Maleness-on-the-Y (MoY) orthologue is a key regulator of male sex determination in Zeugodacus cucurbitae (Diptera: Tephritidae)
2023-02-03FANZizhenMAQinMASiyaCAOFengqinYANRihuiLINXianwu
FAN Zi-zhen ,MA Qin ,MA Si-ya ,CAO Feng-qin ,,YAN Ri-hui,,LIN Xian-wu
1 Key Laboratory of Green Prevention and Control of Tropical Plant Diseases and Pests of Ministry of Education,Hainan University,Haikou 570228,P.R.China
2 School of Plant Protection,Hainan University,Haikou 570228,P.R.China
3 School of Life Sciences,Hainan University,Haikou 570228,P.R.China
4 Hainan Yazhou Bay Seed Lab,Sanya 572000,P.R.China
Abstract The initiation of sex differentiation in insects is regulated by primary sex determination signals.In the Medfly Ceratitis capitata and other Tephritids,Maleness-on-the-Y (MoY) is the master gene for male sex determination.However,the primary signal in Zeugodacus cucurbitae (Coquillett),a very destructive Tephritid pest across the world,remains ambiguous.In this study,we have isolated and characterized the Medfly MoY homolog in Z.cucurbitae,ZcMoY.ZcMOY protein shows high sequence conservation to its homologs in Bactrocera species.ZcMoY transcription begins and peaks at very early embryonic stages and then becomes undetectable except the testes and heads of day 1 male adults.Silencing ZcMoY in early embryos by RNAi causes abnormal external genitalia and interior reproductive organs,giving rise to intersexes and feminization of XY individuals.The expression pattern and knockdown phenotypes of ZcMoY indicate that ZcMoY plays a key role in regulating sex determination of Z.cucurbitae males.Our findings will help the understanding of sex determination in Z.cucurbitae and facilitate the development of genetic sexing strains in its biological control.
Keywords: MoY,Zeugodacus cucurbitae,sex determination,RNA interference,reproductive system
1.Introduction
Sex determination initiates the process of a gene regulatory cascade responsible for sexual differentiation in developing embryos,generating the distinct sexual dimorphic phenotypes and reproductive traits.Although sex determination is an essential component for insect development and reproduction,insects have evolved diverse primary signals to trigger sex determination even in closely related species.This is exemplified by dipteran insects,such as the insect model organismDrosophila melanogasterand the emerging model organism of Tephritidae speciesCeratitis capitata(Medfly).Their primary signals are the ratio of X chromosome to autosome and the dominant male determining factor (M factor),respectively.
The sex determination mechanism ofD.melanogasterhas been studied in detail.The primary sex-determining signal inDrosophilais provided by the ratio of X chromosomes to autosomal sets (X:A ratio).When this ratio is 1 (XX:AA),Sex-lethal(Sxl),a sex determination master gene,is activated and a female-specific functional SXL protein is produced.SXL protein can direct the female-specific splicing oftransformer(tra),generating a functional TRA protein.TRA binds to an RNA-binding protein Transformer 2 (TRA-2),which is encoded by the genetransformer 2(tra-2) that is expressed in both sexes,and forms a TRA/TRA-2 complex to regulate female-specific splicing ofdoublesex(dsx) pre-mRNA.Consequently,the female-specific DSX protein DSXFdirect embryos to female differentiation.On the contrary,Sxlis not activated when the ratio is 0.5 (X:AA),resulting in a truncated nonfunctional SXL peptide.The absence of functional SXL protein promotes male-specific splicing oftra,producing truncated nonfunctional TRA peptide and leading to no formation of TRA/TRA-2 complex and the male-specific splicing ofdsxthat promotes male sexual development (Boggset al.1987;Hoshijimaet al.1991).
