Positive effects of PVP in MIC: Preparation and characterization of Al-Core heterojunction fibers
2023-01-18FuweiLiYuetingWangChengaiWangYunShenZehuaZhangJianChengShuangzhangWuYinghuaYeRuiqiShen
Fuwei Li ,Yue-ting Wang ,Cheng-ai Wang ,Yun Shen ,Ze-hua Zhang ,Jian Cheng ,Shuang-zhang Wu ,Ying-hua Ye ,*,Rui-qi Shen
a School of Chemistry and Chemical Engineering,Nanjing University of Science &Technology,Nanjing,210094,China
b Micro-Nano Energetic Devices Key Laboratory,Ministry of Industry and Information Technology,Nanjing,Jiangsu,210094,China
c College of Field Engineering,Army Engineering University of PLA,Nanjing,210007,China
Keywords:Al-Core heterojunction structure Metastable intermolecular composites(MIC)Al/CuO nanothermites Polyvinylpyrrolidone (PVP)
ABSTRACT The core-shell metastable intermolecular composites (MIC) have attracted much attention in the past few years due to their unique properties.Here,the preparation of Al-Core heterojunction fibers using PVP as a template is proposed.The nano-Al was directly added to the precursor solution of cupric acetate monohydrate (CAM)/Polyvinylpyrrolidone (PVP),and the initial Al@CAM/PVP fibers were obtained via electrospinning.The core-shell MIC fibers are then obtained by calcining the initial fibers.The morphology,structure,and composition of Al-core MIC fibers were characterized,that the energetic fibers calcined at 300 °C,350 °C,and 400 °C have a core-shell structure with shell compositions CuxO and PVP,CuxO and CuO,respectively.The energy release characteristics of Al-core MIC were investigated,and preliminary ignition tests were performed using an ignition temperature measuring instrument and a pulsed laser.The energetic fibers calcined at 300 °C exhibited unique properties.The decomposition of PVP in the shell layer promoted exotherm,and a low-temperature exothermic peak was shown at 372-458 °C.Lower ignition temperatures and higher flame heights were observed in the combustion tests than calcination at 350 °C and 400 °C.An unexpected result was that PVP can play a positive role in Al/CuO nanothermites.Simultaneously,this preparation method provided an idea for the integrated preparation of core-shell Al-Core MIC fibers and tuning the properties of MIC.
1.Introduction
Metastable intermolecular composites (MIC) [1,2] are mainly composed of metal fuels (common metal Al) and various oxidants at the nanoscale.As highly reactive nanomaterials,they have attracted attention with faster reaction rates and higher energy densities [3] than micron-scale Al thermite [4].Due to these advantages,MIC of different systems such as Al/CuO[5],Al/MoO3[6],and Al/Fe2O3[7],are widely used in gas generators [8],microelectromechanical systems(MEMS)[9],explosives[10],propellant.Among them,different forms of MIC,such as microspheres [11],nanowires [12],and thin films [13],were ared by methods like arrested reaction milling [14],sol-gel [15],electrospray [16],printed ink [17],and in situ synthesis [18].
Although higher reactivity is available,the application of nanoscale metal fuels also faces some challenges.The increased surface energy of nanoparticles causes aggregation of particles,which makes the mixing of components difficult [19].Nanoscale particles are highly reactive materials,leading to possible safety issues [20],even though smaller particle sizes have better combustion efficiency.The high reactivity promotes interfacial contact between nanoscale composites,making it difficult to control their performance.More importantly,these particles are susceptible to slow surface oxidation [21].Core-shell structures,formed by the growth of a shell on a nuclear matrix by external components through chemical bonds or intermolecular interaction forces,have three distinct advantages: close contact between components,combination of multiple functions,and improved material properties [22].Besides,the shell layer prevents the oxidation of the nuclear layer and solves the aggregation of nanoparticles,etc.Energetic materials with core-shell structure receive extensive attention.Two main types of Al-based core-shell MIC are currently available: metallic Al as the core and oxide as the shell,and the other in contrast.For the Al-core MIC,the shell layer is usually EMOFs [23] or copper oxide particles [24],obtained by thermal oxidation of Cu2+solution precursors.The exothermic properties of these core-shell MIC are modulated by changing the shell layer,such as coating Al-core with PDA or changing the shell layer's CuO particle size and thickness.CuO-core MICs are usually 2D films.The core-shell MICs are obtained by electrospinning [25] or etching[12,26]to obtain CuO nanowire films,followed by deposition of Al on the substrate.Compared with 1D spheres,2D nanotubes have a larger specific surface area and high homogeneity,and the thin film has potential applications in MEMS.
Electrospinning is a straightforward and effective method to prepare 2D fibers.Polymer fibers,such as NC[27],PVDF[17],PTFE[28,29],etc.,are used as electrospinning precursors to prepare MIC fibers by adding Al and CuO particles.So far,the most commonly used electrospinning template is polyvinyl pyrrolidone(PVP),yet it is rarely used in energetic materials.The cupric acetate monohydrate (CAM)/Polyvinylpyrrolidone (PVP) precursor solution can be used to obtain homogeneous initial fibers,and hollow CuO fibers can be obtained by calcination carried out at relatively high temperature [30].Krishnamurthi Muralidharan [31] reported that he embedded aluminum nanoparticles were protected by PVP from any significant oxidation,but the exposed aluminum nanoparticles are oxidized in the thermal environment after removal of PVP.The diffusion of aluminum cations controls the oxidation of nano-Al below 550°C for the interface between the alumina and the environment[32].PVP-coated aluminum nanoparticles have better high-temperature oxidation resistance before decomposition of PVP.The study by Tao Wu [33] predicted that the heat loss of Al/CuO was only 7% after one-year storage at 300°C.Therefore,it is positive to obtain a 2D MIC fiber via electrospinning with Al as the core and CuO as the shell.
Herein,this paper innovatively added Al nanoparticles directly tothecupricacetatemonohydrate(CAM)/Polyvinylpyrrolidone(PVP) precursor solution.Then initial fibers obtained by electrospinning were calcined at different temperatures to get Al-Core heterojunction MIC fibers.The schematic diagram of the preparation process of Al-Core heterojunction MIC fibers was shown in Fig.1.The MIC fibers were investigated using various characterization techniques such as scanning electron microscopy(SEM),transmission electron microscopy (TEM),X-ray energy dispersive spectroscopy (EDS),X-ray diffraction (XRD),X-ray photoelectron spectroscopy (XPS),thermogravimetry-differential scanning calorimetry (TG-DSC),ignition temperature measuring device and high-speed video camera.The catalytic exotherm of CuO on PVP was found,and the role of PVP in Al-core heterojunction MIC fibers was analyzed.
2.Experiments
2.1.Materials
Aluminum nanoparticles (Al NPs) from Shanghai Haotian Nanotechnology Company with a size of 100 nm had an active aluminum content of 64 wt% from the TG curve of Fig.S1.Polyvinylpyrrolidone (PVP) with a molecular weight of 130,000 was purchased from Aladdin.Cupric acetate monohydrate (CAM) was supplied by Sinopharm Chemical Reagent limited corporation,China.Absolute ethanol (AE) was obtained from Sinopharm Chemical Reagent limited corporation.N,N-Dimethylformamide(DMF) was procured from Kelon Chemical Reagent Factory(Chengdu).All the chemicals are analytical grade.
2.2.Preparation of precursor solution
Assuming that the calcination product of cupric acetate monohydrate is CuO,we determine the equivalence ratio (Ф) of Al and CuO was fixed at 1.5.In a typical preparation procedure,0.530 g of PVP was mixed with a 6 mL mixture solvent of DMF/AE volume ratio (5:1),followed by magnetic stirring for 3 h to ensure PVP dissolution.Then 0.398 g Copper acetate (CAM) was added to the above solution with stirring for 1 h.Finally,0.054 g Al NPs were dispersed in the solution with 1 h of ultrasonic dispersion and 12 h of magnetic stirring to form the precursor.
