (HE) staining and immunofluorescence observation.Another 10 mice were killed by cervical dislocation for electron microscopic observation,oxidative stress and Western blot analysis.
2.2 Blood biochemical analysis
The blood samples were centrifuged at 2000 ×gfor 15 minutes at 4°C to obtain serum samples for detemination the level of alanine aminotransferase (ALT) and aspertate aminotransferase (AST).The samples were sent to the Wuhan Servicebio Technology Co.,Ltd.,for further examination.
2.3 HE,electron microscopy and immunofluorescence observation
Routine HE staining and immunofluorescence staining techniques were used.The liver samples were fixed with 4% paraformaldehyde,embedded in paraffin and made into sections,which were dewaxed with xylene and stained with HE.
Then immunofluorescence staining was performed.Briefly,sections were incubated with primary anti-NLRP3 (1:200;bs-10021R,Bioss,Beijing,China) at 4°C overnight.After washing 5 times with PBS,the sections were incubated with Alexa-488 anti-rabbit secondary antibody (1 ∶200;bs-0295G-AF488,Bioss,Beijing,China).Sections were stained with 2,6-diisopropylaniline (DAPI;D-9106,Bioss,Beijing,China) for 5 min at room temperature for 2 h.
For electron microscopy observation,ultrathin sections of the tissues were obtained and cut into 1 mm spaced pieces,placed in 3% glutaraldehyde phosphate buffer(pH=7.4),and fixed for 2-4 h,followed by 1% osmium tetroxide fixation,ethanol dehydration,and embedding in 812 epoxy resin.Ultrathin sections (60-70 nm) were then obtained from blocks of interest using a ultramicrotome,stained with Uranyl Acetate Lead Citrate stain,and observed with a transmission electron microscopy-1220 (TEM-1220) operated at an accelerating voltage of 80 kV.
2.4 Determination of hepatic oxidative stress
The liver tissue was homogenized in lysis buffer and centrifuged at 10 000 ×gfor 10 min at 4 ℃.Collect the supernatant to detect the content of malondialdehyde(MDA;A003-1-2) and the activity of superoxide dismutase (SOD;A001-1-2) according to the instructions of the kit (Nanjing Jiancheng Bioengineering Institute,Nanjing,China).16
2.5 Western blot analysis
Liver tissues was homogenized in RIPA buffer containing a cocktail of protease inhibitors and centrifuged at 20 000 ×gfor 25 min.Then,the supernatants were harvested,and the protein concentration was quantified by using a BCA protein assay kit.The total protein of sample loading buffer (40 g) was separated by SDS-PAGE followed by PVDF membrane transfer.After blocked in 5% nonfat dry milk for 1 h,membranes were washed with Tris-buffered saline with tween 20 (TBST) for 3 × 10 min.Then,membranes were incubated with the primary antibodies against NLRP3(1:1000),ASC (1:1000;bs-6741R;Bioss,Beijing,China),cysteinyl aspartate-specific proteinase 1(Caspase-1;1:1000;bs-10442R;Bioss,Beijing,China),IL-1β (1:1000;bs-0812R;Bioss,Beijing,China),TXNIP(1:1000;ET1705-72,Huabio,Hangzhou,China),tumor necrosis factor alpha (TNF-α,1:1000;bs-0078R;Bioss,Beijing,China),interleukin 6 (IL-6,1:1000;bs-0782R;Bioss,Beijing,China)and GAPDH (1:1000;K200057M,Solarbio,Beijing,China) overnight at 4 ℃.The membranes were incubated with appropriate secondary antibodies conjugated to horseradish peroxidase (1:2000;SA00001-2,Wuhan,China) for 1 h at room temperature.Color developing agent A and B (34095,ThermoFisher Scientific,Sunnyvale,CA,USA) was mixed at 1:1 ratio and dripped on the membrane for color development.The gray value of the target protein band was analyzed by Image J software.The ratio of target protein band gray value to GAPDH gray value reflected the expression level of target protein.
2.6 Data analysis
Protein data were analyzed by Image J for grey value of the bands.Origin 7.5 was used for mapping,using oneway analysis of variance.All data were subjected to standard error and significance analysis.Data were analyzed using SPSS 17.0 statistical software (SPSS Inc.,Chicago,IL,USA).Measurement data were expressed by mean ± standard deviation (),analyzed by pairedt-test.P< 0.05 was the statistically significant level.