In contrast toDrosophila,dominant male determining factor (M factor) is the primary determinant of male differentiation in non-Drosophilidae species,such as the Tephritid fruit flyC. capitata,Bactrocera dorsalis(Hendel)(Meccarielloet al.2019),the mosquitoesAedes aegypti(Hallet al.2015) andAnopheles gambiae(Krzywinskaet al.2016),and the houseflyMusca domestica(Sharmaet al.2017).In the Medfly,Sxl,however,is expressed in both sexes regardless of whether the M factor is present or absent (Sacconeet al.1998),suggesting thatSxldoes not appear to have a switch function as inDrosophila.Recently,the primary signal M factor:Maleness-onthe-Y(MoY) has been discovered inC.capitata.In XX females ofC.capitata,the female-specific splicing oftraproduces a functional TRA protein that possibly binds to TRA-2 forming a TRA/TRA-2 complex,as previously shown inD.melanogasterto maintain an autoregulatory loop of female-specific splicing oftratranscript.The TRA/TRA-2 complex can also bind todsxpre-mRNA to regulate its female-specific splicing and promote female sexual development and differentiation.In XY males,however,the presence ofMoYinhibits the femalespecific autoregulatory loop oftra,leading to the default male-specific splicing oftrathat generates a truncated nonfunctional TRA peptide (Paneet al.2002;Verhulstet al.2010;Meccarielloet al.2019).The absence of functional TRA results in male-specific splicing ofdsx,andMoYinBactrocera oleaeandB.dorsalisshows sequence and function conservation to the homolog inC.capitata(Meccarielloet al.2019).In fact,all of theMfactors identified so far,such asMdmd,Yob,Nix,andGuy1fromMusca domestica(Sharmaet al.2017),An.gambiae(Krzywinskaet al.2016),A.aegypti(Hallet al.2015),andAn.stephensi(Criscioneet al.2016),respectively,are expressed in early embryos and are required for the initiation of male development.In addition,the autosomal miRNA miR-1-3p was found to require for male sex determination in early embryogenesis inB.dorsalisas an intermediate male determiner (Penget al.2020).
The master roles of M factor andtrain determining sexual development make them potential target genes for genetic control of pests.In fact,trahas been isolated and characterized in many serious agricultural pests,including the Tephritid fruit flies,many of which are designated as quarantine pests in many countries (Scolariet al.2021).Previous studies have shown that transient suppression oftraby microinjecting dsRNA into early embryos can cause XX female embryos to develop into XX pseudomales in many Tephritid fruit flies,such asC.capitata(Paneet al.2002),B.dorsalis(Liuet al.2015),B.oleae(Lagoset al.2007),Bactrocera tryoni(Raphaelet al.2014),andAnastrepha suspensa(Scheteliget al.2012).Fuet al.(2007) have engineered female-specific lethal Medfly by using the sex-specific alternative splicing mechanism ofCctra.To date,the transgenic sexing strains that contain the female-specific intron oftrathat is required for its own sex-specific splicing have been constructed for several species,includingCochliomyia hominivorax(Conchaet al.2016),Drosophila suzukii(Liet al.2021),andLucilia cuprina(Yan and Scott 2020).Recently,dCas9 has been applied to induce masculinization of XX to produce male-only progeny that can be used for SIT(Primoet al.2020).However,M factors in many species have not been identified yet.Although the M factorMoYhas been identified in several Tephritidae species,such asC.capitata,B.dorsalis,B.oleae,andZeugodacus tau(Meccarielloet al.2019;Liuet al.2022),and it has been shown to be conserved by RNAi to downregulateMoYexpression inB.dorsalisandB.oleae(Meccarielloet al.2019),MoYstill needs to be isolated and characterized in other Tephritidae species for understanding of sex determination and genetic control of different pests.
In the present study,we isolated and characterized the geneMoYof the melon flyZeugodacus cucurbitae(Coquillett),which damages over 130 plant hosts representing over 30 families throughout the Pacific,Asia,and Africa (De Meyeret al.2015).The complete cDNA ofZcMoYhas been cloned and the expression ofZcMoYhas been investigated in different developmental stages and tissues with focus on embryonic stages.ZcMoYexpression begins and increases in early embryogenesis,then decreases gradually and maintains at a very low level.Embryonic injection ofMoYdsRNA into earlyZ.cucurbitaeembryos significantly affects their sexual development and causes the occurrence of intersex individuals.Of particular,transient embryonic repression ofZcMoYby RNA interference (RNAi) results in development of XY females.Our results demonstrate thatZcMoYplays a key role in male sex determination ofZ.cucurbitae,as it does in other Tephritidae.The isolation and characterization ofZcMoYwill enrich the understanding of insect sex determination and establish the theoretical basis for its genetic control in the future.