2.3.Preparation of Al-core MIC fibers
The solution was loaded into a syringe equipped with a 23 gauge(inner diameter:0.31 mm;outer diameter:0.63 mm)stainless steel nozzle tip.A positive voltage of 13 kV was applied to the nozzle tip,while the metal collector applied a negative voltage of 3 kV.During electrospinning,the distance between the needle and the metal collector was 18 cm,and a constant feed rate of 0.6 mL/h was used.After 5 h,the initial Al@CAM/PVP fiber was removed from the metal collection plate and then dried at 60°C for 2 h before being calcined at different temperatures maintained for 2 h under air atmosphere.The heating time from atmospheric temperature rise to calcination temperature was 2 h.
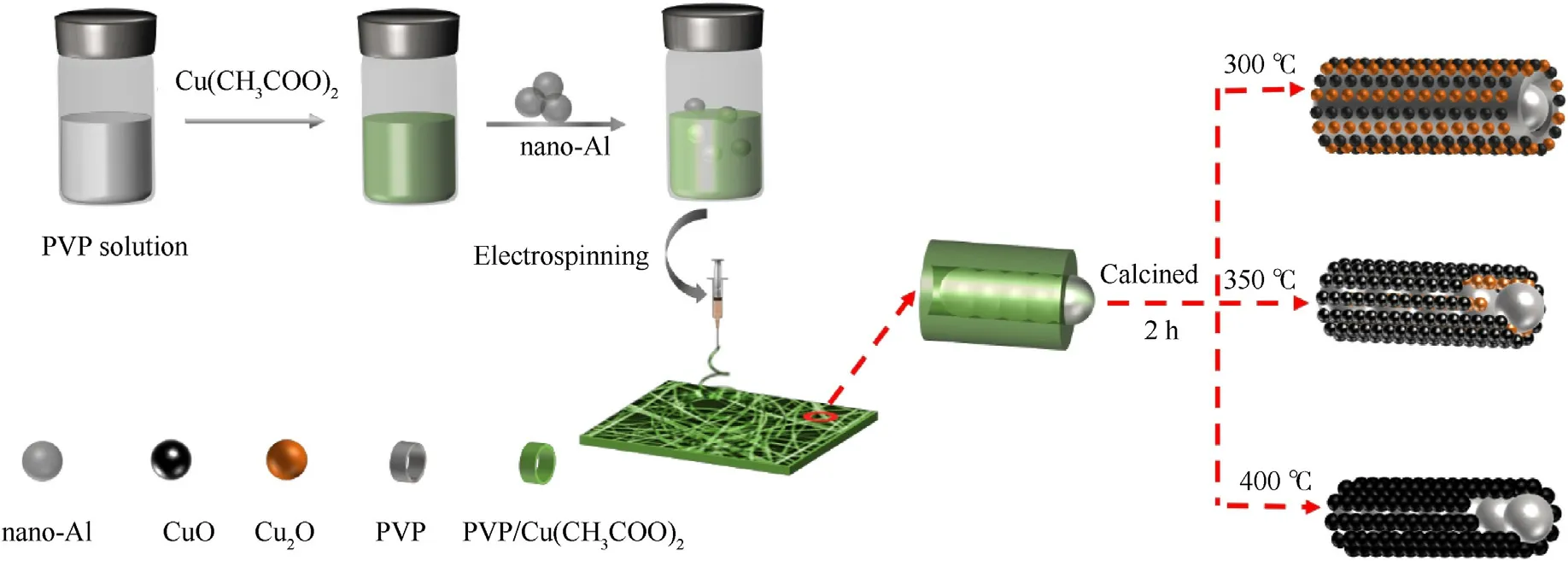
Fig.1.The schematic diagram of the preparation process of Al-core heterojunction MIC fibers.
2.4.Morphology and composition characterization
The morphology of initial Al@CAM/PVP fibers and Al-core MIC fibers were observed by the field-emission scanning electron microscope (FESEM,FEI Quant 250F) at an acceleration voltage of 20 kV.High-resolution transmission electron microscopy(HRTEM,JEM-2100F)and energy dispersive spectrometer(EDS)were used to analyze the structural composition and elemental distribution of MIC fibers.X-ray diffraction (XRD,Bukes D8) was examined for copper acetate calcination at 300°C,and the MIC fibers calcined at 300°C,350°C,and 400°C,respectively.X-ray photoelectron spectroscopy(XPS,AXIS-Ultra OLD)was used to analyze the surface composition of Al-core MIC fibers calcined at 300°C,350°C,and 400°C to determine the oxidation of the fibers calcined at different temperatures.
2.5.Analysis of exothermic behavior
The thermal behavior of Al NPs,cupric acetate monohydrate(CAM),and the initial Al@CAM/PVP was characterized using thermogravimetry-differential scanning calorimetry (TG-DSC,NETZSCH STA 449F3) to determine the appropriate calcination temperature.The samples were placed in an alumina crucible and analyzed at a heating rate of 10°C/min under a flowing air environment (30 mL/min).TG-DSC analyzed the thermal properties of MIC fibers calcined at 300°C,350°C,and 400°C,respectively.The heating rate was 10°C/min from 30°C to 1000°C under an argon atmosphere with a flow rate of 30 mL/min.
2.6.Test of ignition and combustion performance
The ignition temperature of the MIC fibers was tested in the device shown in Fig.2a.Platinum-rhodium wire with a diameter of 0.08 mm and a length of 10 mm,which heats to~1600°C within 10 ms,was placed on the MIC fibers(5 mm×5 mm).A high-speed pulsed current source was connected to both poles,operating at 6.0 A and 10 ms.A photoelectric sensor (THORLABS,DET08CFC/M)collected the light signal during the sample combustion in realtime.An oscilloscope records the current signal on the circuit,the voltage signal between the two poles,and the voltage signal from the photoelectric sensor at the same time.From the signals of current and voltage,a resistance measurement was obtained and related to the instantaneous temperature of the platinum-rhodium wire,which corresponds to the onset of the optical signal and was considered to be the ignition temperature of the thermite composite.
The combustion properties of the MIC fibers were studied using direct irradiation with a pulsed laser,Such as Fig.2b.MIC fibers with a side length of 5 mm were placed inside a transparent plastic ring with an inner diameter of 7 mm.A pulsed laser (Nd: YAG pulsed laser,LS-2147)with a wavelength of 1064 nm,a pulse width of 18 ns,frequency of 10 Hz,and laser energy of 0.164 J/pulse was used to directly irradiate the MIC fibers while taking a high-speed camera at a frame rate of 25,000 fps to record the combustion phenomenon.
3.Results and discussion
3.1.Characterization of initial Al@CAM/PVP fibers
Fig.3(a and c) showed the morphology of the initial Al@CAM/PVP fibers after drying at 60°C for 2 h,and Fig.3b illustrated the morphology of the CAM/PVP fibers.The initial Al@CAM/PVP fibers by electrospinning had an average diameter of 150 nm.Under the same electrospinning condition,the two fibers were distributed randomly but uniformly on the aluminum foil.There were apparent fine copper particles on CAM/PVP fiber,while Al@CAM/PVP fiber's surface is smooth without copper particles.Al@CAM/PVP fibers were more concentrated,and some fibers were bonded together.Raised nodes could be observed on the fibers due to agglomerated nano aluminum particles.It could be seen from the EDS energy spectrum of the as-spun fiber in Fig.3d that most of the nano Al particles were evenly dispersed in the fibers,and only a few nano Al particles aggregated to form nodes.Cu and C elements were completely uniformly distributed.
