Astragaloside IV ameliorates insulin induced insulin resistance in HepG2 cells through reactive oxygen species mediated c-Jun Nterminal kinase pathway
2023-02-15YEXiaomeiXINGXiaoweiYUANKangruiWANGDongmingWUDuduCHENZhiYUZhiqiang
YE Xiaomei,XING Xiaowei,YUAN Kangrui,WANG Dongming,WU Dudu,CHEN Zhi,YU Zhiqiang
YE Xiaomei,XING Xiaowei,YUAN Kangrui,WU Dudu,CHEN Zhi,School of Pharmacy,Guangdong Medical University,Dongguan 523808,China
YE Xiaomei,Department of Clinical Pharmacy,SSL Central Hospital of Dongguan City (the Third People's Hospital of Dongguan City),Dongguan 523326,China
YU Zhiqiang,School of Pharmaceutical Sciences,Guangdong Provincial Key Laboratory of New Drug Screening,Southern Medical University,Guangzhou 510515,China
WANG Dongming,Department of Pharmacy,Central People's Hospital of Zhanjiang,Zhanjiang 524037,China
Abstract OBJECTIVE: To investigate the effects and elucidate the mechanism of Astragaloside IV (AS-IV) for insulin resistance (IR) and type 2 diabet es mellitus (T2DM).METHODS: CCK8 kit was used to detect cell viability,glucose detection kit was used to detect the concentration of glucose in cell supernatant,reactive oxygen species(ROS) detection kit and Western blot were used to explore the mechanism of Astragaloside IV (AS-IV) in improving IR.A diabetic rat model was also established by feeding high sugar and fat diet and streptozotocin(STZ) injection.After treatment with AS-IV,rosiglitazone(ROZ),or normal saline,the fasting blood glucose (FBG),C peptide (C-P),tumor necrosis factor-α (TNF-α),interleukin-6 (IL-6) and the glucose tolerance were assessed.RESULTS: AS-IV could effectively reduce the content of ROS and increase the glucose uptake in high insulintreated IR-type HepG2 cells.The results of molecular mechanisms indicated that AS-IV could improve insulin resistance by reducing JNK phosphorylation and regulating c-Jun N-terminal kinase (JNK) downstream protein expression.Additionally,AS-IV could significantly reduce the levels of FBG,TNF-α,IL-6 and the glucose tolerance in diabetic rats (P < 0.05 or < 0.01).The high and medium dose groups of AS-IV could significantly increase the C-P levels in diabetic rats (P < 0.05 or <0.01).CONCLUSIONS: Our results indicated that AS-IV improve liver IR through the JNK pathway and ROS,which meant a new molecular target for the treatment of diabetes.The AS-IV also helped to prevent and improved the insulin resistance of rats.
Keywords: Astragaloside IV;Insulin resistance;Glucose consumption;ROS;JNK;HepG2 cells
1.INTRODUCTION
In recent years,the prevalence and incidence of diabetes worldwide had risen sharply.Meanwhile,diabetes was increasingly becoming a public health problem around the world and posed a serious threat to human health.1Among them,insulin resistance (IR)-based type 2 diabetes mellitus (T2DM) accounted for more than 90%of diabetic patients.The response to insulin was decreased in T2DM and IR,mainly due to reduction of glucose uptake in insulin-sensitive tissues (liver,muscle,adipose tissue) and the inhibition of hepatic glucose output.2,3Furthermore,studies have illustrated that oxidative stress in the body promoted the development and progression of T2DM and IR.Meanwhile,reactive oxygen species (ROS) was an important factor in causing oxidative stress.4For example,Charlotteet al5found that ROS generation and oxidative stress activation generation,were proposed contributors to the onset of skeletal muscle.Akashet al6proposed that oxidative stress and ROS were responsible for impaired glucose utilization,abnormalities in hepatic glucose and causing T2DM and IR ultimately.These findings suggested that,as one of the essential factor in oxidative stress,the accumulation of ROS could lead to T2DM and IR.
