A review on the high energy oxidizer ammonium dinitramide:Its synthesis,thermal decomposition,hygroscopicity,and application in energetic materials
2023-01-30FuyoChenChunleiXunQingqingLuLeiXioJunqingYngYuingHuGungPuZhngYingleiWngFengqiZhoziHoWeiJing
Fu-yo Chen ,Chun-lei Xun ,Qing-qing Lu ,Lei Xio ,Jun-qing Yng ,Yu-ing Hu ,Gung-Pu Zhng ,Ying-lei Wng ,Feng-qi Zho ,G-zi Ho ,**,Wei Jing ,*
a National Special Superfine Powder Engineering Technology Research Center,Nanjing University of Science and Technology,Nanjing,China
b Xi'an Modern Chemistry Research Institute,Xi'an,China
Keywords:Ammonium dinitramide High energy oxidizer Synthesis Properties Applications
ABSTRACT Ammonium dinitramide (ADN) is considered as a potential substitute for ammonium perchlorate in energetic materials due to its high density,positive oxygen balance,and halogen-free characteristics.However,its application has been severely limited because of its strong hygroscopicity,difficult storage,and incompatibility with isocyanate curing agents.In order to better bloom the advantages of the highly energetic and environment-friendly ADN in the fields of energetic materials,an in-depth analysis of the current situation and discussion of key research points are particularly important.In this paper,a detailed overview on the synthesis,thermal decomposition,hygroscopic mechanism,and antihygroscopicity of ADN has been discussed,its application in powdes and explosives are also presented,and its future research directions are proposed.
1.Introduction
Energetic materials are either intrinsic explosives or mixtures of an oxidizer with one or several fuels,whose self-sustained reaction can release energy.They have a wide range of applications in the military and civil fields [1-3].In the military field,energetic materials are often used in the launch of rockets and missiles,and they can also be used as explosives for military blasting.In the civil field,energetic materials are often used in mining,geological prospecting,and festive fireworks.Based on the different uses of energetic materials,they can be divided into propellants,pyrotechnics,and explosives[4,5].A solid propellant is an energetic composite material that contains an oxidizer and a combusting agent and can burn in an oxygen-free environment to produce a large amount of gas [6].It is mainly composed of oxidizers,fuels,adhesives,plasticizers,and other main components,as well as auxiliary components such as curing agents,catalysts,and antiaging agents.Solid propellants are widely used in aerospace,tactical and strategic missile launches,underwater blasting,and other fields.For a long time,high energy has always been the goal pursued by solid propellants,and the energy level of solid propellants is closely related to their composition.The use of high energy oxidizers,fuels,and energetic binder components is the key way to increase the energy level of solid propellants [7].The oxidizer represents a large proportion of the composition of a propellant.It mainly provides the oxygen necessary to release the energy of the fuel,but also makes a great contribution to the energy of solid propellant [8].
Nowadays,the most widely used oxidizers in solid propellants include ammonium nitrate (AN),ammonium perchlorate (AP)[9,10],and several nitramine explosives (such as hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX),1,3,5,7-tetranitro-1,3,5,7-tetrazocane(HMX),and2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane (CL-20)) [11-14].However,these oxidizers have their own limitations in practical applications.For example,r AP produces a large amount of chlorine-containing gas during the decomposition process,which can cause ozone layer depletion,acid rain,and even thyroid cancer by contaminating soil and water[15-17].AN has multiple crystal phase transitions in practical applications,which greatly reduces the burning rate and specific impulse of solid propellants.RDX and HMX have poor mechanical properties and combustion characteristics.CL-20 has a high mechanical sensitivity that makes its handling hazardous(the impact sensitivity is 4 N m and the friction sensitivity is 54 N) [18-20].Therefore,the development of a new type of high energy oxidizer is of great significance.So,far,new oxidizers have been reported,such as ammonium dinitramide (ADN),hydrazinium nitroformate(HNF),and 1,3,3-trinitroazetidine (TNAZ) [21].Among these oxidizers,only the research and preparation of ADN is relatively mature,and it has been used by Russia in Topol intercontinental ballistic missiles (SS-24 and SS-27) [22].
AlgorithmStage reward mechanismMultihead attention mechanism PPO-GNA✓✓PPO-S✓✘PPO-M✘✓PPO✘✘
In 1971,the Zelinsky Institute of Organic Chemistry synthesized ADN for the first time in Moscow,USSR.The synthesis technology of dinitramide ammonium salt in the USSR was strictly confidential.It was in 1988 that ADN has attracted wide attention for it was synthesized “again” by the Stanford Research Institute in the USA[23,24].
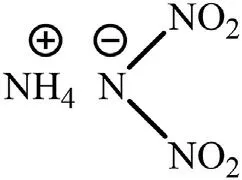
Fig.1.Molecular structure of ADN.
ADN is a chlorine-free inorganic oxidizer that is composed of NH4+and N(NO2)2-(Fig.1).It is not only an important component in solid propellants,but it is also a kind of explosive with excellent performance (the explosive heat of pure ADN is 3.92 kJ/g,and the detonation velocity of ADN is 6000 m/s when the charge diameter is 100 mm)[25,26].Compared with the currently used oxidizers in solid propellants,it has the advantages of low characteristic signal,high gas yield,and excellent enthalpy of formation(the gas yield is 1084 L/kg and the enthalpy of formation is -1207.4 kJ/kg) [27,28].Table 1 shows the comparison of the performance of ADN and some commonly used high energy oxidizers.
According to the reports,when ADN is used instead of AP in composite propellant formulations,the specific impulse of a rocket can be increased by 20%,with a 40%reduction in the total weight of the rocket motor.The National Aeronautics and Space Administration had calculated that using ADN as a potential candidate for replacement of AP in composite propellant formulations,the thrust of the space shuttle's booster can be increased by 14%and the load can be increased by 4 tons.In addition,by using ADN in low characteristic signal propellants,their total specific impulse can be increased by 7% [39].Flon et al.[40,41] used ADN in/Al/AP/Hydroxyl-terminated polybutadiene (HTPB) solid propellant instead of AP to study its energy and combustion performance.The theoretical specific impulse of the propellant increased by 3%,and its combustion temperature decreased by 4%.In addition,as a high energy oxidizer,ADN can be used not only for surface-to-air missiles in a small range but also for intercontinental missile boosters[42].It will play an indispensable role in the development of tactical and strategic missiles in the future.
The preparation method and application technology of ADN have been the research focus in the field of energetic materials,which include synthesis and prilling technology,surface coating technology,and application of ADN in powder and explosive formulations [43].
2.Synthesis of ADN
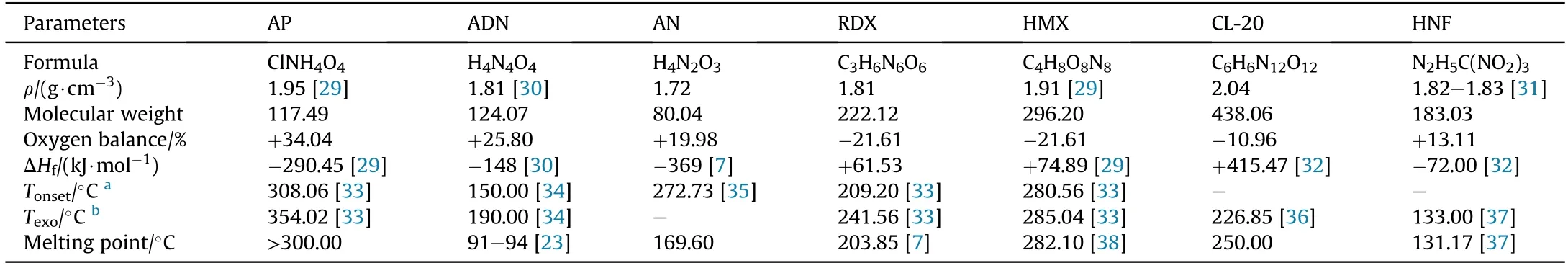
Table 1 Comparison of the physicochemical properties of some high energy oxidizers.