Thermal analysis of CAM,PVP,Al NPs,and initial Al@CAM/PVP fibers was performed to obtain the appropriate calcination temperature.The results were shown in Fig.4a.The TG curve of CAM showed that the decomposition was basically in two stages.The first stage occurred in the temperature range of 110°C-160°C,and the weight loss of 8.52% was the dehydration of bound water.The second stage occurred at 219°C-291°C.At this stage,CAM started to decompose with a weight loss of 52.46%.CuO and Cu2O became the decomposition products,as shown by the XRD results in Fig.S2 obtained after calcination of CAM at 300°C for 2 h.Cu2O was oxidized to CuO with increasing temperature in an oxygen atmosphere.When the temperature reached 450°C,the residual mass was maintained at 41.65%.As previously reported [34],the mechanism of decomposition was as follows:
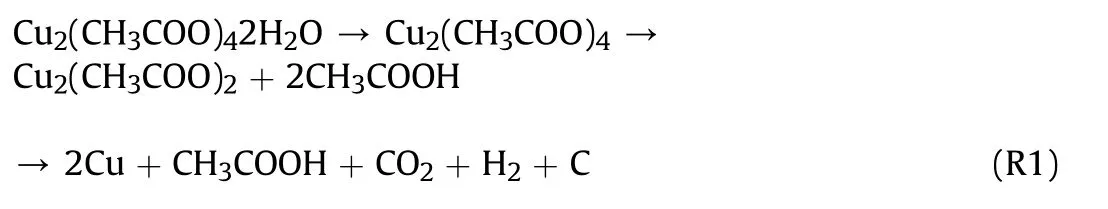
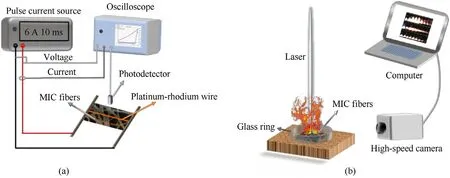
Fig.2.(a) T-jump ignition temperature test device;(b) Devices for combustion performance testing.
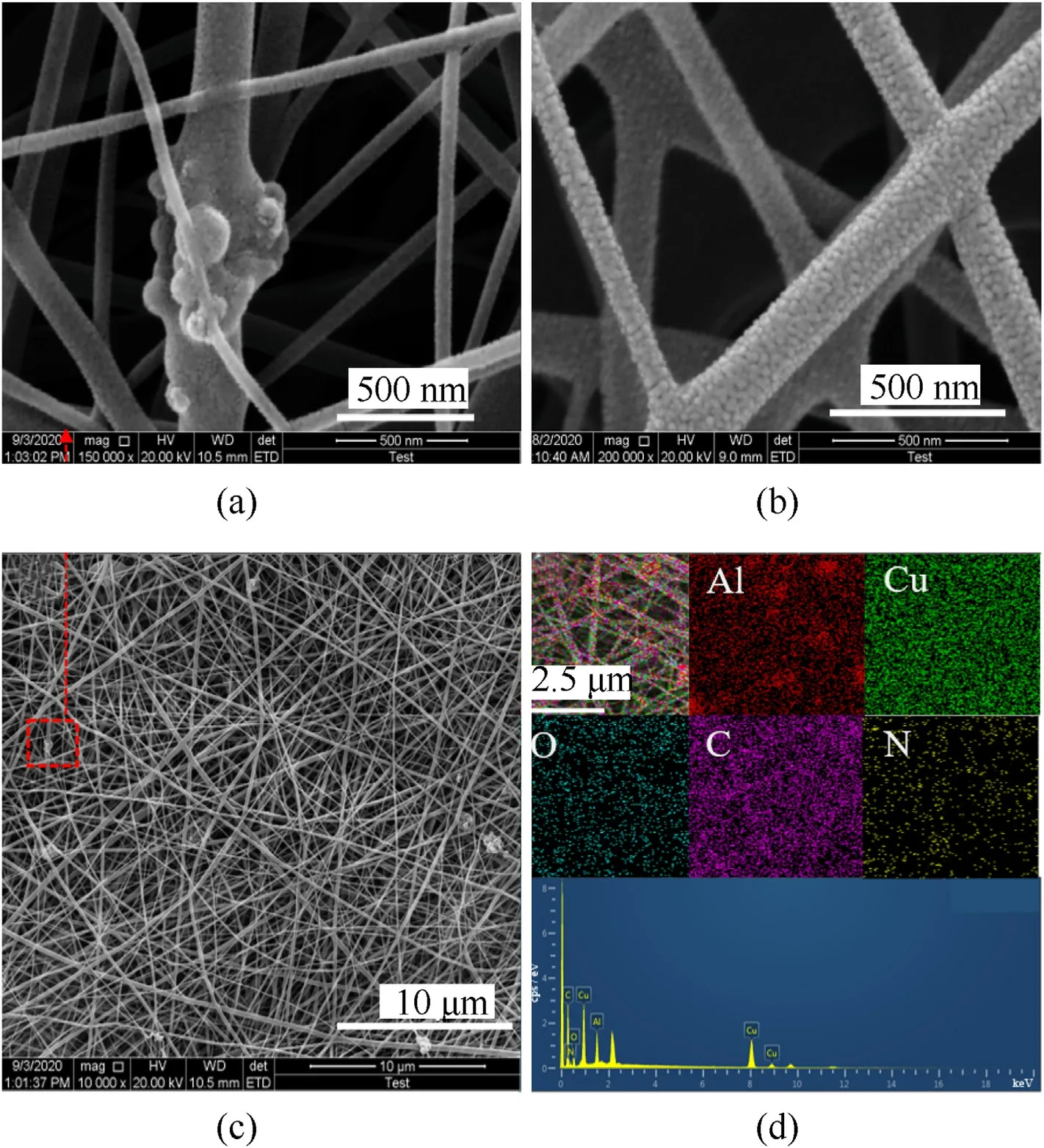
Fig.3.SEM images of (a) initial Al@CAM/PVP fibers.(b) CAM/PVP fibers.(c) Initial Al@CAM/PVP fibers.and (d) the EDS mapping images of initial Al@CAM/PVP fibers.
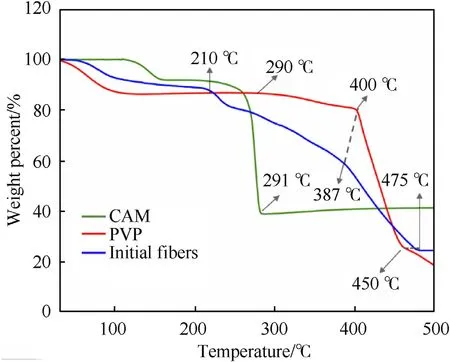
Fig.4.TG curves of CAM,PVP,and initial Al@CAM/PVP fibers.
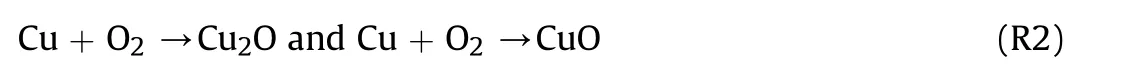
The TG curve of PVP showed that the degradation started at 290°C with a slight decrease.The main degradation products were VP monomer and pyridine 4-methylpyridine [35],while the decomposition started at 400°C,ended at 450°C.Unlike the decomposition of PVP in a nitrogen environment[31],the residual carbon continues to be released as CO2after 450°C.Similar to previous reports,the mechanism of decomposition is as follows:
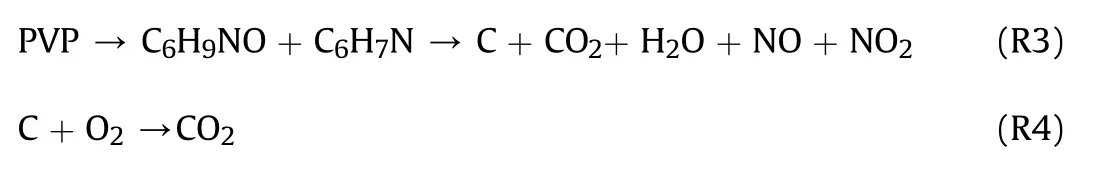
The TG curve of Al NPs from Fig.S1 showed that the oxidation started from 512°C,and rapid oxidation occurred from 551.7°C to 595.3°C,then a pseudo-oxidation plateau appeared.It could be understood that the oxidation takes place through the oxide layer due to the diffusion of oxygen or aluminium.This process was limited by the thickening of the oxide shell layer,which allowed the reaction to reach a false plateau.After 660°C,the aluminium core melted and broke the oxide layer to react with oxygen atoms.The result was consistent with previous studies [32].The TG results of the initial Al@CAM/PVP fibers showed that the weight loss of 7.53%at 50°C-110°C indicated some adsorbed water on the surface.At 210°C,the copper acetate began to decompose.The initial temperature of PVP accelerated decomposition decreased from 400°C to 387°C,while ended at 475°C.The decomposition of the initial Al@CAM/PVP fibers turns out to be very complicated and its mechanism still not well understood.As could be seen,the decomposition of PVP was advanced in the initial fibers.However,the reaction ended at 475°C.The calcination temperature was at least 300°C to obtain the CuO shell layer.Because the Al powder oxidized after 512°C,the calcination temperature should be 300°C-500°C.A further discussion of this issue will follow in the next section.