2.Materials and methods
2.1.Insect culture
The melon flies used in this study were collected on City West Campus of Hainan University and maintained in the laboratory at 26°C,70% RH,and a photoperiod of 14 h:10 h (L:D).The larvae were reared on artificial diet (Liuet al.2020),and the grown larvae were transferred into small plastic boxes with sand before pupation.After 7 d,the pupae were transferred into insect cultivation cages for eclosion.The adults were fed water and a protein-rich food consisting of brewer yeast powder/sugar (1:3,w/w).
2.2.RNA extraction,DNA amplification and rapid amplification of cDNA ends (RACE)
Total RNA was extracted from eggs using TRI Reagent(Sigma,USA) as reported in Luoet al.(2017).A total of 1 μg of total RNA was used to synthesize the first strand of cDNA following the manufacturer’s instructions of TaKaRa Prime Script RT-PCR Kit (TaKaRa,China).
The original sequence ofZcMoY(GenBank:MK165754.1) was obtained from NCBI.Two primers(ZcMoY-F: 5´-ATGGGATCAGTTTGGGTATTG-3´;ZcMoY-R: 5´-TTTATAATAAAATTTTTTAGTCAACAGACA C-3´) were designed and used to amplify theZcMoYsequence from embryonic cDNA.A PCR product of 210 bp was obtained and sequenced.For 5´-and 3´-RACE,the first strand cDNA was synthesized using the SMART RACE cDNA Amplification Kit according to the manufacturer’s protocol (Clontech,USA).The two primers used forZcMoY5´-RACE are TTTATAATAAAATTTTTTAGTCAACAGACAC and GTGTGTTCTTTGCATTCC,and the two primers forZcMoY3´-RACE are ATGGGATCAGTTTGGGTATTG and GAAAACCAAATATAATTCAAGAACG.5´-and 3´-ZcMoYcDNAs were amplified from embryonic cDNA using nest PCR,respectively.
2.3.Expression of ZcMoY mRNA over development
The temporal and spatial expression ofZcMoYmRNA was investigated using semiquantitative RT-PCR.To analyze the temporal expression ofZcMoYin embryos,1 μg of total RNAs from eggs at 0,1,2,3,5,8,12,18,24 h after egg laying were used to synthesize the first strand of cDNA,respectively.PCR was performed with the primers 5´-GATATCCAATGTTAGCTGTCAG-3´ and 5´-GTGTGTTCTTTGCATTCC-3´ under the following conditions: 3 min at 94°C;35 cycles of 30 s at 94°C,30 s at 55°C,30 s at 72°C;and 5 min at 72°C.The reference geneα-tubulinwas used as a control.
To investigate the expression ofZcMoYin different developmental stages,we collected eggs at 3 h after egg laying;the mixed sexes of 1st and 3rd instar larvae;the mixed sexes of day 1 and day 7 pupae;the day 1 and day 10 adult females and males for RNA extraction and RT-PCR.The different tissues (head,thorax,midgut,fat body,testis and malpighian tubule) of day 1 adult males were dissected in cold 1× PBS and used to detectZcMoYexpression.
2.4.RNA interference
The dsRNAs targetingZcMoYandGFPwere synthesized using the T7 RiboMAX Express RNAi System (Promega,USA).Primers containing T7 promoters at the 5´ ends were used to amplify theZcMoYandGFPtranscripts(272 and 700 bp,respectively).Recombinant plasmids containingZcMoYandGFPsequences were used as templates for PCR amplification.The primer sequences are listed in Table 1.