Since the USSR used the aminopropionitrile method to synthesize ADN in 1971,the USA,Switzerland,and Germany had successively carried out research on its synthesis.So far,there are at least 20 different methods for the synthesis of ADN that have been publicly reported,such as the aminopropionitrile method,urea method,carbamate method,and sulfamate method.
2.1.Aminopropionitrile method
As early as the 1970s,the USSR had studied the synthesis of ADN by the aminopropionitrile method[24],although the literature only reportedthelaststepofthesynthesismethod:(NO2)2NCH2CH2CN+NH3→ADN.Wang et al.[44]conducted an indepth study on the synthesis of ADN by the aminopropionitrile method (Fig.2),and the experimental steps are as follows:
a.An aqueous solution of β-aminopropionitrile and sodium hydroxide is cooled below 5°C.Then,propyl chloroformate is slowly added into the solution,and the resulting mixture is stirred for an hour.White solid N-n-propyl ester-β-aminopropionitrile(I)is prepared after filtration,washing,and drying.
b.Under stirring conditions,N-n-propyl ester-β-aminopropionitrile is added into pure nitric acid that is precooled below 10°C.After completion of the reaction,the solution is added to cold water.White solid N-nitro-β-aminopropionitrile ammonium (II) is obtained by extraction,concentration,and ammonization.
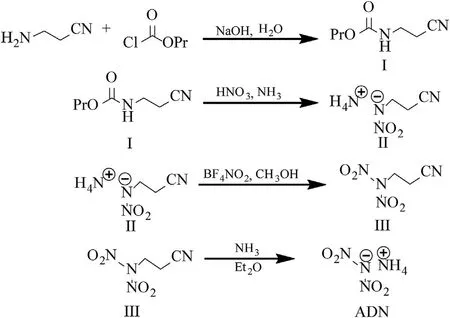
Fig.2.Synthesis of ADN by the aminopropionitrile method.
c.Under stirring conditions,nitronium tetrafluoroborate(NO2BF4)and N-nitro-β-aminopropionitrile are added into anhydrous acetonitrile that is precooled to -10°C.Yellow solid N,Ndinitro-β-aminopropionitrile (III) is prepared after extraction,washing,and drying.
d.N,N-dinitro-β-aminopropionitrile is dissolved in anhydrous ether at low temperature.Afterwards,ammonia is added in excess into the solution,and the reaction is continued for 30 min.After concentration,cooling,and crystallization,pale yellow solid ADN is obtained.Then,the crude product is crystallized twice with n-butanol to purify the crystals,and finally,pure white solid ADN is prepared.
2.2.Urea method
The urea method involves nitrating urea first to form nitrourea[45-47],then treating nitrourea with NO2BF4to produce dinitrourea (DNU),and finally mixing DNU with NH3to prepare ADN(Fig.3)[27].The advantage of this method is that the raw materials are very cheap and the synthesis steps are relatively simple.However,the reaction process needs to be carried out in low temperature and anhydrous conditions to prevent a very low yield(20%-45%).
2.3.Carbamate method
Stern et al.[27,48,49] proposed an efficient method for the synthesis of ammonium dinitramide from carbamate(Fig.4),which consists of the following steps:
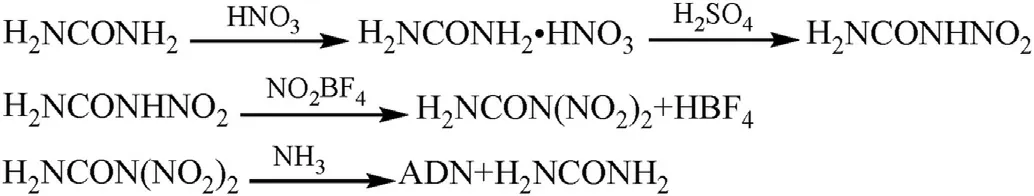
Fig.3.Synthesis of ADN by the urea method.
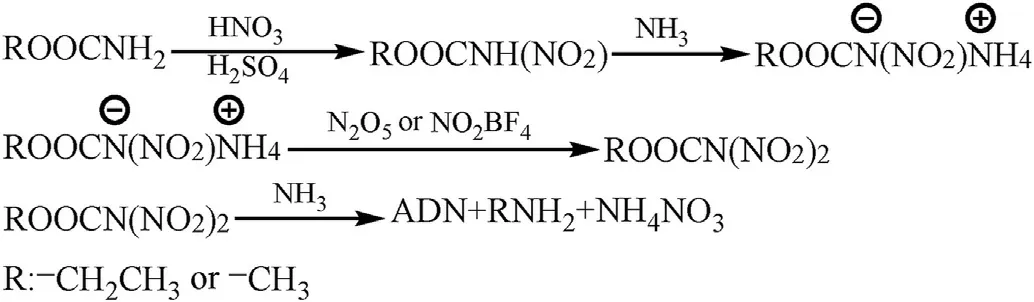
Fig.4.Synthesis of ADN by the carbamate method.
a.N-nitrocarbamate (ROOCNH(NO2)) is formed by reacting carbamate with mixed acid (e.g.,98% concentrated nitric acid and 98% concentrated sulfuric acid).
b.Then,ROOCNH(NO2) is treated with anhydrous ammonia to obtain the corresponding ammonium N-nitrocarbamate salt(ROOCNNO2-NH4+).
c.Finally,ROOCNNO2-NH4+is reacted with N2O5or NO2BF4to prepare the intermediate N,N-dinitrocarbamate(ROOCN(NO2)2)that is not isolated but is directly treated with ammonia to prepare ADN.
This method has a relatively high yield(40-60%).However,it is not suitable for large-scale production of ADN because it requires a large amount of solvent and strict reaction conditions caused by instability of intermediate products.
2.4.Sulfamate method
Langlet et al.[50] disclosed a method of preparing ammonium dinitramide from sulfamate.Dinitramidic acid (HN(NO2)2) is formed by the reaction of sulfamate with a sulfonitric mixture at a low temperature,and ADN is synthesized by neutralizing HN(NO2)2with an excess of ammonia (Fig.5).The final product can be obtained after filtration,washing,and drying.
The shortcomings of this method are lots of acid that is required and the large amounts of pollutants (such as nitrate and sulfate)that are produced after the reaction [51].In order to solve these problems,the European Energetic Material Company (EURENCO)had improved the method as summarized in the follow steps[52,53]:
a.On the basis of mixed acid nitration sulfamate,N-guanylureadinitramide(FOX-12)is prepared by adding amidinoureas salt to the nitration solution of dinitroamine.
b.Then,potassium dinitramide (KDN) is formed by reacting FOX-12 with alcoholic solution of potassium hydroxide.
c.Finally,ADN is obtained by the metathesis reaction of KDN with ammonium sulfate ((NH4)2SO4).
The improved process by the EURENCO significantly reduces the waste generated from the reaction,and the water consumption is reduced by more than 75% compared with the Swedish Defence Research Agency (FOI) process [43,54].The waste generated from the production of 1 ton ADN by the FOI process and the EURENCO process is shown in Table 2.
Although the EURENCO process can greatly reduce the consumption of materials,it still has obvious disadvantages,which are as follows:
a.K+ions may be mixed in the final product,which will not only increase the characteristic signal of ADN-based propellants,but they will also even cause catalyst poisoning during the combustion process of propellants.
b.For the intermediate product FOX-12 to be converted into ADN,a two-step reaction is necessary,and this process consumes a lot of materials and time.
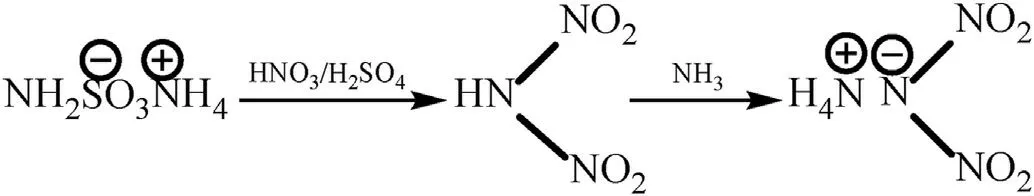
Fig.5.Synthesis of ADN by the sulfamate method.