3.2.Morphology and microstructure of Al-core MIC fibers after calcination
The morphology of Al-core MIC fibers was studied by calcination at 300°C,350°C,400°C,450°C,500°C,and 550°C for 2 h under air environment.The results were shown in Fig.5.From the results of Fig.5(a-c),after calcined at 300°C,350°C,and 400°C for 2-h under air,intact MIC fibers morphology remained.At calcination temperatures of 350°C and 400°C,the fiber was made up of CuxO nanograins by removing PVP.For the fibers calcined at 300°C,the fibers merged and had larger diameters than the fibers calcined at 350°C and molten PVP might be the cause of fiber merging.With the increase of calcination temperature,the grains particles became coarser and fiber morphology changed from tight to porous from the results in Fig.5(b-f).It was seen that the higher the calcination temperature,the more the fiber was destroyed when the calcination temperature was higher than 400°C.The fibers were destroyed,and the product was made of irregular nanoparticles when the calcination temperature was 550°C.In addition,no nodes were found in these broken fibers from 400°C-550°C.Previous work [33] mentioned that the alumina layer of Al/CuO425C_air(calcined in air at 425°C for 2.6 h) thickens and the aluminum reserves are depleted.The Al NPs might have reacted during the calcination process above 400°C.Considering that the MIC fibers still maintained the nanofiber morphology after calcination below 400°C,calcination temperatures of 300°C,350°C,and 400°C were chosen to maintain the activity of MIC fibers in further experiments.
The Al-core MIC fibers calcined at 300°C,350°C,and 400°C were further analyzed by HRTEM and EDS.The results in Fig.6(a-c)showed that the MIC fibers were core-shell structures.With CuxO nanotubes as shell,Al particles as the core,obtained from the EDS results of three fibers.Obviously,the distribution of Cu element is consistent with O elements,and Al elements were inside the fiber.Fig.6(d and e) showed that the CuxO nanoparticles with a size about 20 nm in the fibers,some of the particles were stacked as shown in the circle.Detailed structure of the interface of Al nanoparticles was further observed.The interface of MIC fibers calcined at 300°C could be seen in Fig.7.The interplanar spacing of 0.252 nm and 0.275 nm Corresponded to the (0 0 2) and (-1 1 0)plane of CuO(ICDD/JCPDS No.04-0937).The interplanar spacing of 0.302 nm and 0.295 nm Corresponded to the(110)plane of Cu2+1O(ICDD/JCPDS No.05-0667) and Cu2O (ICDD/JCPDS No.34-1354),respectively.The interplanar spacing of 0.203 nm Corresponding to the(2 0 0)plane of Al(ICDD/JCPDS No.04-0787)was also observed.Obvious and amorphous areas might be considered as PVP cladding.In Fig.S3,no amorphous PVP was observed at the interface of MIC fibers calcined at 350°C.The fiber shell consisted of CuO and Cu2O,overlapping of 2-3 grains could be observed from the circled lattice region.The plane of(-11 0)was seen at the interface of MIC fibers calcined at 400°C in Fig.S4.The partial oxidation of Al in MIC fibers calcined at 400°C led to the thickening of the amorphous Al2O3layer,leaving the Al particle region without corresponding crystal information.
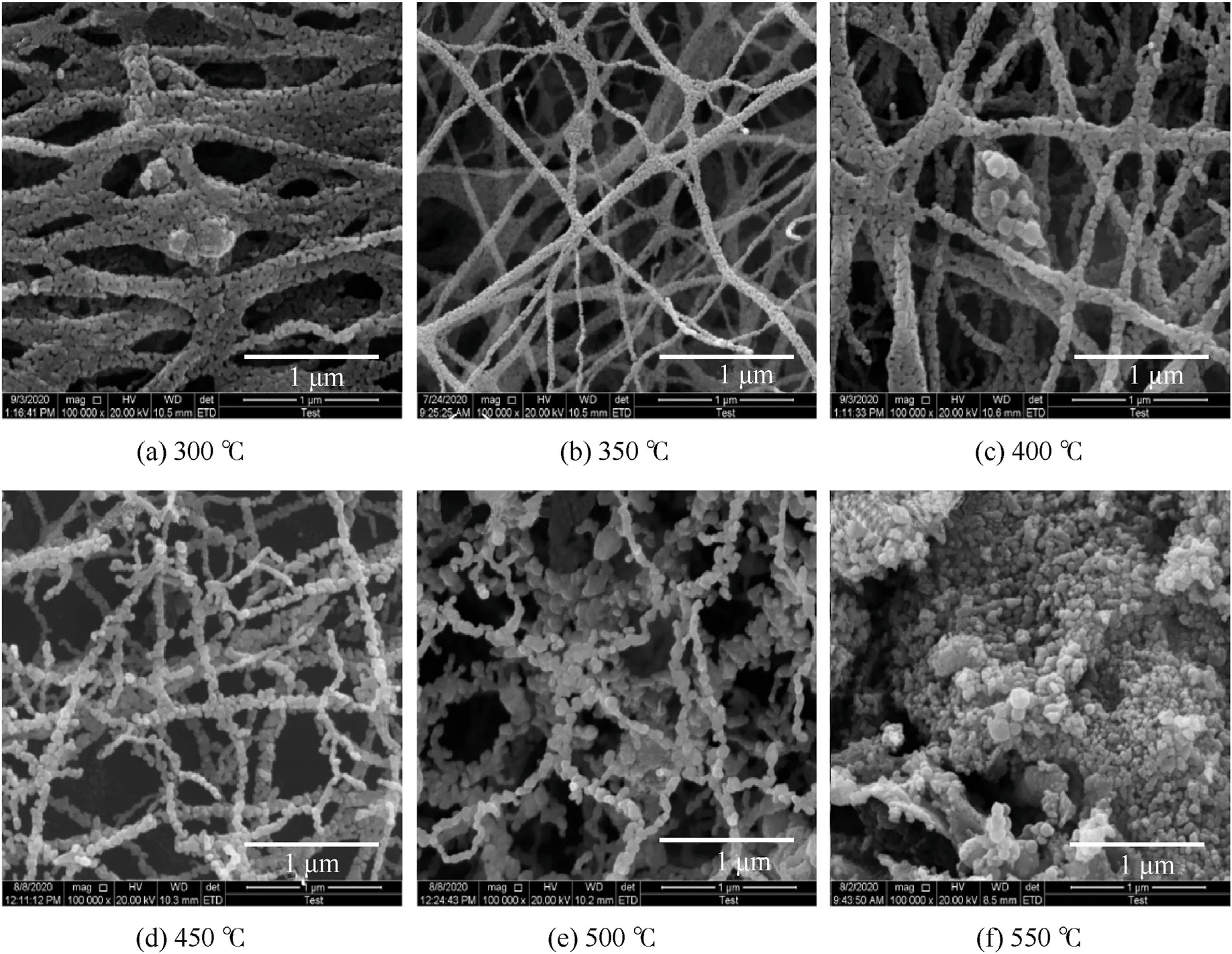
Fig.5.SEM images of Al-core MIC fibers calcined at (a) 300 °C,(b) 350 °C,(c) 400 °C,(d) 450 °C,(e) 500 °C and (f) 550 °C.