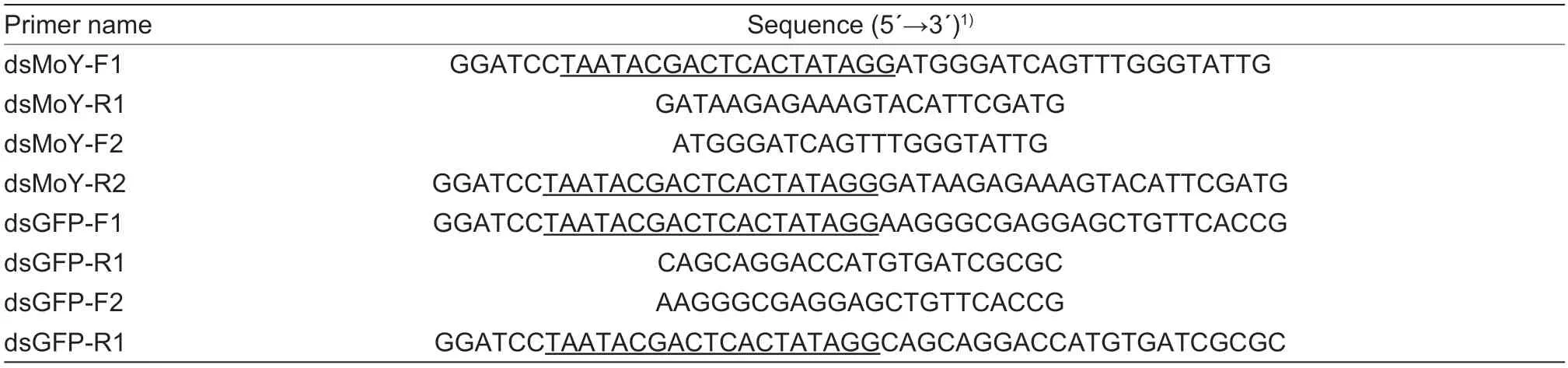
Table 1 Primers for RNA interference (RNAi)
Embryos were collected and microinjected with 1 μg μL-1dsRNA within 1 h after egg laying according to Zhaoet al.(2019) with modification.Briefly,eggs laid within 15 min were collected,and then treated with 1%sodium hypochlorite and 0.02% Triton-X 100 for 45 s,respectively.After washing with deionized H2O,the eggs were aligned on a slide with double-sided sticking tape left to dry for 10 min in the desiccating chamber,and covered with a mix of halocarbon 700/27 oils (1:1 (v/v);Sigma,USA).Embryos were injected with 1 μg μL-1dsRNA through the chorion membrane into the posterior end of pre-blastoderm embryos under an inverted microscope,using a micromanipulator M-152 (Narishige,Japan) and a microinjector FemtoJet 4i (eppendorf,Germany).After draining off excess oil,the injected embryos were culturedat 26°C within a humid chamber.Larvae were then transferred to an artificial diet for further development under the same conditions as wild-type flies.After eclosion,sexual dimorphism was examined,and progeny were classified by sexes.
2.5.Dissection of internal reproductive organs
The external genitalia of intersex flies that developed from embryos injected with ZcMoYdsRNA were observed under a stereomicroscope SZX16 (Olympus,Japan).After phenotypic observation,RNAi female adults and intersex individuals were dissected to observe their internal genitalia in 1× PBS.
2.6.Genomic DNA isolation and ZcMoY detection
The genomic DNA was isolated from dsMoY females using TIANamp Genomic DNA Kit (TIANGEN,China).ZcMoYgene was detected with PCR using the primersZcMoY-F andZcMoY-R.PCR was performed with the following parameters: 3 min at 94°C;32 cycles of 30 s at 94°C,30 s at 55°C,30 s at 72°C;and 5 min at 72°C.A PCR product of 210 bp would be obtained ifZcMoYexists in the fly.
3.Results
3.1.Isolation and molecular characterization of Zc-MoY cDNA
The originalZcMoYsequence that is 180 bp in length and encodes 59 amino acids was obtained from NCBI(GenBank: MK165754.1).However,most of the MOY proteins contain about 70 amino acids in other Tephritidae species,indicating that some nucleotides may be missing in this sequence.Thus,we designed one pair of primers within the 180 bp sequence to amplifyZcMoYgene from embryonic cDNA and sequenced the amplification product.The full-lengthZcMoYcDNA was amplified using RACE.TheZcMoYcDNA is 438 bp in full-length (GenBank:OK086041) that contains a 131-bp 5´-untranslated region (UTR),an open reading frame (ORF) encoding a 70 amino acid protein,and a 94-bp 3´-UTR.The amino acid sequence of ZcMOY is conserved and shares high sequence similarity with MOY ofBactroceraspecies:B. dorsalis(72.6%),Bactrocera zonata(72.6%),B.tryoni(69.9%),andBactrocera jarvisi(68.5%);but relatively low with MOY ofC. capitata(43.9%) (Appendix A).Two regions of MOY (KXNSRT and EXXKL) seem to be widely conserved,however,they exhibit no significant homologies to any functional or structural domain or motif against current sequence databases.