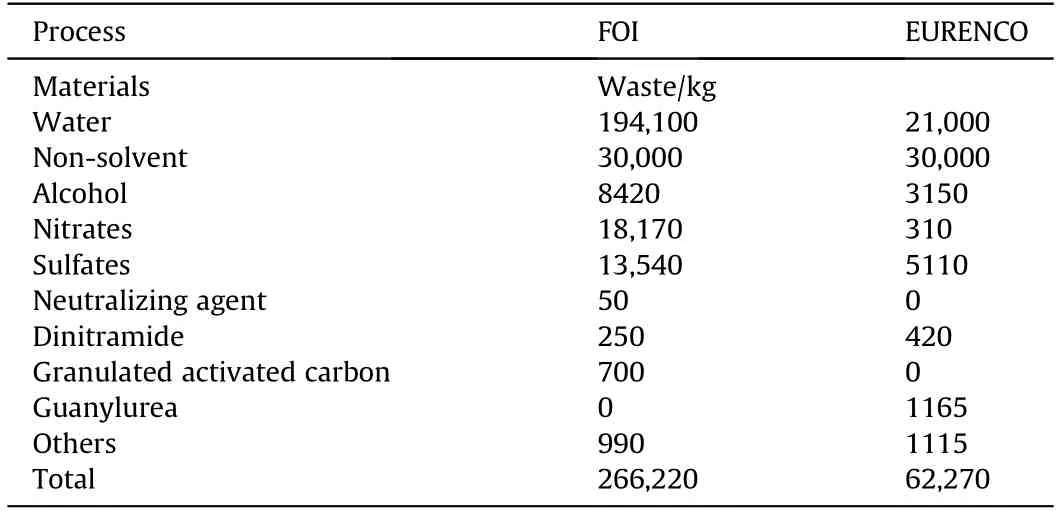
Table 2 Comparison of the waste for the production of 1 ton of ADN using the FOI process and the EURENCO process.
In 2014,a one-step method for the direct synthesis of ADN from FOX-12 was proposed by Stefan [15,55-57].ADN is formed by the metathesis reaction of FOX-12 with ammonium salts (such as(NH4)2SO4and NH2SO3NH4)in ethanol or isopropanol(Fig.6).This method is a more ideal synthesis method for it has fewer synthesis steps and does not introduce potassium ions,so the purity of the final product is higher.However,due to the low solubility of FOX-12 in alcohol,the consumption of organic solvents is high,and more than 500 L of organic solvents is required to produce 1 kg of ADN.To solve this problem,Stefan et al.[58]improved the method by using acetone instead of ethanol as the solvent of FOX-12,which greatly reduces the consumption of organic solvent.Only 50-100 L of organic solvent is needed to prepare 1 kg of ADN.In addition,the viscosity of the byproduct guanyl urea sulfate precipitate in acetone is lower than that in alcohol,which simplifies the filtration process and makes it more feasible to expand the production scale of ADN.
Liming Research&Design Institute of Chemical Industry[59,60]prepared ADN with a purity higher than 99.7% from FOX-12 by a one-step synthesis method,and the yield reached 80%.Lei et al.[61,62]used FOX-12 as the raw material to react with KOH solution and (NH4)2SO4solution,respectively,for preparing ADN with a purity of 99.8%.They also upscaled the synthesis process with a batch output up to 3 kg.
Among the methods described for preparing ADN,the sulfamate method is the most commonly used method for the synthesis of ADN.Comparison of the described methods is shown in Table 3.
3.Thermal decomposition of ADN
The melting point of ADN is 91-95°C,which is considerably higher than the data reported in the literature (83-95°C),mainly due to the different purity of ADN.It is found some impurities(e.g.,water and ammonium nitrate) in ADN significantly affect its melting point even at very low concentrations [20].After ADN is completely melted,it begins to decompose further,which is a process that largely depends on the experimental conditions and analytical methods.In addition,it is worth noting that molten ADN is extremely dangerous,and the impact sensitivity threshold of molten ADN is similar to that of nitroglycerin (<0.25 J),far lower than that of solid ADN (4J) [63].Therefore,it is necessary to pay attention to safety when dealing with molten ADN or working with experimental devices in which molten ADN is formed.
Rossi et al.[64] studied the thermal decomposition behavior of ADN in a vacuum chamber and monitored the evolved gas using coupled thermal analysis techniques (TGA-QMS).The authors pointed out that the thermal decomposition reaction path of ADN has three types:
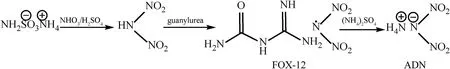
Fig.6.One-step synthesis of ADN from FOX-12.
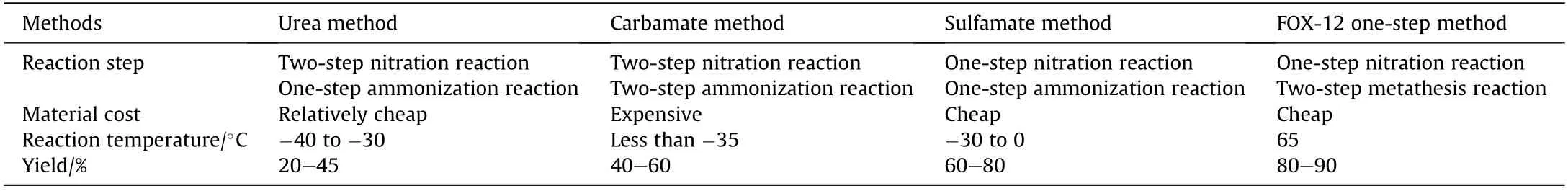
Table 3 Comparison of the different methods for preparing ADN.
NH4N(NO2)2→NH3+HN(NO2)2→NH3+HNO3+N2O
NH4N(NO2)2→2NH2NO2→2N2O+2H2O
NH4N(NO2)2→NH4NO3+N2O
After the decomposition of ADN is completed,the other side reactions still proceed,and the final products include NH3,HNO3,N2O,H2O,NO,HN(NO2)2,HNO2,and N2,among others.
In general,the differential scanning calorimetry (DSC) curve of ADN consists of two exothermic peaks and two endothermic peaks(Fig.7)[23,65,66].The endothermic peak at 91-94°C is attributed to the melting of ADN,whereas the exothermic peak at 150-190°C is caused by the thermal decomposition of ADN into NH4NO3and N2O.Oxley et al.[67]attributed the second exothermic peak to the dissociation of NH4NO3when the temperature is higher than 270°C.Finally,another endothermic peak is produced due to the sublimation of water (heating rate is 20°C/min).
Yang et al.[35] conducted an in-depth research on the thermal decomposition and combustion mechanism of ADN.The author pointed out that the thermal decomposition of ADN is strongly related to the initial sample,pressure,temperature,and experimental methods.It is found that the decomposition of ADN is an acid-catalyzed and self-accelerating process,which is hindered by alkali and water [68-70].