On the other hand,the mainstay of treatment for IR was oral insulin sensitizers such as rosiglitazone (ROZ),pioglitazone and so on.7However,these drugs could cause anemia,edema,and side effects of liver and kidney damage in clinical applications.8Recently,Chinese medicine had received extensive attention in the treatment of T2DM due to its unique advantages,with minimal adverse effects and low toxicity.9Astragaloside-IV (AS-IV) was one of the main active ingredients extracted from medicinal Huangqi (Radix Astragali),which had diverse pharmacologic actions,such as antiinflammatory,anti-oxidation,and improved endothelial cells and angiogenesis.10In recent years,AS-IV was also used has been reported to exert hypoglycemic pharmacological and antidiabetic effects.Lǖet al11reported that AS-IV could inhibit liver glycogen phosphorylase (GP) and glucose-6-phosphatase (G6P)activity,thereby reducing blood glucose levels in diabetic mice.And Yinet al12indicated that AS-IV had an effects on regulating blood glucose.However,the molecular mechanism about the hypoglycemic effects on AS-IV were still not clear.Therefore,in this paper,the hypoglycemic effect of AS-IV were studied,and the molecular mechanism of AS-IV was further explored to improve IR.
2.MATERIALS AND METHODS
2.1.Animals
Forty-eight male Sprague-Dawley (SD) rats (8 weeks old)of specific pathogen-free grape,weighing (190 ± 10 g),were purchased from Southern Medical University Laboratory Animal Center (certification number SCXK2016-0041;Guangzhou,China).The study protocol was approved by the Guangdong Medical University Animal Care Committee.
2.2.Reagents and antibodies
Human HepG2 cells were obtained from China-America Cancer Research Institute of Guangdong Medical University (Dongguan,China).High-glucose Dulbecco’s modified Eagle’s medium (DMEM) and Fetal calf serum (FBS) were purchased from Gibco (Carlsbad,CA,USA).MTT Cell Proliferation and Cytotoxicity Assay Kit,Reactive Oxygen Species (ROS) Assay Kit,and Secondary Antibody Dilution Buffer were purchased from Beyotime (Beijing,China).Glucose assay kit was purchased from Shanghai RongShengBiological Technology Co.,Ltd.(Shanghai,China).Dimethyl sulfoxide (DMSO),Radio-Immun-oprecipitation Assay(RIPA),Phenylmethanesulfonyl fluoride (PMF),SDSPAGE Gel Kit were obtained from Solarbio (Beijing,China).Rosiglitazone (ROZ) was purchased from Shanghai Yuanye Biological Technology Co.,Ltd.(Shanghai,China).BCA protein assay kit was purchased from Thermo (Waltham,MA,USA).JNK,phospho-JNK(Thr183/Tyr185).Pyruvate dehydrogenase kinase(PDK1),Protein kinase B (AKT) and glycogen synthase kinase 3β (GSK-3β) antibodies were obtained from Cell Signaling (Shanghai,China).Streptozotocin (STZ) was provided by Sigma-Aldrich (Steinheim,Germany).Interleukin-6 (IL-6),tumor necrosis factor-α (TNF-α)and C peptide (C-P) radio immunoassay detection kit were bought from Kainuo Bio Ltd.(Beijing,China).
2.3.Cell culture and insulin treatment
Human HepG2 cells were grown in DMEM supplemented with 10% FBS and the following two antibiotics: penicillin and streptomycin (50 mg/L).The cells were maintained at 37 ℃ in a humidified atmosphere of 5% CO2.A total of 80% influent cells were inoculated into 96-well plates (1.0 × 104cells/well),and assigned into control group,model group,treatment group and positive group.The model group was treated with 1.0× 10-6M insulin for 24 h.The treatment group and positive group were cultured with AS-IV (0.1,5 and 100 μM) and rosiglitazone (30 μM) respectively after being pretreated with 1.0 × 10-6M insulin for 24 h.
2.4.Cell viability assay
HepG2 cells were inoculated in 96-well plates at a density of 3 × 104cells/well,the medium was replaced after 24 h and the cells were treated with AS-IV (0.1,1,5,10,100 and 150 μM) for 24 h.Cell viability was assessed by MTT assay.After the treatment of AS-IV,20 μL MTT (5 mg/mL) was added into each well to be incubated further for 4 h at 37 ℃.Next,medium containing MTT in each well was removed and 100 μL DMSO was added to dissolve the formazan crystals.Absorbance was measured at 490 nm using a microplate reader and all the measurements about cell viability were performed in triplicate.