Further analysis found that there are at least twoZcMoY-related sequences (ZcMoY-RS1andZcMoY-RS2)within Y chromosome,showing 91.9 and 50.3% sequence identity or similarity with theZcMoYgene.The ORF ofZcMoY-RS1has two more nucleotides (TT) thanZcMoYORF,generating a truncated MOY peptide.ZcMoY-RS2contains an incompleteZcMoYORF that can only be translated a short peptide.Therefore,the products of bothZcMoY-related sequences should be nonfunctional(Appendix B).
3.2.ZcMoY expression during Z.cucurbitae development
AsMoYhas been identified as a key primary sex determination factor that begins to express in early embryos and becomes undetectable by 48 h until adulthood inC.capitata,we examined the expression pattern ofZcMoYduring the embryogenesis.RT-PCR was performed to analyzeZcMoYexpression in eggs within 0-24 h after egg laying asZ.cucurbitaeeggs develop into larvae within 28-30 h at 26°C.The results revealed thatZcMoYexpression began at 1 h after egg laying,peaked at 3 h and decreased gradually and maintained at a low level (Fig.1-A).We also examinedZcMoYin the mixed sexes of larvae,pupae and male or female adults.The result showed thatZcMoYwas not detected in all stages except in the eggs (Fig.1-B).However,ZcMoYwas detected in different tissues of day 1 adult males,especially in the testes and heads whereZcMoYexhibited higher expression levels than other tissues (Fig.1-C).
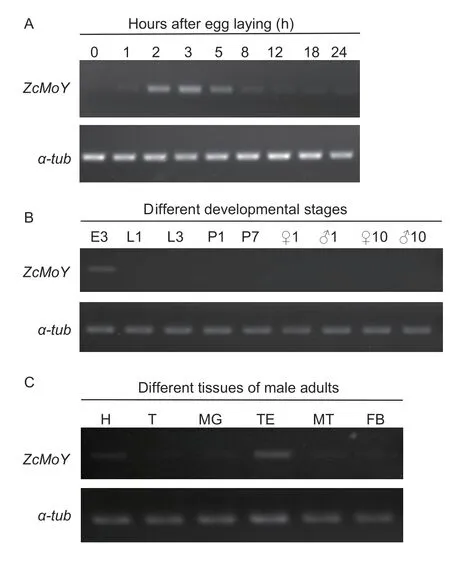
Fig.1 Expression of ZcMoY at different developmental stages and tissues of male adults.A,ZcMoY expression in embryogenesis.Total RNA was extracted from the eggs of mixed sexes at 0,1,2,3,5,8,12,18 and 24 h after egg laying and used for PCR.B,ZcMoY expression at different developmental stages.Total RNA was extracted from different developmental stages and used for PCR.E3,3 h eggs of mixed sexes;L1 and L3,1st and 3rd instar larvae of mixed sexes;P1 and P7,day 1 and day 7 pupae of mixed sex;♀1,♀10,♂1,and♂10,day 1 and day 10 female and male adults,respectively.C,ZcMoY expression in different tissues of male adults.Total RNA was extracted from different tissues of day 1 male adults and used for PCR.H,head;T,thorax;MG,midgut;TE,testis;MT,Malpighian tubule;FB,fat body.α-tub,the internal control gene α-tubulin.
3.3.Suppression of ZcMoY in embryos causes abnormal male development
To assay the function ofZcMoYinZ.cucurbitaesexual development,RNAi ofZcMoYwas performed.The dsRNA containing 272 bp ofZcMoYtranscript was constructed and injected into the newly laid eggs within 1 h.More than 70.9% of embryos developed into females or intersexes after dsMoY injection and only 29.1% of embryos developed into males.The male ratio significantly decreased in theZcMoYRNAi group and the ratio was much lower than the dsGFP injection one(50.94%),suggesting that the sexual development of males is disrupted (Table 2).