Morphologically,the Al-core MIC fibers were successfully obtained.The choice of calcination temperature was important.When the calcination temperature was 300°C and 350°C,the Al2O3layer on the surface of Al particles did not thicken drastically.However,when the calcination temperature was 400°C,the Al particles have been partially oxidized.
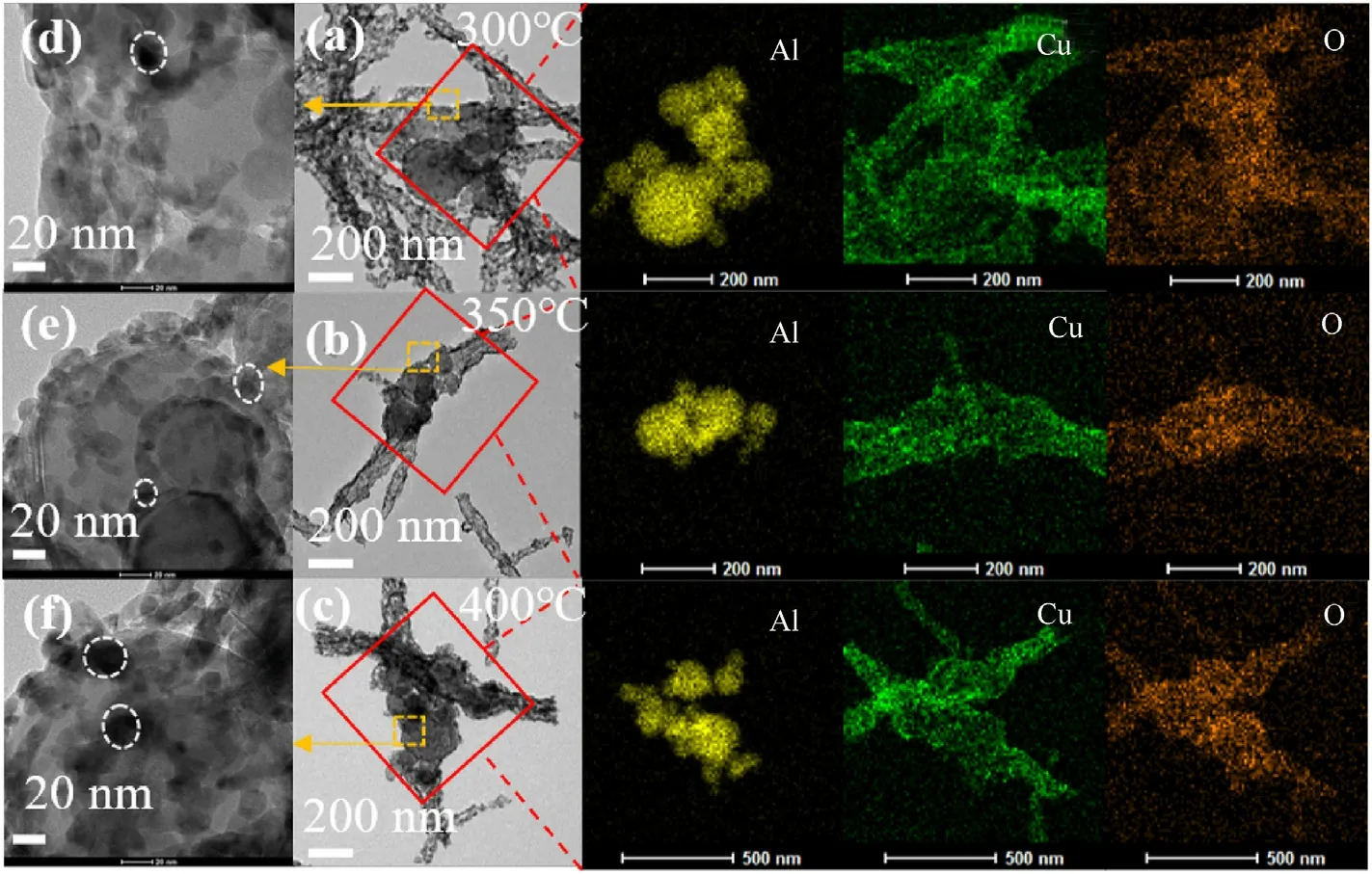
Fig.6.TEM and EDS mapping images of Al-core MIC fibers calcined at(a)300 °C,(b)350 °C,and(c)400 °C;The interface of Al in fibers calcined at temperatures of(d)300 °C,(e)350 °C,and (f) 400 °C.
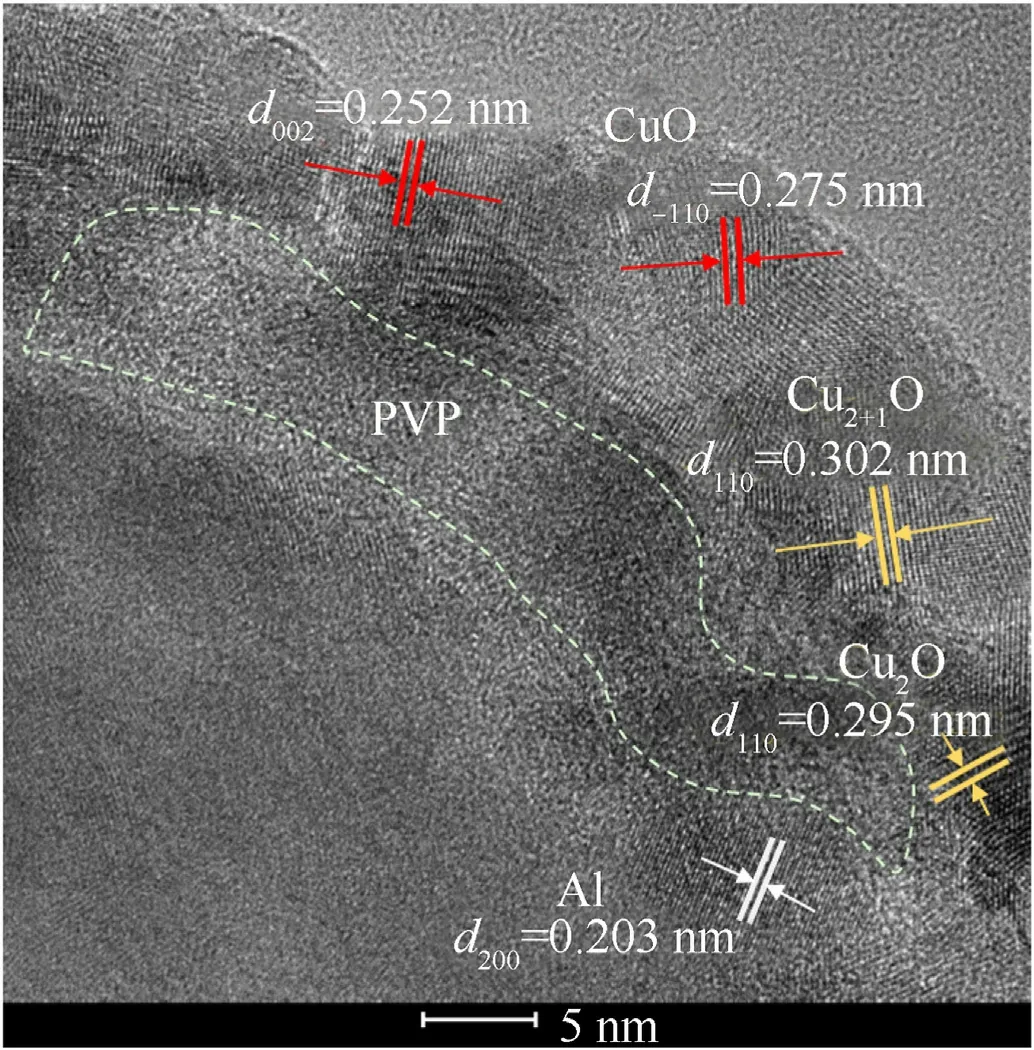
Fig.7.HRTEM images of the MIC fibers the interface calcined at 300 °C.