2.5.Glucose consumption
After the treatment of insulin and AS-IV for 24 h in 96-well plates,HepG2 cells were washed with PBS and cultured in DMEM.Glucose concentration in the medium was under determination by the glucose assay kit.On the other hand,glucose consumption was calculated using the difference between initial glucose concentration and remaining glucose concentration in the medium.
2.6.Measurement of ROS production
For the detection of intracellular ROS level,3 × 105cells were incubated in 6-well plates and replaced into the serum-free medium containing DCFH-DA after the treatment of insulin and AS-IV.After calculating for 30 min at 37 ℃,the cells were collected and fluorescent intensity was detected by flow cytometry at the condition of excitation wavelength in 488 nm and emission wavelength in 525 nm.
2.7.Western blotting analysis
HepG2 cells were washed in cold PBS and lysed in RIPA buffer containing PMSF.The protein concentration from the whole cells extracts was determined using BCA protein assay kit.Equally 90 μg protein was separated on 6% to 12% SDS-PAGE Gel Kit and transferred onto PVDF membrane.The blots were blocked in blocking buffer of 5% skim milk for 2 h,and washed with PBS buffer for three times.Subsequently,membranes were incubated overnight at 4 ℃ with the primary antibodies.The following day,the secondary antibodies were diluted 1:5000 in Secondary Antibody Dilution Buffer and then the membranes were incubated with the secondary antibodies for 2 h at room temperature.To measure the expression of different proteins,β-actin was used as a loading control.The results about the densities of the bands were analyzed using Image J software.
2.8.Animals and treatment
Forty eight SD rats were assigned randomly to six groups by random number table method after one week's acclimation: control group (N),model group (M),lowdose AS-IV group (A1,0.01 g/kg),medium-dose ASIV group (A2,0.04 g/kg),high-dose AS-IV group (A3,0.08 g/kg),and ROZ group (X,5 mg/kg) (n=8 rats per group).The control group was provided conventional feed,and the remaining groups accepted high sugar and fat diet for 6 weeks.The rats with high sugar and fat feed were subsequently fasted for 12 h and intraperitoneally injected with streptozotocin (STZ) solution (35 mg/kg).It was considered to be diabetic with random blood glucose (RBG) ≥ 16.7 mmol/L after 72 h.Afterwards,all rats were then treated with corresponding drugs intragastrically (ig) for 8 weeks.The normal group and the model group were offered the equal volume of normal saline.13
After 8 weeks of intragastric administration,the rats were fasted for 12 h and the blood from rat tail vein was collected for fasting blood glucose (FBG) measurement with glucose meter.The IL-6 and TNF-α levels were detected by ELISA Kit,and the C peptide (C-P) levels were measured using the radio immunoassay detection following manufacturer's instructions respectively.14
After 8 weeks of intervention,the oral glucose tolerance test (OGTT) was performed.The rats were fasted for 12 h and orally treated with glucose (2 g/kg).The blood glucose levels at 0,0.5,1,and 2 hours after gavage were measured and the area under the curve(AUC) was measured: AUC=(0 h blood glucose+0.5 h blood glucose) × 0.5/2+(0.5 h blood glucose+2 h blood glucose) × 1.5/2.15
2.9.Statistical analysis
The data were calculated as the mean ± standard deviation unless otherwise indicated.All experiments were repeated in three independent trials.The one-way analysis of variance (ANOVA) test was performed to determine the differences between experimental groups,andP <0.05 was considered to be statistically significant.
3.RESULTS
3.1.Effects of AS-IV on the viability of HepG2 cells
In order to investigate the effects of AS-IV treatment on HepG2 cells viability,HepG2 cells were exposed to various concentrations of AS-IV for 24,48,72 or 96 h,and detected using a MTT assay.As demonstrated in Figure 1,the cells viability in 0.1,5,10 and 100 μM of AS-IVtreated cells was slightly affected compared with the control cells.The low cells viability was found in 150 μΜ AS-IV-treated cells which compared with the control cells (P <0.05).Furthermore,the IC50 values of AS-IV in HepG2 cells were calculated to be (179.6 ± 3.2) μM(24 h),(173.4 ± 3.7) μM (48 h),(167.1 ± 2.0) μM (72 h),and (139.5 ± 2.6) μM (96 h).