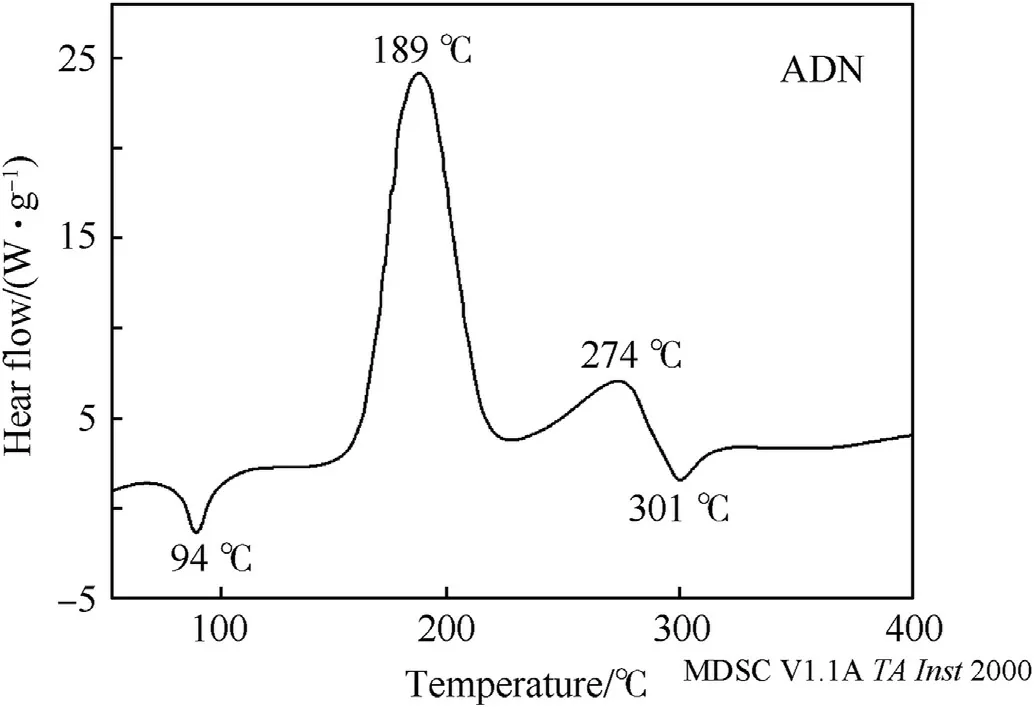
Fig.7.The DSC curve of ADN.
Matsunaga et al.[71,72]pointed out the thermal decomposition process of ADN can be summarized as follows:
a.NH3and HN(NO2)2are formed by the decomposition of ADN.
b.Then,HN(NO2)2decomposes into N2O and HNO3.
c.Under acid catalysis condition,NH4NO3is formed by the reaction of HNO3with NH3.
d.The side reaction of HN(NO2)2gradually dominates under high temperature conditions.
e.The decomposition of NH4NO3into N2O and H2O is the last step of the thermal decomposition reaction.
NH4N(NO2)2→NH3+HN(NO2)2
HN(NO2)2→HNO3+N2O
HNO3+NH3→NH4NO3
HN(NO2)2→NO2++HNNO2-
NH4NO3→N2O+2H2O
Löbbecke et al.[73] investigated the thermal decomposition behavior of ADN by DSC,TGA,and evolved gas analysis(EGA).DSC plot shows that the melting point of ADN is 91.5°C,and its initial decomposition temperature is 127°C.ADN first dissociates into NH3 and HN(NO2)2,which is immediately followed by the exothermal decomposition of HN(NO2)2 to produce N2O and HNO3.TGA plot shows that the mass loss of the initial sample is 30%in the first stage (ADN→NH4NO3+N2O).In the second stage,NH4NO3begins to decompose into N2O and H2O.The decrease in NH4NO3and the increase in N2O at T>200°C were also confirmed by the EGA plot.The thermal decomposition of ADN is as follows:
NH4N(NO2)2→NH3+HN(NO2)2
→NH3+HNO3+N2O
→NH4NO3+H2O
NH4NO3→N2O+2H2O
In addition to the above main reactions,the author also proposed some side reactions in the process of thermal decomposition,including the following steps:
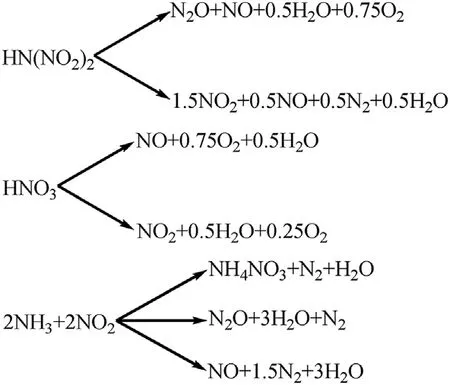
2NH3+3N2O→3H2O+4N2
2NO2+H2O→HNO3+HNO2
Park et al.[74] studied the thermal decomposition behavior of ADN by pyrolysis mass spectrometry under low pressure and in a temperature range of 373-920 K.They found that under lowpressure conditions,the following reactions play a major role.
Chain initiation reactions:
NH4N(NO2)2→NH3+HN(NO2)2
HN(NO2)2→HNNO2+NO2
HNNO2+M→OH+N2O+M
Chain propagation reactions:
OH+NH3→H2O+NH2
NH2+NO2→H2NO+NO
NH2+NO→H+N2+OH
The authors also pointed out that when the thermal decomposition of ADN is carried out under high pressure conditions,the radical-radical reaction involving HNNO2will gradually play a major role due to the increasing concentration of radical species.
HNNO2+OH→HNOH+NO2
→HNO+HONO
HNNO2+NO→HNNO+NO2
→HONO+N2O
HNNO2+NO2→HNO+N2O3(NO+NO2)
→HNO3+N2O
From the results of various studies,it is not difficult to see that the thermal decomposition mechanism of ADN largely depends on the initial sample,experimental temperature,experimental pressure,and analysis method used by the researcher.In general,the thermal decomposition process of ADN can be divided into two stages.In the first stage,ADN begins to dissociate and finally produces N2O and NH4NO3.In the second stage,NH4NO3is further decomposed to produce N2O and H2O.In addition,the thermal decomposition process of ADN is accompanied by some side reactions.The final decomposition products include NH3,HNO3,N2O,H2O,NO,HN(NO2)2,HNO2,and N2,among others.
4.Research on hygroscopic mechanism of ADN
The research on the hygroscopicity of ADN has already made some achievements,but the published reports are rarely known.It is only known that the treatment condition of ADN must be at a relative humidity (RH) of lower than 55% [75].Hatano et al.[46]indicated that ADN will deliquescence rapidly when the relative humidity is higher than 70%,but they did not explain the reason for its strong hygroscopicity.Teipel [20] reported that ADN with a lower water content has better stability,whereas completely anhydrous ADN is highly unstable.He also pointed out that when the water content of ADN increases from 0.1 to 0.5%,its thermal stability will not deteriorate.
The moisture absorption of ADN is closely related to its structure.X-ray single-crystal diffraction data shows that the threedimensional structure of ADN joined by the fourth longer hydrogen bond is an unusual two-fold three-dimensional interpropagation network structure [76,77].This special structure makes the hydrogen bonds in ADN crystals (Fig.8) stronger than those in AP crystals,which causes ADN to be more hydrophilic.Cui et al.[76]compared the DSC plot of moisture-containing ADN with that of AP crystals.They found that a large amount of bound water is present in ADN crystals,which is caused by the strong hydrogen bonds between ADN and water molecules.Hence,the special hydrogen bond in ADN crystal is the main factor leading to the increase in its hygroscopicity,which was confirmed by Wang et al.[78].
In addition to hydrogen-bonding actions,the strong hygroscopicity of ADN is also due to the following factors:
a.ADN is a very polar inorganic salt,and its solid surface has a very strong electrostatic interaction with water molecules in the air.These factors allow ADN to strongly attract moisture in the air.
b.ADN easily decomposes when exposed to light,and the decomposition process will produce highly hygroscopic ammonium nitrate covering its surface.NH4NO3continuously absorbs water from the environment,further aggravating the moisture absorption of ADN.
c.In line with the theory of surface chemistry,due to the high surface energy of ADN,it has a strong tendency to adsorb water molecules to reduce its own surface energy.Therefore,ADN is more likely to absorb moisture.
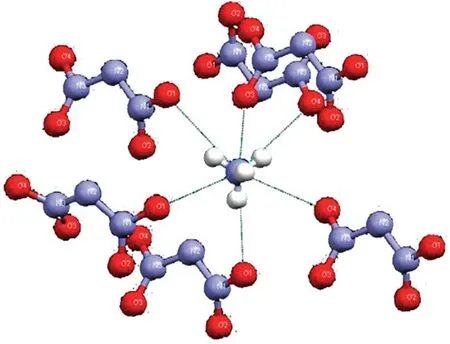
Fig.8.Hydrogen bonds among ADN molecules around NH4+.
Fig.9.Hygroscopic mechanism of ADN.