3.3.Compositions and chemical bonding analysis of Al-core MIC fibers
Fig.8a showed the XRD patterns of Al-core MIC fibers calcined at 300°C,350°C,and 400°C,respectively.The XRD pattern of the fibers calcined at 300°C only showed the partial peak of Al,Cu2O,and CuO due to the remaining PVP-coated MIC fibers.However,peaks of Al,CuO,and Cu2O were evident on the curve of the fibers calcined at 350°C for 2 h,indicating a complete decomposition of PVP,which was different from the thermal analysis results.At the calcination temperature of 400°C,the characteristic peak of Cu2O disappeared,and the MIC fibers consisted of Al and CuO at this time.In particular,the fibers calcined at 350°C seemed to have better crystallinity,which was consistent with the morphology from Fig.5(b-c).Maybe the oxidation of Al during calcination at 400°C increased the amorphous composition.The blocking effect covered by PVP[36]resulted in a low crystallinity and the intensity of the fibers calcined at 300°C was so weak that was difficult to analyze further.
XPS analysis were carried out on Al-core MIC fibers calcined at 300°C,350°C,and 400°C to obtain information on the surface layer.In the full XPS spectra Fig.8b,the N peak was present only for fibers calcined at 300°C,indicating that some undecomposed PVP was still present on the fiber surface.The high-solution XPS spectra of O 1s showing in Fig.8(c-e)illustrated the change in copper oxide oxidation during the calcination process.The O 1s of the MIC fibers at 300°C showed a small residual amount of PVP due to the peaks around 531.8 eV.These peaks 529.7 eV,530.6 eV,and 531.6 eV indicate CuO,Cu2O,and Al2O3,respectively,with peak 533.1 eV being the peak of adsorbed O.The presence of adsorbed O on the energetic fibers' surface at 300°C,350°C,and 400°C may be related to the fiber surface's porous structure.Comparing the CuO content of the three fibers from Table S1,it was found that CuO content increased as the calcination temperature increased,with Cu2O present on the surface of the calcined fibers at 300°C and 350°C and the fibers at 400°C by only CuO.At 400°C,the O 1s of Al2O3shifted,and its value changed from 531.5 eV to 531.2 eV.At this time,the outer layer of aluminium powder started to react with CuO so that the electron binding energy of the oxide layer on the surface of the nano aluminium powder became smaller.Al-core MIC fibers with different structures and compositions could be obtained by controlling the calcination temperature in a specific temperature range.
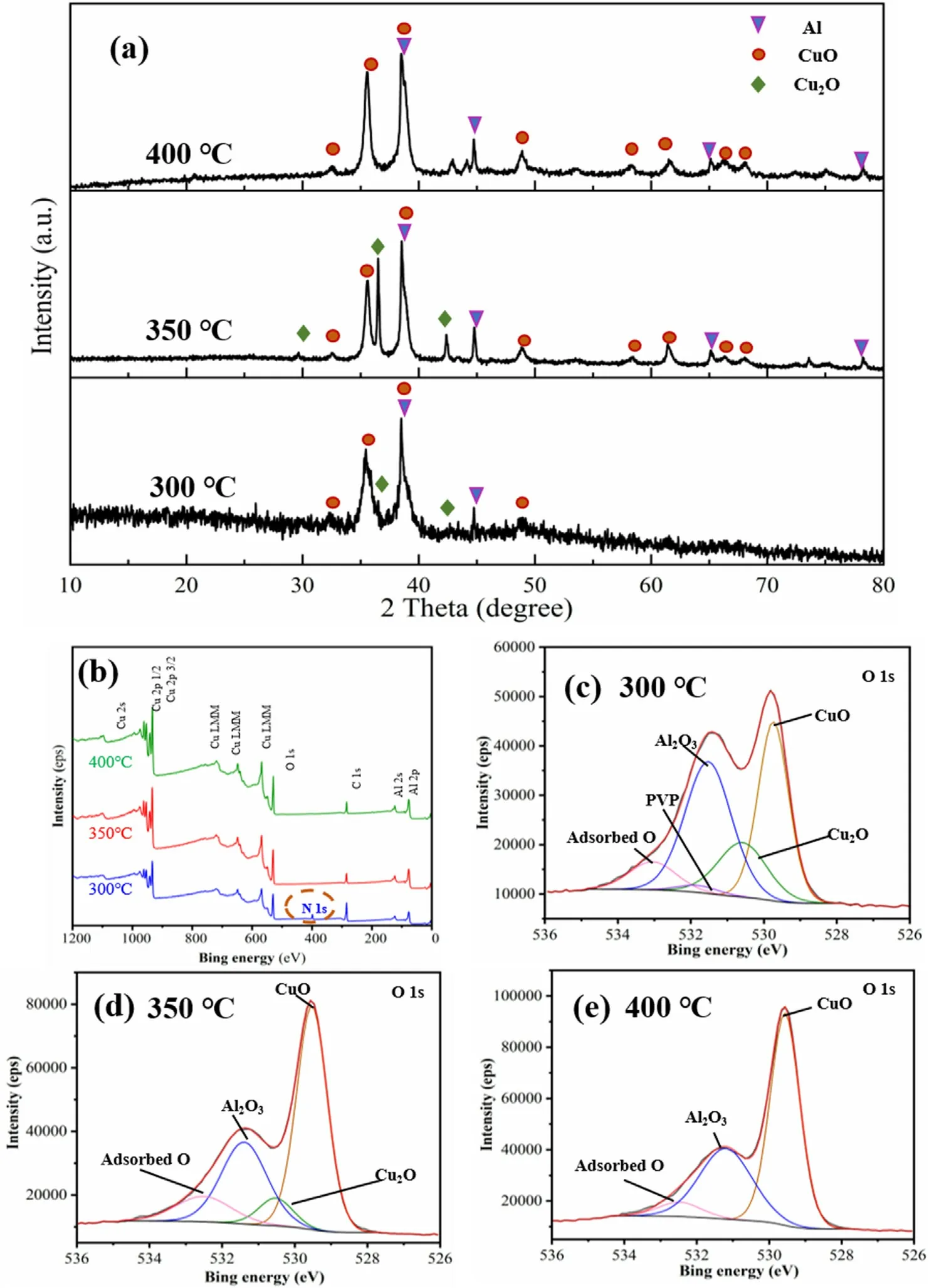
Fig.8.(a)XRD patterns of Al-core MIC fibers calcined at 300 °C,350 °C,and 400 °C.(b)Full XPS spectra of Al-core MIC fibers calcined at 300 °C,350 °C,and 400 °C.High solution XPS spectra of O 1s peaks for Al-core MIC fibers calcined at (c) 300 °C,(d) 350 °C,and (e) 400 °C.
3.4.Thermal analyses of Al-core MIC fibers
The thermal reactivity of Al-core MIC fibers calcined at different temperatures(300°C,350°C,and 400°C)was studied by thermal analysis.Fig.9a showed the DSC results of MIC fibers,and the DSC curve of fibers with different calcination temperatures was shifted in the y-axis for clarity.Two main exothermic peaks were observed for three MIC fibers.As seen in Table 1,the main exothermic peak before the melting point of Al started around 512°C.The peak temperatures are 574.6°C,575.6°C,and 574.6°C,respectively,with the exothermic values of 718.1 J/g,886.7 J/g,and 754.3 J/g,respectively.After the melting point of Al at 660°C,another exothermic peak with exotherms of 251.1 J/g,362.1 J/g,and 193.6 J/g,respectively.The exothermic heat of the MIC fibers calcined at 350°C is 1248.8 J/g,higher than the exothermic heat of 1160.7 J/g and 947.9 J/g at 300°C and 400°C.