3.2.Effects of AS-IV on glucose consumption in insulin resistance HepG2 cells
The effects of AS-IV on glucose consumption was investigated on the insulin resistant HepG2 cell model(Figure 2).It was discovered that AS-IV at 5 and 10 μM significantly improved glucose consumption compared with the insulin resistant HepG2 cell in the IR-HepG2 cell model (P <0.05).ROZ treatment at the dose of 30 μM also induced a significant reduction in the supernatant glucose concentration (P <0.05).However,the glucose consumption of the 0.1 μM AS-IV treatment was similar to the control (P <0.05) and the high dose of AS-IV(100 μM) reduced the glucose consumption compared with the insulin resistant cell model (P <0.05).These results indicated that AS-IV displayed a dose-dependent manner in ameliorating the insulin resistance status in the IR-HepG2 cells.
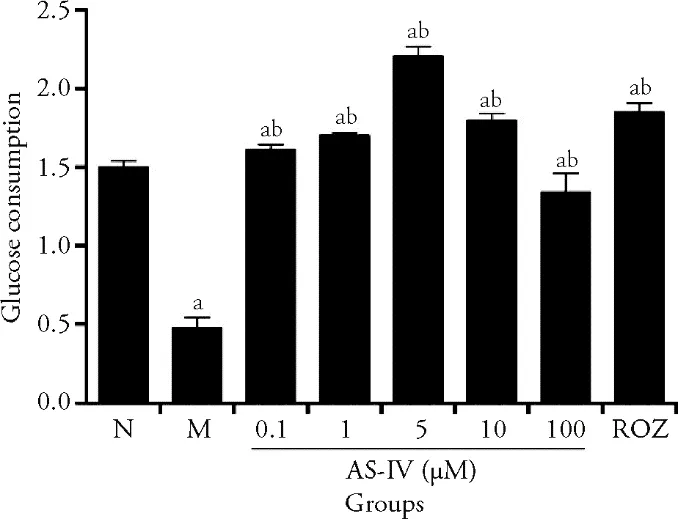
Figure 2 Effects of AS-IV on ameliorating glucose consumption in insulin resistance
3.3.Effects of AS-IV on the FBG,IL-6,TNF-α and C-P levels in diabetic rats
Compared with the normal group,the levels of FBG,TNF-α,and IL-6 in the model group were increased significantly (P <0.01),and the level of C-P was descended obviously (P <0.01).Compared with the model group,the levels of FBG,TNF-α,and IL-6 in all treatment groups were remarkably reduced (P <0.05 or<0.01).The C-P levels in the high and medium dose groups of AS-IV and the ROZ group were significantly higher than the model group (P <0.05 or<0.01).On the contrary,there were no significant differences in the C-P levels between the low dose group of AS-IV and the model group (P> 0.05) (Table 1).
3.4.Effects of AS-IV on the glucose tolerance in diabetic rats
The blood glucose levels of the model group at 0,0.5,1,2 and 3 h were significantly higher than the control group(P <0.01).The blood glucose levels of the intervention group (the high,medium,low dose groups of AS-IV,and the rosiglitazone group) at 0,0.5,1,2 and 3 h significantly lower than the model group (P< 0.05 or<0.01).The AUC of the model group increased greatly compared with the control group (P< 0.01).Compared with the model group,the AUC of the high,medium and low dose groups of AS-IV,and the rosiglitazone group reduced significantly (P <0.01) (Table 2).