Table 2 Phenotypic statistics of ZcMoY knockdown by RNA interference (RNAi)1)
While wild-type female adult has no bristle on the 3rd tergum and possesses female-specific external genitalia(Fig.2-A and B),wild-type male adult has male external genitalia and a row of bristles on each side of the third tergum (Fig.2-C and D).Intersex individuals,which exhibit normal or abnormal female external genitalia but have a row of bristles on each side of the 3rd tergum(Fig.2-E-H),were observed in theZcMoYRNAi group.Conversely,there were no intersex individuals in the dsGFP control group.We further examined the internal reproductive system of intersexes.Compared to wild-type adult testes and ovaries (Fig.3-A and B),intersex adults showed malformed interior reproductive organs and both aberrant testes and ovaries were observed in the intersex individuals (Fig.3-C-E).Taken together,these results demonstrate thatZcMoYknockdown in the early embryos affects male development and causes abnormal external genitalia and interior reproductive organs,indicatingZcMoYplays a key role in sex determination ofZ.cucurbitae.

Fig.2 Phenotypic analysis of intersex individuals induced by ZcMoY knockdown.A and C,dorsal view of wild-type female (A)and male (C).Wild-type males exhibit short black pigmented bristles (arrows) on both lower sides of the third tergum.B and D,ventral view of normal female ovipositor (B) and male genitalia (D).E-H,intersex individuals show female-specific ovipositor with male-specific black-colored bristles (E and F) and deformed genitalia with bristles (G and H).Scale bar: 500 μm.
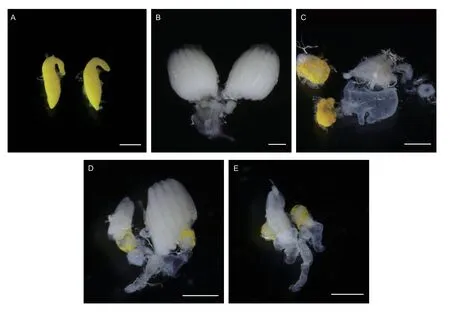
Fig.3 The internal genital structures of intersex flies.A,wild-type testes.B,wild-type ovaries.C-E,ovotestes of intersex individuals.Scale bar: 500 μm.
3.4.Suppression of ZcMoY in embryos causes feminization of XY individuals and leads to producing abnormally developed reproductive organs
MoYis a Medfly Y-chromosome linked gene and the gene disruption ofMoYby CRISPR/Cas9 or transient repression of its expression can feminize XY embryos (Meccarielloet al.2019).Thus,we speculated that the suppression ofZcMoYmay cause feminization of XY individuals.To test this,we dissected the internal reproductive system of 28 random selection RNAi females.Compared to wild-type adult ovaries (Fig.4-A),three main phenotypic categories of ovaries were observed for the RNAi females: i) 25females (No.1-4,6-21,23-25,27,and 28) exhibit a pair of normal ovaries (Fig.4-B);ii) 1 female (No.22) exhibits a pair of malformed ovaries with different size (Fig.4-C);and iii) 2 females (No.5 and 26) exhibit a pair of ovaries with both in small size (Fig.4-D).Furthermore,genomic DNA of all 28 females was extracted and used to amplifyZcMoYgene by PCR (Fig.5) to verify the karyotype.ZcMoYwas specifically detected in the 5 females (No.2,4,5,22,and 26),but not in other 23 females,suggesting these 5 females are pseudofemales (XY karyotype).Our results therefore demonstrate that transient repression ofZcMoYexpression may produce completely feminized XY individuals and result in malformed ovaries.