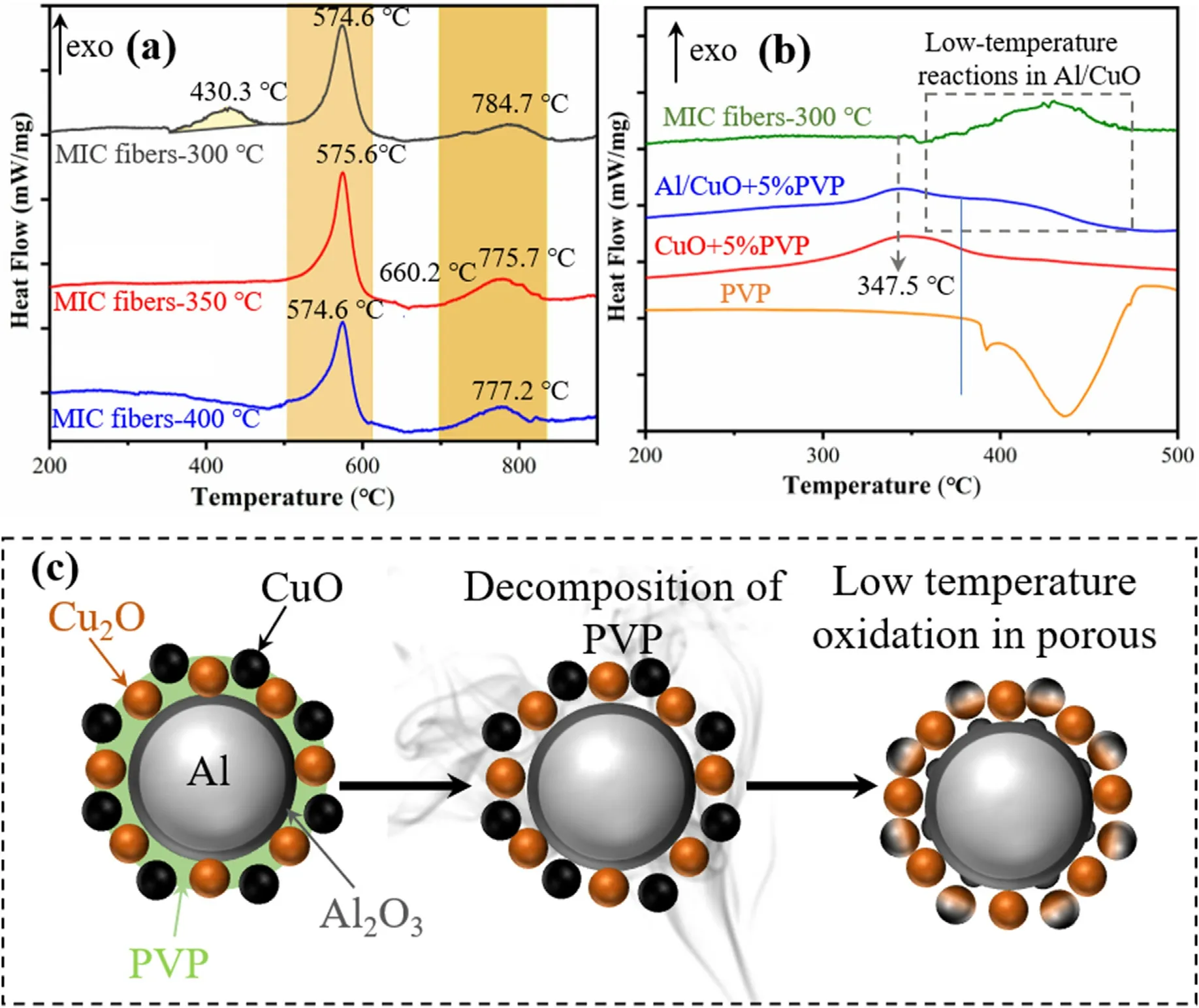
Fig.9.(a) DSC curves of Al-core MIC fibers calcined at 300 °C,350 °C,and 400 °C.(b) DSC curves of Al-core MIC fibers calcined at 300 °C,PVP,CuO-PVP5%,Al/CuO-PVP5%.(c)Reaction process at the Al interface of Al-core MIC fibers calcined at 300 °C.
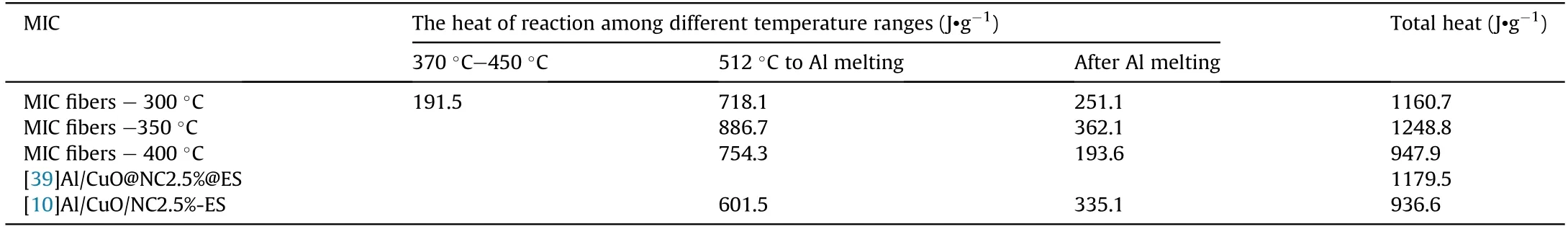
Table 1 Heat of reaction of Al/CuO MIC obtained in this work and reported in the literature.
However,a low-temperature exothermic peak with a value of 191.5 J/g in the range of 372°C-458°C appeared on the MIC fibers-300°C.The TG curves of the three MIC fibers in the argon atmosphere could be found in Fig.S5.This fiber had a significant weight loss of 6.29% between 372°C-458°C.This process could not consider as the decomposition of remaining PVP or lowtemperature exothermic reactions in Al/CuO directly.Further analysis of the reaction,the thermal reactivity of PVP,CuO-PVP5%,Al/CuO-PVP5% in Ar atmosphere were measured (experimental details were in supplementary material Experiment S1).As seen in Fig.9b,the decomposition temperature of PVP decreased from 380°C to 300°C in the condition of copper oxide,while an exothermic decomposition peak was observed.Comparing the lowtemperature exothermic behavior of CuO-PVP5% and Al/CuOPVP5%,it was found that the low-temperature exotherm of Al/CuO-PVP5% consisted of two peaks.The first peak at 347.5°C was attributed to the decomposition of PVP.A similar low-temperature exothermic peak was reported for Al/CuO prepared by arrested reactive milling(ARM)and vacuum layer deposition,Referring to a previous study[37,38].Defects and porous structures on the surface of CuO/Al would accelerate the decomposition of CuO to produce gaseous oxygen.For the Al/CuO-PVP5%,the second exothermic peak would be the low-temperature reaction in Al/CuO.It was proposed that decomposition of PVP produced porous before Al and CuO and the redox reaction occurring via release of oxygen from CuO that oxidizes Al at the nearby exposed Al2O3surface explains the formation of the low-temperature peak observed in MIC fibers-300°C.As noted above,the morphology illustrated schematically in Fig.9c was just an example.Compared to the previous work [9,39],the heat releases of Al-core MIC fiber were higher for its high uniformity and intimate contact of the reactants.Insufficient oxidation of precursors led to CuO and Cu2O as oxidants.However,it was foreseen that higher heat release could be achieved when suitable precursors or better calcination temperatures were found.Of course,the optimization of precursors and calcination temperature were not the focus of this work,and therefore was not further investigated.