From a microscopic point of view [79],the interior of ADN particles presents a porous structure,which is composed of many capillaries.Due to the effect of capillary adsorption force,the saturated vapor pressure of the capillary meniscus is lower than the external vapor pressure,which provides conditions for the diffusion of water vapor into ADN crystals.Finally,ADN crystals incline to deliquesce and even agglomerate.
The hygroscopic process of ADN also follows the double-film theory of diffusion and mass transfer process [8,80].The surface of ADN crystals adsorbs water molecules through electrostatic force and hydrogen bonding.When the amount of adsorbed water is sufficient,the crystal surface begins to dissolve and forms a saturated solution film that continuously diffuses into the crystal and forms a saturated aqueous solution of ADN [81].Fig.9 shows the hygroscopic mechanism of ADN.
Wang et al.[82] studied the moisture absorption process of prilled ADN by dynamic analysis and pointed out that the process can be divided into the following three stages:
a.The surface moisture absorption process is the first stage where ADN absorbs moisture relatively smoothly,which mainly occurs on the spherical surface.This stage belongs to multimolecular layer adsorption and conforms to the BET adsorption theory.
b.The second stage is the destruction process of the surface morphology.In this process,the hygroscopicity of ADN increases sharply,and the spherical macroscopic morphology of ADN particles changes sharply,which can be explained by the capillary condensation theory.
c.The third stage is the gradual deliquescent liquefaction process.
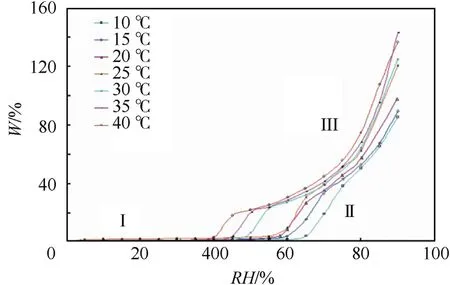
Fig.10.Isothermal hygroscopic curves of ADN at different temperatures.
In the first two stages,the hygroscopic process of ADN is mainly the result of the interaction between water vapor and the spherical ADN surface,whereas in the third stage,the deliquescent liquefaction process of ADN is mainly the result of the interaction between water vapor and ADN liquid interface.The isothermal hygroscopic curve of ADN is shown in Fig.10.
Nie et al.[83] pointed out that the moisture absorption of ADN largely depends on relative humidity,temperature,and sample purity.It is found that when the relative humidity is higher than 50%,ADN will deliquesce within 10 h.Wang et al.[84] indicated that the critical relative humidity of ADN is 59%,which is similar to the result reported by Wingborg[75](the critical relative humidity is 55.2%).
5.Research progress on anti-hygroscopicity of ADN
A newly synthesized ADN generally has needle or flake structures,which can easily absorb moisture and agglomerate [85,86].Directly using this raw material in propellant and explosive formulations will seriously affect their mechanical properties and combustion performance[87].In order to solve these problems,it is necessary to conduct an anti-hygroscopic treatment for ADN before it is used in practical applications.So far,various methods have been utilized to reduce the hygroscopicity of ADN,such as prilling,surface coating,and crystal modification.
5.1.Prilling technology of ADN
Prilling technology is the most commonly used method for the anti-hygroscopic treatment of ADN,which reduces its hygroscopicity by optimizing its surface morphology.Currently,this technology mainly includes emulsion crystallization,spray prilling,ultrasonic-assisted prilling,jet milling,micro-reaction,and film emulsification crystallization technologies [88].This technology can improve the stability and the safety of ADN,by increasing its thermal decomposition temperature,and reducing its mechanical sensitivity and its strong hygroscopicity.
5.1.1.Emulsion crystallization technology
Emulsion crystallization technology is a method where raw ADN is heated and melted in a nonpolar solvent,and then,ADN droplets are crystallized to form spherical particles by means of rapid cooling (Fig.11 and Fig.12).As early as the 1990s,the FOI had carried out research on the preparation of prilled ADN by emulsion crystallization technology [32].The steps are as follows:
a.Raw ADN is added to a nonpolar solvent with stirring to emulsify the solution.
b.The emulsion is heated to above the melting point of ADN,cooled,and crystallized to form spherical ADN particles.
c.The final product is obtained after filtration,washing,and drying.
The spherical ADN particles prepared by this method have a particle size of 25-250 μm,and the single output is 20-30 g.
Teipel et al.[89,90] proposed the preparation of prilled ADN from the emulsion crystallization method that consists of the following steps:
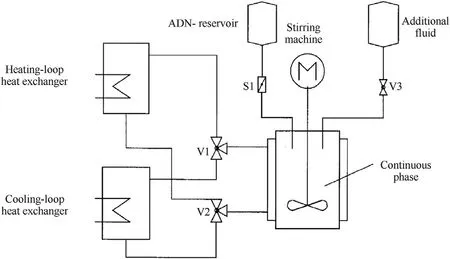
Fig.11.Emulsion crystallization process.
a.ADN is dissolved in a polar solvent.Then,the ADN solution is emulsified with silicon oil or paraffin oil.
b.The emulsion is cooled below the melting point of ADN,so as to promote the crystallization of ADN droplets to form spherical particles.
In order to suppress the supercooling phenomenon of droplets,a stirring device is also used in the cooling and crystallization process.The prilled ADN prepared by this method is uniform in shape with a particle size of 10-600 μm (Fig.13).
Guimont et al.[91] heated ADN powder with a stabilizer in a container in order to form molten droplets,which are dripped into the cooling tank (containing a coolant) under the action of gravity and crystallized to form spherical particles.The prilled ADN particles prepared using this process can meet the particle size requirements for general oxidizers in propellants and can be produced on a large scale (in kilogram level).
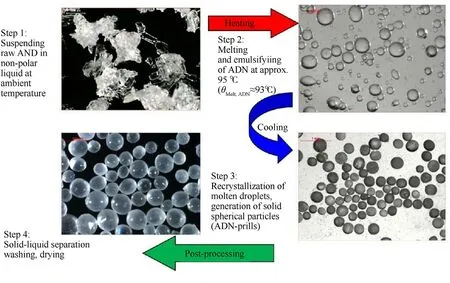
Fig.12.Principal steps of the emulsion crystallization process.
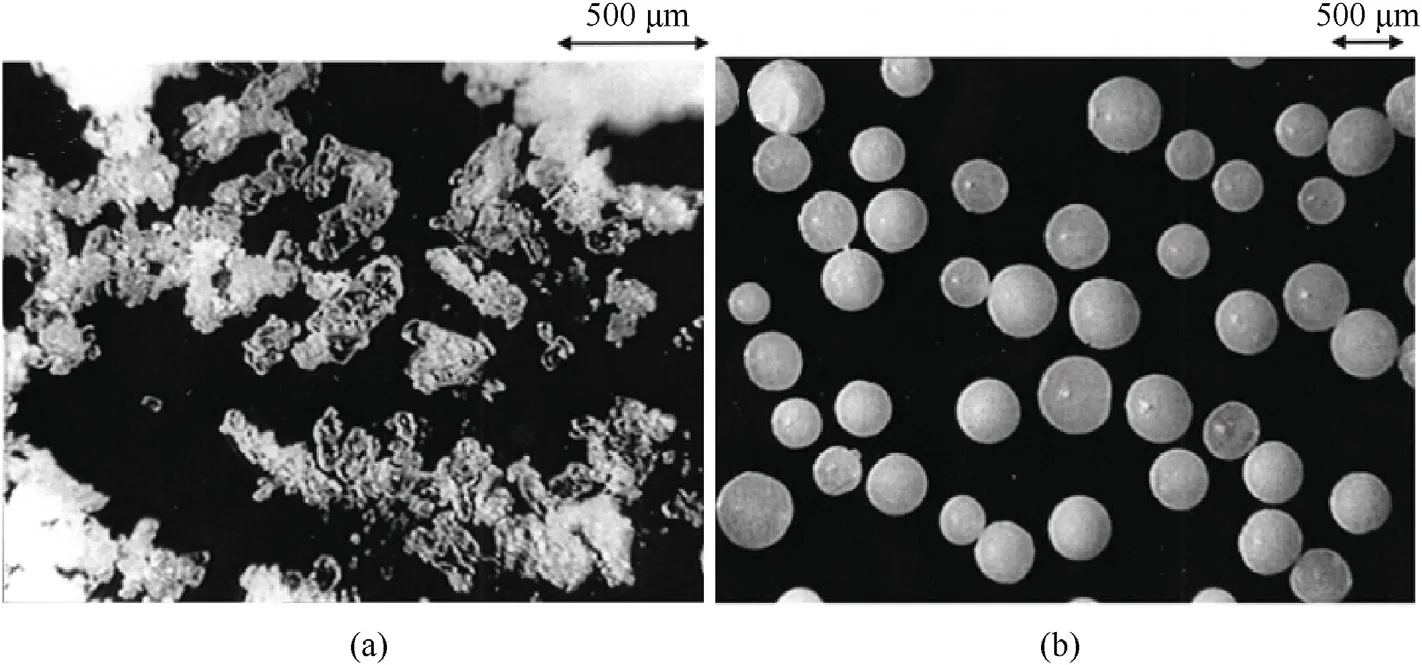
Fig.13.(a) Newly synthesized ADN (b) Prilled ADN after the emulsion crystallization process.