3.RESULTS
3.1.AS-IV alleviates radiation-induced liver injury
Radiation may cause injury to healthy tissue,so we measured the levels of ALT and AST in serum to evaluate the liver function after modelling.In addition,we want to investigate the effect of AS-IV on liver function after radiation.It was evident that the levels of ALT and AST (Figure 1A) in the IR group were significantly higher than those in the control group and the DMSO group (P< 0.001).Compared with the IR group,the levels of ALT and AST were significantly decreased in the IR+AS-IV groups (P< 0.001).The results suggest that AS-IV can effectively restore liver function.
HE staining was carried out in liver tissue (Figure 1B).The result showed that compared with the control group,hepatic sinus and vacuolar degeneration of the liver showed a significantly wider trend in the IR group.However,AS-IV administration reduced pathological features,and the liver histological characteristics of the IR+40 mg/kg AS-IV group and the control group were no difference.Furthermore,transmission electron microscopy was used to observe the morphology of hepatocyte (Figure 1C).Electron microscopy of hepatocytes revealed vesicles of different sizes and shapes.In IR group,the nuclear membrane was indistinct and endoplasmic reticulum was dilated,bile duct lumen enlarged and electron dense substance could be seen in the lumen.Compared with the IR group,the endoplasmic reticulum was significantly decreased in the IR+AS-IV groups.
3.2.AS-IV alleviates radiation-induced inflammatory response and oxidative stress
Here,we explored further the alleviation by AS-IV on the oxidative stress and inflammatory response in radiation-induced liver injury,with the determination of levels of the oxidative stress markers MDA and SOD in liver tissue (Figure 2A),the expression of IL-6 and TNFα in liver as the representative inflammatory cytokines(Figure 2B).The content of MDA in the IR group was significantly higher than that in the control group and DMSO group (P< 0.01).Compared with the IR group,the content of MDA in the IR+AS-IV groups significantly decreased (P< 0.001).Conversely,the content of SOD in the IR group was significantly lower than that in the control group after irradiation (P< 0.001).Compared with the IR group,the content of SOD in the IR+AS-IV groups increased significantly (P< 0.001).In addition,the effect of 40 mg/kg AS-IV administration was better than that of 20 mg/kg AS-IV administration both in MDA and SOD measurement.
Compared with the control group,levels of IL-6 and TNF-α in the IR group increased significantly (P<0.001).Compared with the IR group,IL-6 and TNF-α levels in the liver of the IR+AS-IV groups showed a significant downward trend (P< 0.001),and it was in a dose-dependent manner.These showed that AS-IV can inhibit the levels of radiation-induced inflammatory factors and play a protective role,and has a certain dose dependence.
3.3.AS-IV blocks radiation-induced activation in TXNIP/NLRP3 inflammasome pathway
We further performed immunofluorescence detection on the expression of NLRP3 inflammasome in liver of each group.As shown in Figure 3A,the positive expression of NLRP3 inflammasome is mainly in the cytoplasm.There were few NLRP3 inflammasome positive cells in the control group and DMSO group.The positive expression in the IR group appeared to be higher than that in the control group,DMSO group,and the IR+AS-IV groups.In order to further explore whether AS-IV plays a protective role against radiation-induced liver injury through the TXNIP/NLRP3 inflammasome pathway,we further examined the expression of TXNIP,NLRP3 inflammasome,ASC,Caspase-1 and IL-1β in mouse liver in each group (Figure 3B).The result of Western blot analysis showed that the expression of TXNIP,NLRP3 inflammasome,ASC,Caspase-1 and IL-1β were significantly increased in IR group than in the control and DMSO group (P< 0.01,P< 0.001).However,compared with the IR group,AS-IV administration significantly decreased the expression of these proteins (P< 0.001),and the protective effect of the AS-IV 40 mg/kg group stronger than that of AS-IV 20 mg/kg group.Therefore,these data indicate that AS-IV protect radiation-induced damaged liver by reducing the expression of TXNIP and NLRP3 inflammasome.