3.5.Analysis of rapid ignition process
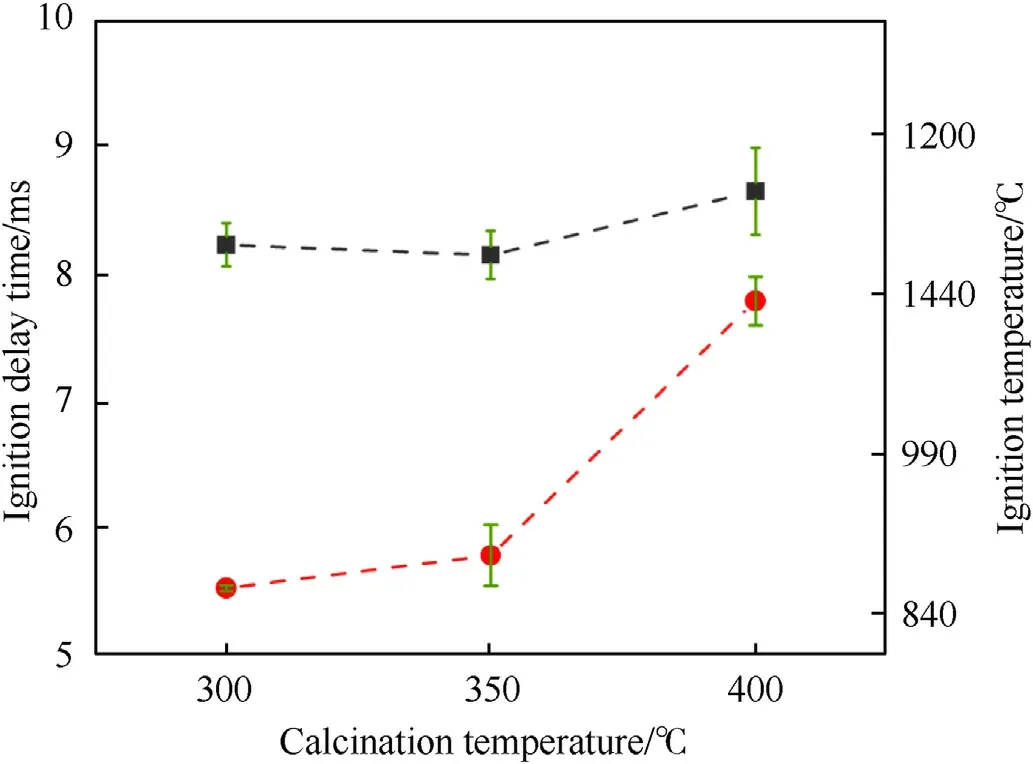
Fig.10.The ignition temperature and ignition delay times of the Al-core MIC fibers calcined at 300 °C,350 °C,and 400 °C.
The ignition temperature and ignition delay time for the Al-core MIC fibers were shown in Fig.10.The ignition temperatures of the energetic fibers calcined at 300°C,350°C,and 400°C were 863.83°C,894.55°C,and 1133.65°C,respectively.The ignition delay time was 8.22 ms,8.14 ms,and 8.64 ms,respectively.It was evident that the ignition temperature of MIC fibers increased with the increase of calcination temperature.Interestingly,the ignition delay time of MIC fibers calcined at 300°C was higher than the MIC fibers calcined at 350°C.In the MIC fibers calcined at 300°C,a part of the input energy was used to decompose PVP rapidly,and this might cause the input energy to increase so that the ignition delay time became larger.Previous work [39] showed that more gas products were beneficial for convective heat transfer.In the ignition stage,the porous structure was formed due to PVP decomposition,where the gas products promoted convection heat transfer between Al and CuO.And the O2produced by CuO was sufficiently diffused on the surface of Al powder.Therefore,it could be concluded that the activity of MIC fibers increased and the ignition temperature was low due to the presence of PVP.When the calcination temperature was 400°C,the thickening of the oxide layer of Al particles in MIC fibers caused a significant increase in ignition temperature and ignition delay time.It could be found that the decomposition of PVP contributed to the promotion of the reaction,although more heat needed to be absorbed in the heating phase.
3.6.Analysis of combustion performances
To analyze the combustion performance,open combustion experiments were carried out on Al-core MIC fibers calcined at 300°C,350°C,and 400°C under the same conditions.Fig.11 showed the combustion process of three MIC fibers.The combustion times of the MIC fibers calcined at 300°C and 350°C were 3.12 ms and 3.40 ms,respectively.The combustion time of 1.88 ms was significantly shorter for the fibers calcined at 400°C.The initial moment was the laser acting on the energetic fibers.The MIC fibers calcined at 300°C started to react with a flame expansion of 19 m/s,after which the maximum flame area was reached at 0.48 ms (fourth panel)with a flame height of 2.18 cm at this time,the combustion flame area was beyond the image acquisition range of the camera.The maximum flame height was maintained until 0.72 ms,then the combustion gradually decreased and ended at 3.12 ms.Unlike the 300°C calcined fibers with a significant combustion enhancement process,the 350°C calcined fibers had only a slight acceleration of combustion,with a maximum flame area at 0.28 ms and a flame height of 1 cm.The flame started to weaken at 1.00 ms and ended at 3.40 ms.The Al-core MIC fibers calcined at 400°C had no combustion diffusion phase,and the maximum flame height was 0.75 cm after 0.48 ms.The combustion started to diminish and ended at 1.88 ms.
The detailed ignition mechanism can be derived from the combustion phenomenon.The MIC calcined at 300°C had a larger flame area and shorter burn time.On the one hand,the remaining PVP rapidly decomposed and released a large amount of gas upon ignition.The thrust generated by the gas pushes the reaction zones to spread rapidly,creating a larger flame area and height.On the other hand,from a previous study [40],the products generated outside the flame to be removed faster by the gas so that the flame moves closer to the surface of the particles.This process accelerated the evaporation of aluminum and the transport of oxygen to the surface of the aluminum particles.Obviously,over-calcined 400°C MIC fibers showed a reduced reactivity and shorter burning time.
4.Conclusion
In this paper,PVP was used as a template to prepare Al-Core heterojunction MIC fibers by calcining the initial Al@CAM/PVP fibers of electrospinning.The morphology,thermal reactivity,and ignition and combustion properties of the MIC fibers were investigated.The following conclusions were obtained.MIC fibers with different morphologies,compositions,and properties can be obtained by controlling different calcination temperatures.The shell layers of MIC fibers calcined at 300°C,350°C,and 400°C were CuxO and PVP,CuxO,and CuO,respectively.Thermal analysis showed that the MIC fibers calcined at 300°C had unique properties,and a different reaction(occurring from 372°C to 458°C)was found.It could be ascribed to a low-temperature reaction between CuO and Al resulting from PVP decomposition.Ignition and combustion tests indicated that MIC fibers calcined at 300°C have lower ignition temperatures and higher flame height.All these indicated that a small amount of PVP can play a positive role in nanothermites,such as serving as a template,the protection of Al during calcination,and the promotion of combustion.The optimization of calcination preparation conditions and the precise mechanism of PVP in MIC fibers are future works.

Fig.11.Sequential burning images of Al@CuxO MIC fibers calcined at 300 °C,350 °C,and 400 °C.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.10.006.
杂志排行
Defence Technology的其它文章
- A review on the high energy oxidizer ammonium dinitramide:Its synthesis,thermal decomposition,hygroscopicity,and application in energetic materials
- Blast wave characteristics of multi-layer composite charge:Theoretical analysis,numerical simulation,and experimental validation
- Task assignment in ground-to-air confrontation based on multiagent deep reinforcement learning
- Deep learning-based LPI radar signals analysis and identification using a Nyquist Folding Receiver architecture
- A small-spot deformation camouflage design algorithm based on background texture matching
- Quasi-static and low-velocity impact mechanical behaviors of entangled porous metallic wire material under different temperatures