Wang et al.[92,93] prepared spherical ADN with a particle distribution d50=177 μm by the emulsion crystallization method.Ma et al.[87,94] used this method to obtain prilled ADN with a particle distribution d50=352 μm.Hygroscopic test results show that at a temperature of 30°C and a relative humidity of 74.9%,raw ADN has completely transformed into an aqueous solution after 6 h,whereas prilled ADN can still maintain a relatively intact morphology after 96 h,and its water content is only 1.948%.
Compared with other prilling technologies,emulsion crystallization technology has some disadvantages.For example,the surface of prilled ADN particles may contain oil residues,and cleaning the particles will generate a large amount of liquid organic waste.In addition,the purchase and maintenance costs of the equipment are high when the production scale is expanded.The effective solution of these problems will be the key to the wide application of this method.
5.1.2.Spray prilling technology
Spray prilling technology involves creating a two-phase equilibrium environment with the interface energy approaching to a minimum in order to achieve spherical granulation.Using this technology,most liquid materials (such as solution,emulsion,and molten liquid,etc) can be quickly prepared into micron and nano particles with high sphericity.
5.1.2.1.Spray prilling technology (ADN is processed in molten state).
In recent years,the FOI had developed spray prilling technology and used it in the preparation of spherical ADN.The general process is as follows [95-97]:
a.Raw ADN is added into a stainless-steel reactor,and a mixture of ethylene glycol and water is used as a heating medium of the reactor.
b.When all the raw materials are melted,the pneumatic valve at the bottom of the reactor is opened.ADN droplets are ejected from the spray nozzle and fall into a coolant under pressurized nitrogen.
c.Prilled ADN is collected after filtration and drying.
In order to reduce the melting time of ADN,a pneumatic stirring device is used for stirring during the heating process.The advantage of this method is that the nozzle diameter and nitrogen pressure in the process can be adjusted to control the particle size of prilled ADN.The schematic and device diagrams of the spray prilling method are shown in Fig.14.
After morphological characterization of prilled ADN,it is found that the surface of the particles is relatively rough with a lot of holes,and the particle size distribution is non-homogeneous(Fig.15).This is mainly due to the fact that thermal stabilization is not used in the prilling process,and part of ADN is decomposed at high temperatures.In addition,the lack of vacuum during the process will also cause more pores on the surface of ADN.Table 4 shows the properties of raw ADN and prilled ADN.
5.1.2.2.Spray prilling technology (ADN is processed in solution).
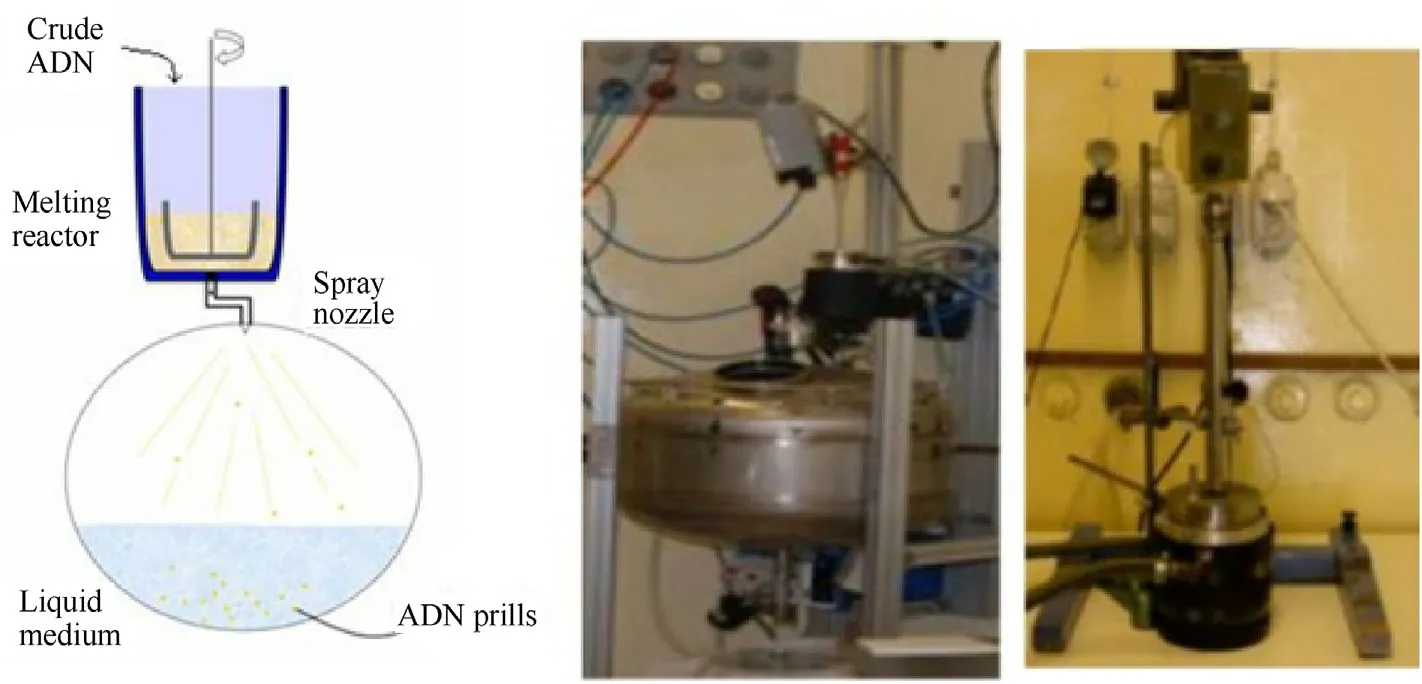
Fig.14.Diagram and process setup of spray prilling technology.

Fig.15.SEM images of (a) raw ADN and (b) prilled ADN prepared by the spray prilling process.
Spray flash evaporation (SFE) is another spray prilling technology which atomizes the solution by means of a hollow heating nozzle,and evaporates the solvent by heating at constant pressure to crystallize the solute into spherical particles[98].As a novel prilling technology,it has been gradually applied to the pharmaceutical engineering,energetic materials and other fields due to the advantages of short drying period,high efficiency,and less particle aggregation [99-102].Berthe et al.[103] recrystallized ADN in different solvent (methyl acetate and ethyl acetate) by the SFE process to obtain nanoparticles of ADN(nano-ADN) and study the shape factor effect on its properties.Compared with the crude ADN,a noticeable reduction of the particle size of the crystallized ADN is observed from SEM images (Fig.16 and Fig.17).The average diameters of ADN crystallized in methyl acetate and ethyl acetate are 32 nm and 34 nm,respectively (Fig.18) which indicates that SFE technology can effectively ultrafine ADN particle.Concerning the sensitivity tests,the nano-ADN is less sensitive to impact with a value measured at 4.5 J,but is more sensitive to electrostatic discharge (ESD) with a value of 996 mJ,and the sensitivity of the nano-ADN to friction remains unchanged(Table 5).All these results show that SFE technology is very suitable for the preparation of submicron and nanometer ADN particles with excellent performance.