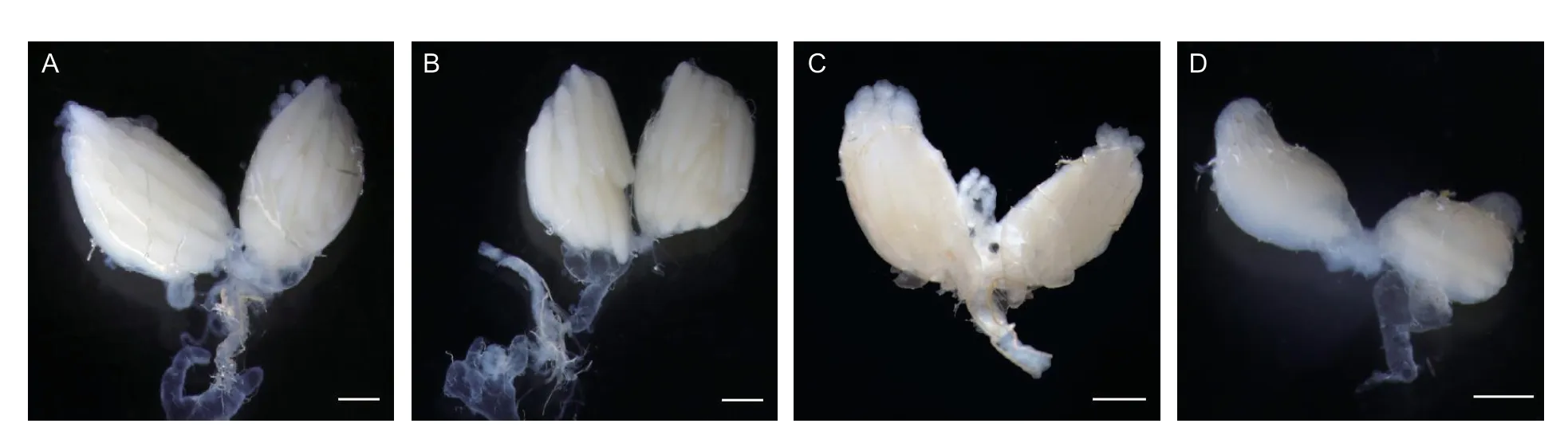
Fig.4 Ovarian structures of RNA interference females.A,wild-type females.B,No.1-4,6-21,23-25,27,and 28.C,No.22.D,No.5 and 26 RNAi females.Scale bar: 500 μm.
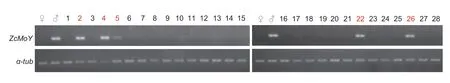
Fig.5 Molecular karyotyping of RNAi females.The genomic DNA of 28 adult females was extracted and used to detect the Y-chromosome linked gene ZcMoY.XY karyotype was detected in 5 out of 28 adult females (shown in red).α-tub,the internal control gene α-tubulin.♀,female adult;♂,male adult.
4.Discussion
The primary signals of sex determination are diverse and evolve rapidly across different insects.Identifying the primary signals in insects holds significant promise for development of novel genetic sexing strains using modern genetics.Previous studies have identified several primary signals in dipteran species (Hallet al.2015;Krzywinskaet al.2016;Sharmaet al.2017),especially the M factor of MedflyMoY,which has been demonstrated to be conserved in some Tephritidae species (Meccarielloet al.2019).In this work,we have isolated and characterized the M factor ofZ.cucurbitae,ZcMoY,and found thatZcMoYis required for sexual development ofZ.cucurbitaemales.
Three similar replicates ofZcMoYsequences were identified in the Y chromosome,but two of them are not able to encode the correct ZcMOY protein(Appendix B).This may due to the composition of widely heterochromatic and highly repetivite sequences in Y chromosome (Buchman and Akbari 2019).TheZcMoYgene has only one exon which encodes a protein of 70 amino acids.More than 68.5% of ZcMOY protein sequence is similar to the MOY of otherBactroceraspecies,but the similarity is only 43.9% with MOY ofC.capitata.Although it is speculated that the function ofMoYwill be conserved in Tephritidae species,we cannot find any other conserved domain between different MOY sequences except two regions of KXNSRT and EXXKL.Thus,they may play an important role in the function of MOY.