Table 1 Comparison of the levels of FBG,C-P,TNF-α,IL-6 in different groups ()
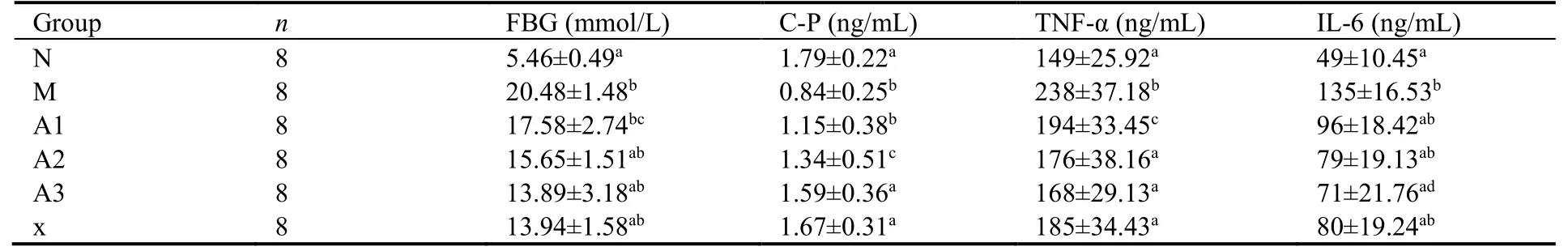
Table 1 Comparison of the levels of FBG,C-P,TNF-α,IL-6 in different groups ()
Notes: N: control group,given conventional feed without other interfering factors.M: model group,given high sugar and fat feed but no other interfering factors.A1: intervention group,fed high sugar and fat as well as low doses of AS-IV;A2: intervention group,fed high sugar and fat feed and medium doses of AS-IV;A3: intervention group,fed high sugar and fat feed and high doses of AS-IV;X: rosiglitazone group,fed high sugar and fat feed and rosiglitazone (200 mg/kg);FBG: fasting blood glucose;C-P: C peptide;TNF-α: tumor necrosis factor-α;IL-6:interleukin-6;compared with the model group,aP < 0.01,cP < 0.05,compared with the control group,dP < 0.05,bP < 0.01.
Table 2 Comparison of the levels of blood glucose during OGTT in different groups ()
Notes: N: control group,given conventional feed without other interfering factors.M: model group,given high sugar and fat feed but no other interfering factors.A1: intervention group,fed high sugar and fat as well as low doses of AS-IV;A2: intervention group,fed high sugar and fat feed and medium doses of AS-IV;A3: intervention group,fed high sugar and fat feed and high doses of AS-IV;X: rosiglitazone group,fed high sugar and fat feed and rosiglitazone (200 mg/kg);AUC: the area under the curve;OGTT: oral glucose tolerance test;compared with the model group,aP < 0.01,cP < 0.05,compared with the control group,bP < 0.01.
3.5.Effects of AS-IV on ROS level in insulin resistance HepG2 cells
For IR-HepG2 cells model,insulin pretreatment for 24 h increased the intracellular ROS content compared with the control group (Figure 3,P <0.05),AS-IV and ROZ treatment reduced the intracellular ROS content compared with the insulin-pretreated group (Figure 3,P<0.05).These results suggested that AS-IV could ameliorate IR by decreasing the intracellular ROS accumulation.
3.6.Effects of AS-IV on the phosphorylation of JNK in insulin-resistant HepG2 cells
Since it has been found that ROS was involved in the induction of the JNK pathway,16,17the effects of AS-IV on the phosphorylation of JNK were investigated (Figure 4 A,B).The pretreatment of cells with 1.0 × 10-6μM insulin markedly increased the phosphorylation of JNK compared with the control group (P <0.05).When ASIV (0.1,5 and 100 µM) and ROZ (30 μM) were added,the insulin-induced increase of JNK phosphorylation was significantly decreased compared with the IR-HepG2 cells model group (P <0.05).
3.7.Effects of AS-IV on PDK1 in insulin-resistant HepG2 cells
When cells were pretreated with 1.0 × 10-6μM insulin for 24 h,the protein expression levels of PDK1 was higher than these in the control group (Figure 4 C,D,P <0.05).After being subsequently treated by AS-IV (0.1,5 μM)and ROZ (30 μM) for 24 h,the protein level of PDK1 in HepG2 cells increased compared with that in insulinpretreated group (Figure 4 C,D,P <0.05).
3.8.Effects of AS-IV on AKT in insulin-resistant HepG2 cells
As a downstream molecule of phosphatidylinositol 3 kinase (PI3K),Akt was involved in the regulation of a variety of signaling pathways,including the insulin signaling pathway.18Furthermore,high expression level of Akt protein was found in insulin-sensitive tissue skeletal muscle,fat and liver.As can be seen in Figure 4 E,F,the pretreatment of cells with insulin downregulated the AKT level compared with the control group(P <0.05).When AS-IV (0.1,5 and 100 µM) was added,the insulin-induced decrease of AKT was significantly increased compared with the IR-HepG2 cells model group (P <0.05),while ROZ did not up-regulated compared with insulin resistant cells model group (P <0.05).The results of AKT level indicated that AS-IV could correct the down-regulation of AKT level in IRHepG2 cells.