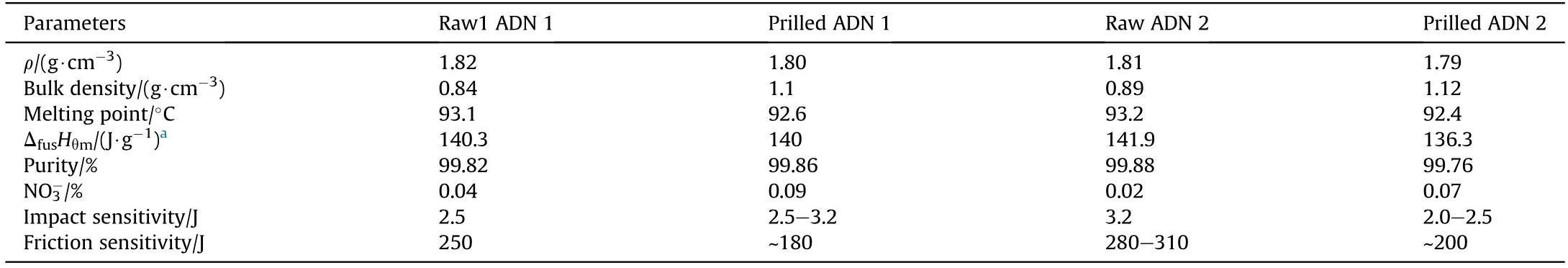
Table 4 Properties of raw ADN and prilled ADN.
5.1.3.Ultrasonic-assisted prilling technology

Fig.16.SEM image of crude ADN.
Although the spray prilling method has the advantages of simple operation and high efficiency,it also has obvious disadvantages such as the production of ADN with a wide particle size distribution,and a density which is lower than the theoretical density by 1-2%.In order to overcome these problems,Liljedahl et al.[104]proposed an ultrasonic-assisted prilling method that uses an ultrasonic nozzle instead of an ordinary nozzle to release energy so that molten ADN is more uniformly dispersed in the medium,thereby preparing spherical ADN with a high density.
Langlet et al.[105]prepared spherical ADN particles by another ultrasonic-assisted prilling method.The raw materials are heated and melted in a nonpolar medium,and a special nozzle is used to release ultrasonic waves to refine ADN in the nonpolar medium.After filtration,washing,and drying,the product is finally collected and packaged.The authors indicated that adding a small amount of surfactant to the nonpolar medium can make the surface of the final product more uniform.This method can produce tiny particles with a particle size of less than 40 μm,which can be used in the production of dense ADN-based propellants and explosives.Fig.19 shows the images of the ultrasonic nozzle and the morphology of the spherical ADN.
5.1.4.Jet milling prilling technology
Jet milling technology [92,106] is a method that crushes raw ADN into spherical particles through a preset airflow(Fig.20).Raw materials enter the bottom of a funnel along a preset track and are driven by the compressed grinding air into a vortex grinding chamber.Under the action of centrifugal force,large particles are thrown to the boundary of the grinding chamber,whereas the small particles move to the center of the grinding chamber and finally exit through a vortex overflow pipe.Because the driving force of the jet milling equipment comes from the compressed air,it can avoid the danger of accidental discharge.The particle sizes of the final product can be controlled using different raw materials and adjusting the value of the grinding pressure.Using this method,spherical ADN with a particle size of 15-30 μm can be easily prepared from crude ADN within a few minutes(Fig.21).It is found that ADN particles produced by jet milling equipment still have high fluidity within 2 years after storage experiments.Therefore,the jet milling prilling method can be regarded as an efficient and low-cost process for preparing prilled ADN.
5.1.5.Micro-reaction prilling technology
Micro-reaction technology emerged in the 1990s as it can achieve precise control of reaction temperature,reaction time,and material ratio.This technology can be applied in many fields such as biopharmaceuticals,fine chemicals,and energetic materials.In 2010,the Fraunhofer Institute for Chemical Technology (ICT)applied micro-reaction technology to the granulation production of energetic materials and combined it with emulsion crystallization technology to develop a micro-reaction prilling method (Fig.22)[107,108].The steps are as follows:

Fig.17.SEM images of (a) ADN crystallized in ethyl acetate and (b) crystallized in methyl acetate.
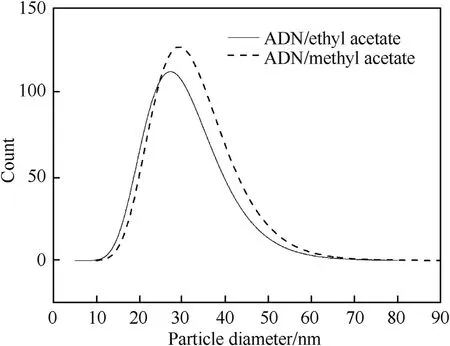
Fig.18.Log-normal size distribution of ADN crystallized in methyl acetate and ethyl acetate.
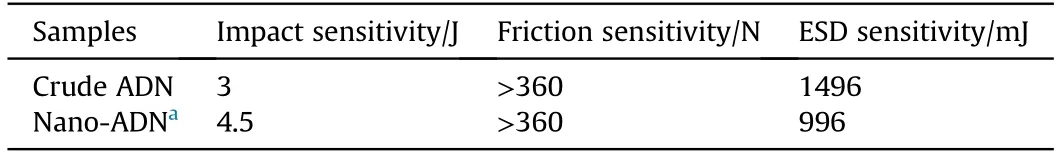
Table 5 Values of measured sensitivities of different ADN samples.
a.ADN is suspended in a decane solution,and the suspension is transferred from a sample tank into a heating plate through a tube pump.
b.The suspension is heated and melted to form numerous droplets of expected size,which pass through a very small cross-section with many baffles in the microreactor and spitted into smaller liquid particles.
c.Finally,the smaller liquid particles enter the receiving tank,which cool to form tiny solid particles under stirring.
In this process,the microreactor is placed in a glycol bath to prevent the solidification of the molten droplets,thereby blocking the connecting pipe.Spherical ADN particles with a particle size of 76-373 μm can be prepared by this method (Fig.23).
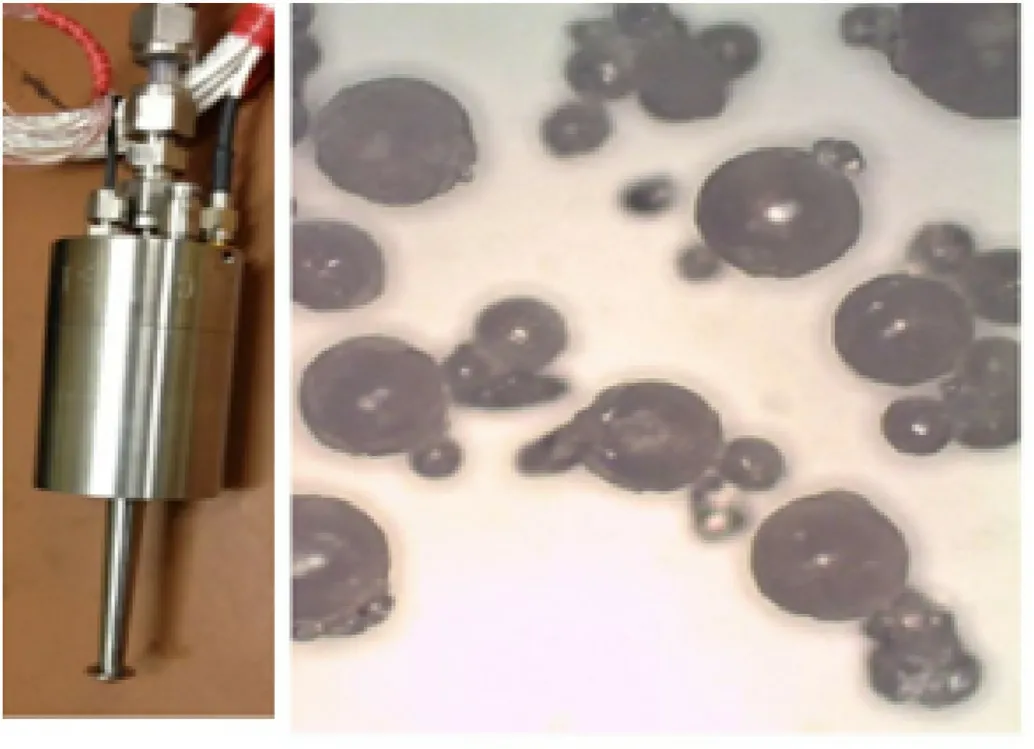
Fig.19.Ultrasonic prilling process setup and ultrasonic prilled samples.