A previous study has demonstrated thatMoYfunction as an inhibitor of the female-specific autoregulatory loop oftra,leading to the male-specific splicing oftra(Meccarielloet al.2019).Thus,the expression ofZcMoYshould be earlier than the male-specific splicing oftra.The expression pattern ofZcMoYin embryos shows that theZcMoYtranscript appears and peaks at very early embryonic stage,which is consistent with the expression period of male-specificZctratranscript as previously reported (Luoet al.2017).Although its expression level decreases rapidly,but ZcMoYstill maintains at a low level,suggestingZcMoYis needed for sex determination during the whole embryonic stage.Compared withC.capitata,ZcMoYis expressed for a shorter period of time in the embryos,which may be due to the different embryonic development timing of these two species.In general,embryos ofZ.cucurbitaebegin to develop into larvae at 28 h after egg laying at 26°C,whileC.capitataembryos hatch at 48 h after egg laying at 25°C (Meccarielloet al.2019).Furthermore,theZcMoYtranscript can be detected in the testes and heads of day 1 adult males.It impliesZcMoYmay also play a role in these tissues.It will be interesting to explore the role ofZcMoYin these tissues in the future.
Generally,the sex ratio of wild-type progeny is nearly 1:1,as shown in the dsGFP treatment.By contrast,in the adults emerged from the dsMoY-injected embryos,male development was significantly disrupted as more than 64.02% of individuals developed into females,some of which were XY females that were confirmed byZcMoYdetection.Some embryos fromZcMoYRNAi developed into intersex individuals (6.88%),which might be due to insufficient knockdown ofZcMoY.Our results substantially suggest that the knockdown ofZcMoYleads XY embryos that are presumptively males to develop into XY females and intersexes.These results are consistent with the previous reports in which the knockdown ofMoYresults in the reversal of males to females in Tephritidae including otherBactroceraspecies flies (Meccarielloet al.2019).Many previous studies have shown that knockdown oftraby RNAi causes more than 90% individuals to develop into males or intersexes (Paneet al.2002;Lagoset al.2007;Liuet al.2015),however,knockdown ofZcMoY,the upstream regulator oftra,only produced 70.9% females or intersexes.This may be due to the following two reasons: (I)tra-dsxaxis is a well-known cascaded signal in sex determination.The suppression oftraresults in male-specific splicing ofdsxand directly induces embryos to develop into males or intersexes (Paneet al.2002).However,as the transient repression ofMoYthat is the upstream regulator oftraaffects sex determination through influencing female-specific splicing oftra(Meccarielloet al.2019),the efficiency on sex reversion is higher by knockdown oftrathan by suppression ofMoY.(II) It is unclear howMoYregulates the splicing oftraso far (Meccarielloet al.2019).There may be some unknown factors participating in the regulation of this process,which may affect the efficiency of sex reversion by knockdown ofMoY.Taken together,we suggest thatZcMoYhas regulatory and functional similarity with its Tephritid homologs.
5.Conclusion
Our work demonstrates that the transient suppression ofZcMoYby RNAi significantly disturbs the sexual development ofZ.cucurbitae,and produces XY pseudofemales.As the overexpression ofMoYin XX embryos induces masculinization,andMoYis conserved in Tephritidae species,MoYmay be an ideal tool of the transgenic sexing system for the control of Tephritid fruit flies.Thus,combining with Tet-off system,aMoYoverexpression transgenic strain can be constructed and may be used for the population suppression of the pests.
Acknowledgements
This study was supported by the the National Natural Science Foundation of China (31702059,31860523,and 31660339),and the Hainan Provincial Natural Science Foundation of China (321CXTD435).
Declaration of competing interest
The authors declare that they have no confilict of interest.
Appendicesassociated with this paper are available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
杂志排行
Journal of Integrative Agriculture的其它文章
- Analyses and identifications of quantitative trait loci and candidate genes controlling mesocotyl elongation in rice
- OsMas1,a novel maspardin protein gene,confers tolerance to salt and drought stresses by regulating ABA signaling in rice
- Marker-assisted selection to pyramid Fusarium head blight resistance loci Fhb1 and Fhb2 in the high-quality soft wheat cultivar Yangmai 15
- Identification,evolution,expression and protein interaction analysis of genes encoding B-box zinc-finger proteins in maize
- Characterization of transgenic wheat lines expressing maize ABP7 involved in kernel development
- Increasing the appropriate seedling density for higher yield in dry direct-seeded rice sown by a multifunctional seeder after wheatstraw return