3.9.Effects of AS-IV on GSK3β in insulin-resistant HepG2 cells
As shown in Figure 4 G,H,GSK3β was increased in cells with the pretreatment of insulin compared with the control group (P <0.05).Meanwhile,both AS-IV and ROS could significantly decreased the insulin-induced increase of GSK3β compared with the IR-HepG2 cells model group (P <0.05).
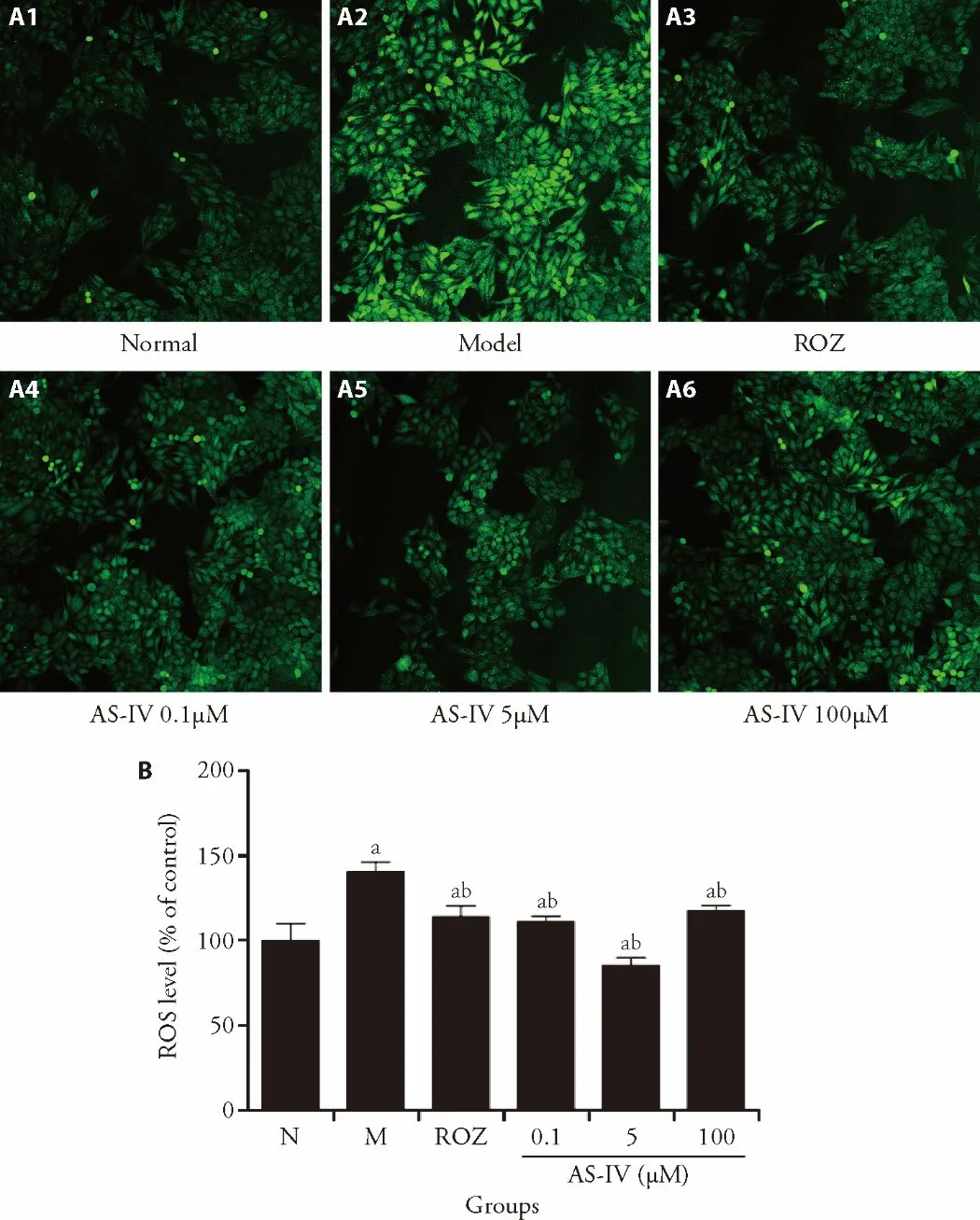
Figure 3 AS-IV ameliorates ROS accumulation in HepG2 cells
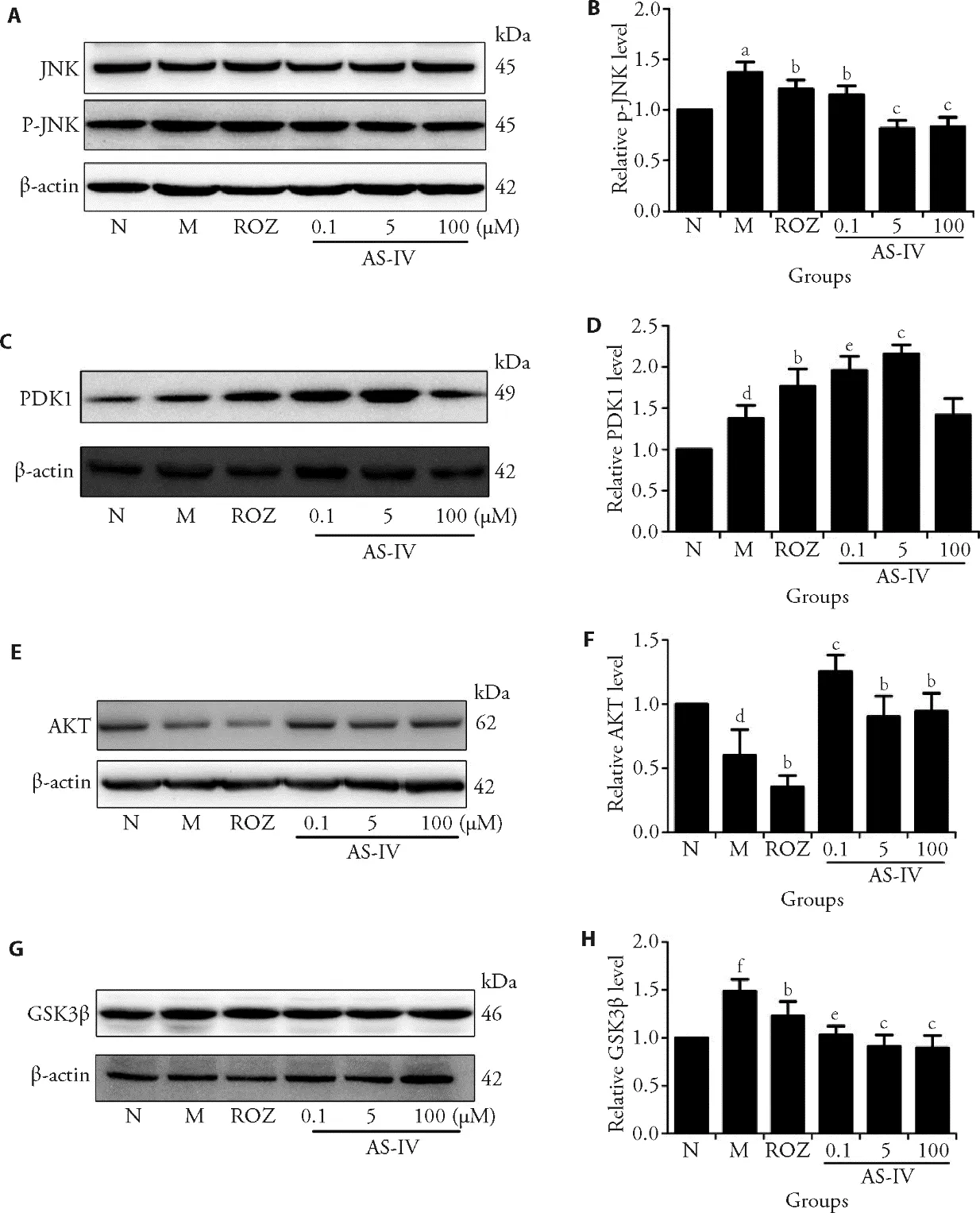
Figure 4 Effects of AS-IV on regulation of JNK-AKT-GSK3β signaling pathway in insulin-resistant HepG2 cells
4.DISCUSSION
In this study,we investigated the effects of AS-IV on regulating the ROS accumulations in insulin-resistant HepG2 cells,and elucidated the underlying mechanism for the effects.It was found that AS-IV attenuated IR and ROS accumulation in HepG2 cells.AS-IV could reduce phosphorylated-JNK thus causing positive or negative regulation of proteins in the corresponding insulin signaling pathway,and ultimately regulating intracellular glycogen synthesis to regulate blood sugar.These findings proved that AS-IV may act as a promising therapeutic agent for treating hepatic insulin resistance.It is known that IR is closely associated with oxidative stress.19Due to the oxidative stress,the excessive production of ROS could not only cause JNK phosphorylation through insulin signaling pathway,but also interfere with cellular insulin receptor signal transduction and downstream signaling pathways,and then affect the utilization of sugar ultimately.