Figure 1 AS-IV alleviates radiation-induced liver injury
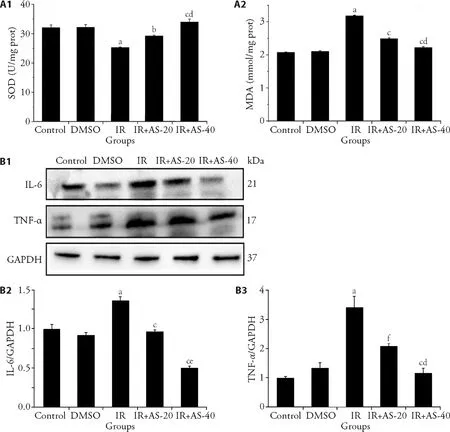
Figure 2 AS-IV alleviates the oxidative damage and the expression of inflammatory factors in radiation-induced liver injury mice

Figure 3 AS-IV blocks radiation-induced activation in TXNIP/ NLRP3 inflammasome pathway
4.DISCUSSION
Ionizing radiation is more and more widely used in our life and it may cause injury to healthy tissue,with both short-term and long-term side effects.As the largest parenchymal organ in human body,liver is sensitive to radiation,so the research on radiation-induced liver injury and its protective mechanism is of great significance.
AS-IV,the bioactive compound derived from Huangqi(Radix Astragali Mongolici),has been indicated to suppress acetaminophen-induced,and cisplatin-induced liver injuries in animal models.10,12AS-IV was also shown to effectively suppressed hepato cellular carcinoma cell proliferation,invasion and anti-apoptosis in vitro.17Our previous studies have shown that AS-IV can effectively reduce radiation-induced apoptosis and senescence of brain cells.16,18However,the protective effect of AS-IV on radiation-induced liver have not yet been elucidated.Therefore,the aim of the present study was to explore the effects of AS-IV against radiationinduced liver injuryin vivo,which provided basic materials for innovative development of anti-radiation active molecules of Traditional Chinese Medicine and health products.
Studies have shown that radiation energy can damage the microstructure and ultrastructure of liver tissue and affect the liver function.Studies have shown that 4.5 GHz radiation from mobile phone electromagnetic field caused serious damage to rat liver tissue: hepatic sinusoidal expansion,focal filtration,reduction of Kupffer cells and hepatic steatosis can be observed in liver slices.19After mature rats were exposed to electromagnetic field of frequency 2.45 GHz,it can be observed that the liver tissue presented moderate hyperemia,hepatic sinus expansion,small inflammatory balloon itis in the liver lobule,and the proliferation of vesicles,lipid droplets and smooth endoplasmic reticulum.20In the liver cell of mice irradiated by low dose (30 and 120 mGy) for 60 weeks,the result showed increased damage to mitochondrial ultrastructure and lipid deposition in hepatocytes.21Ultraviolet irradiation can affect the ultrastructure of rat liver cells involved the reduction of organelles,the expansion of the rough endoplasmic reticulum,the damage of the nuclear membrane,the widening and the vacuolation of the mitochondria in the cytoplasm.22When the body is exposed to an electromagnetic field of 900 MHz,the ALT,AST,urea,creatinine and corticosterone were significantly increased.23Our structural and ultrastructural study showed that AS-IV treatment can significantly ameliorate the pathological morphology of liver.In addition,AS-IV could significantly decrease the level of ALT and AST in serum after radiation suggested that AS-IV could effectively restore liver function in radiation-induced liver injury.
It has been proved that radiation can trigger the production of oxidative metabolites,ionizations,free radicals and ROS reactions of water molecules,and damage the structure and function of DNA,lipids and proteins,leading to metabolic and functional damage.In addition,evaluation of normal tissue toxicity mechanisms suggests that inflammatory responses play an important role in the adverse reactions of early and late IR.24So,inflammation and oxidative stress are the key features in the early stage of liver failure.25,26In fact,studies have shown that AS-IV has a protective effect on liver function in some different disease models.AS-IV can fight inflammation through various pathways,and it can regulate inflammatory cytokines such as TNF-α,IL-1β and other inflammatory mediators related to the NFκB signaling pathway to inhibit inflammatory injury.27In the present experiments,we demonstrated that AS-IV treatment could decrease the level of oxidative stress indicators and the release of inflammatory proteins including TNF-α and IL-6 induced by irradiation.The result is consistent with many reports.24-27Inflammatory responses play an important role in the adverse reactions of ionizing radiation.NLRP3 inflammasome can give rise to the proinflammatory cytokines.Studies have shown that up-regulation of NLRP3 inflammasome have a big impact on radiation damage.5,28The NLRP3 inflammasome is a multiprotein complex consisted of NLRP3,ASC and caspase-1.The first signal that activates the NLRP3 involves the initiation signal,which is induced by the toll-like receptor/nuclear factor NF-κB pathway and up-regulates the expression of NLRP3;the second signal is transduced by various