In our study,a reduced uptake of glucose,an increased in ROS level and a phosphorylation of JNK were found in HepG2 cells that were exposed to high concentrations of insulin,which was in agreement with the results of the study carried out by Gaoet al.20After treatment with ASIV,the glucose uptake was increased and ROS levels were decreased in high concentrations of insulin-induced HepG2 cells,and AS-IV could reduce JNK phosphorylation levels.In addition,AS-IV could significantly reduce the levels of FBG,TNF-α,IL-6 and the glucose tolerance,and increase the C-P levels in diabetic rats.These results demonstrated that AS-IV may further regulate the insulin signaling pathway by affecting ROS and P-JNK,thereby increasing the utilization of glucose and improving insulin resistance.On the one hand,our study confirmed the role of ROS in hepatocyte IR,and investigated the effects of AS-IV on JNK expression and phosphorylation in insulin-resistant HepG2.JNK played a crucial role in insulin signaling pathway in insulin-resistant HepG2 cells,due to the phosphorylation of JNK caused by stress.For example ROS,could significantly down-regulate the expression of IRS-1.21Also,the resulting abnormal expression of downstream proteins in insulin signaling pathways would cause insulin resistance.22Our study showed that AS-IV could reverse the expression and phosphorylation of JNK,and the underlying mechanism might be that ASIV could reduce the excessive ROS production.
On the other hand,GSK-3β had been researched,and it is an important function to maintain glucose homeostasis by negatively regulating glycogen synthesis in the insulin signaling pathway.23As an important substrate for AKT,the activity of GSK-3β was negatively regulated by AKT.24Here,it was observed that the expression of AKT was downregulated and GSK-3β was up-regulated in HepG2 cells that were exposed to high concentrations of insulin,which was consistent with the previous researches.25After the treatment of AS-IV,the abnormal expression of AKT and GSK3β could be reversed and regulate the glucose homeostasis.The underlying mechanism might be that AS-IV affected the JNK-AKTGSK3β cell pathway.
Based on our findings,AS-IV could be used to improve insulin resistance.Meanwhile,its pharmacological mechanism is related to the reduction of ROS,and regulates glycogen synthesis by JNK-AKT-GSK3β insulin signaling pathway.Since ROS could activate various stress kinases and multiple oxidative stress sensitive signaling pathways through the second messenger signaling molecule function,it was agreed that Chinese herbs could be applied to a wider range of human diseases with the further research on the antioxidant stress and oxygen free radical elimination.
杂志排行
Journal of Traditional Chinese Medicine的其它文章
- Effects of the Huangkui capsule (黄葵胶囊) on chronic kidney disease: a systematic review and Meta-analysis
- Effectiveness of moxibustion alone on lumbar disc herniation: a Meta-analysis of randomized controlled trials
- Efficacy of acupuncture therapy for post-stroke fatigue: a systematic review and Meta-analysis
- Efficacy of luteolin on the human gastric cancer cell line MKN45 and underlying mechanism
- Tilianin extracted from Xiangqinglan (Herba Dracocephali Moldovicae) inhibits apoptosis induced by mitochondrial pathway and endoplasmic reticulum stress in H9c2 cells after oxygenglucose deprivation/reoxygenation
- Comparing the effects of three decoctions for coronavirus disease 2019 on severe acute respiratory syndrome coronavirus 2-related tolllike receptors-mediated inflammations