pathogenassociated molecular patterns or injury-related molecular patterns and mitochondrial-derived ROS.The activation of the NLRP3 results in the recruitment of the adaptor protein ASC,which results in the activation of the Caspase-1 precursor into its cleaved form,and then give rise to the proinflammatory cytokines such as IL-18 and IL-1β maturation.28The accumulation of IL-18 and IL-1β acts as a proinflammatory mediator by recruiting neutrophils and accelerating liver pathology.29,30In addition,after the activation of inflammasome,secreted IL-1β and IL-18 induce the expression of some other downstream cytokines such as IL-6,IL-12,TNF-α,and interferon gamma.29These cytokines contribute to innate immune responses to stress or infection resulting in a generalized pro-inflammatory environment.29,30Several studies have also shown that AS-IV can inhibit NLRP3 inflammasome signaling in different models,31,32but its effect on NLRP3 inflammasome in radiation-induced liver injury model has not been reported so far.In this experiment,we tested the expression of NLRP3 fluorescence and the protein levels of NLRP3,ASC,Caspase-1,and IL-1β,the results showed that the radiation caused the increase of the expression of the above proteins,and the AS-IV prevention group can significantly reduce the trend.The results indicated that AS-IV can inhibit the activation of NLRP3 inflammasome in radiation-induced liver injury model.TXNIP is the endogenous negative regulator in TRX system belonging to α-arrestin protein family.TXNIP is widely expressed almost in all normal tissue cells33that can mediate oxidative stress,inhibit cell proliferation,and induce apoptosis by inhibiting the function of the thioredoxin system.34Many different cellular stress factors positively or negatively regulate TXNIP expression.These include UV light,heat shock,hypoxia,ROS,nitric oxide,transforming growth factor β,calcium channel blockers,etc.35Several previous studies have also shown that exposure to ultraviolet-A radiation might increase ROS,which could potentially decrease the activity of Trx associated with enhanced expression of TXNIP protein.35,36TXNIP regulates inflammatory responses by modulating different switch points along inflammatory cascades in different cell populations.However,there is accumulating evidence suggesting that the all-inclusive mechanism describing TXNIP proinflammatory role is the direct activation of NOD-like receptor protein 3 (NLRP3) inflammasomes in several cell types.33TXNIP is a key signaling molecule that links oxidative stress to the activation of NLRP3 inflammasomes.37Studies have shown that in the presence of excess ROS,TXNIP interaction with TRX can be reversed,which then allows released TXNIP to bind and activate NLRP3 inflammasome,resulting in enhanced inflammation.6,35It should also be noted that the combination of TXNIP with TRX or NLRP3 is competitive,and the activation of caspase-1 is not completely blocked in absence of TXNIP.This indicates the existence of other pathways (along with ROS pathway) regulating the inflammasome activity initiating inflammatory response with TXNIP as a major key role player.38Some studies have demonstrated that AS-IV protected cell from inflammation,and some molecular mechanisms,such as anti-oxidative and TXNIP/NLRP3 pathways,have been proposed.39In the present study,we observed increased levels of ROS,expression of TXNIP,NLRP3 inflammasome and its downstream factors in liver after the body exposed to irradiation,but AS-IV treatment reversed these alternations,demonstrating the inhibitory effect of AS-IV on TXNIP/NLRP3 NLRP3 inflammasome activation.
In conclusion,our findings suggest that AS-IV might be a potential protector against radiation-induced excessive inflammation and oxidation in liver via the suppression of the excessive activation of the TXNIP/NLRP3 inflammasome signals.This study provided theoretical evidence for the clinical usage of AS-IV for radiation damage therapy.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Effects of the Huangkui capsule (黄葵胶囊) on chronic kidney disease: a systematic review and Meta-analysis
- Effectiveness of moxibustion alone on lumbar disc herniation: a Meta-analysis of randomized controlled trials
- Efficacy of acupuncture therapy for post-stroke fatigue: a systematic review and Meta-analysis
- Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism
- Tilianin extracted from Xiangqinglan (Herba Dracocephali Moldovicae) inhibits apoptosis induced by mitochondrial pathway and endoplasmic reticulum stress in H9c2 cells after oxygenglucose deprivation/reoxygenation
- Comparing the effects of three decoctions for coronavirus disease 2019 on severe acute respiratory syndrome coronavirus 2-related tolllike receptors-mediated inflammations