In 2018,the Fraunhofer ICT used another micro-reaction prilling method to prepare spherical ADN with a particle size of 80-120 μm[109,110].This method requires heating ADN through an oil bath to form molten ADN (Fig.24).Then,the molten ADN (dispersed phase)and paraffin(continuous phase)are transported to a droplet generator through a microfluidic pressure controller(Fluigent)and a NEMESYS syringe pump(Fig.25).ADN droplets are collected in a heated collecting vessel and are subsequently redirected to a second syringe pump through a ball valve in order to prevent mixing of the components containing different droplet sizes.Finally,the droplets are cooled and crystallized to form spherical particles under stirring.In this process,the second syringe pump is placed between the droplet generator and external collecting vessel,which can avoid agglomeration of the prills and subsequent blocking of the tubes.The obvious advantage of this method is that it can accurately control the flow rate of the dispersed phase and continuous phase to obtain prilled ADN with expected size.
Compared with traditional prilling technology,the microreaction prilling method has the advantages of simple operating conditions,continuous production,and excellent safety performance.The prilling technology of the Fraunhofer ICT is close to the industrial level.It can produce 2 kg of prilled ADN per hour,and the particle size can be controlled in a range of 76-373 μm (Fig.26).
5.1.6.Membrane emulsification prilling technology
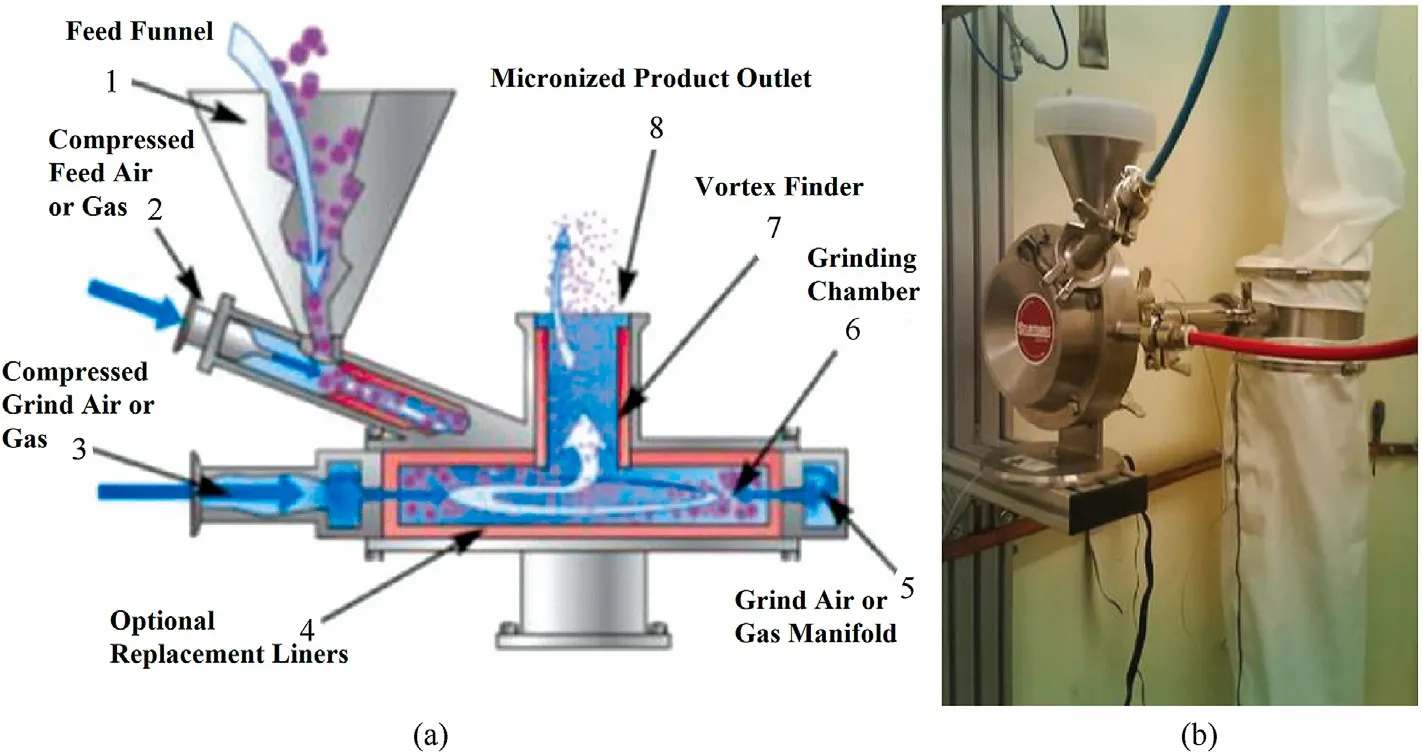
Fig.20.(a) Schematic diagram and (b) experimental setup of grinding prilling technology.
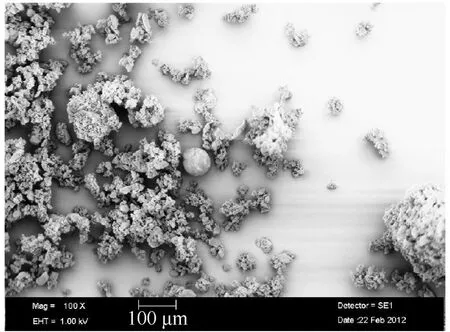
Fig.21.SEM images of ADN prepared by the grinding prilling process.
Membrane emulsification prilling technology refers to the method where a porous membrane containing a hydrophobic structure is used to promote the formation of ADN emulsion and to prepare spherical ADN by cooling the temperature rapidly(Fig.27).
The British Roxel company[111,112]used this method to prepare spherical ADN with a particle size of 5-50 μm (Fig.28).The steps are as follows:
a.A specific pump is used to pass the solution of ADN through a porous membrane to form tiny liquid in the continuous phase.
b.Then,the liquid is emulsified by mechanical stirring and heating and ADN droplets are solidified by rapid cooling to form prilled ADN.
c.Finally,spherical ADN particles are collected through filtration,drying,and washing.
Currently,the Roxel company has scaled up this process from laboratory research,and the production scale can reach to the kilogram level.
5.1.7.Comparison of prilling methods
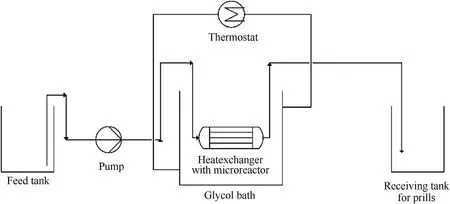
Fig.22.Micro-reaction prilling process.
The differences of the discussed prilling methods are shown in Table 6.It can be found that emulsion recrystallization technology is still the most common method in the prilling process of ADN.However,this technology is greatly affected by process conditions and is only suitable for small-scale production of ADN.Jet milling technology can prepare tiny spherical ADN in a short time,is safe and low cost,and is very suitable for further industrialization and use.Spray prilling method of molten ADN has the advantages of high efficiency and low cost,but the particle size distribution of ADN produced by this way is wide,and the processing of molten ADN is hazardous.Spray flash evaporation,as a newly emerging spray prilling method in recent years,has a wide prospect in the preparation of sub-micron and even nano-sized ADN particles.Ultrasonic-assisted spray prilling,micro-reaction prilling,and membrane emulsification crystallization technologies are all new methods that emerged in recent years,which have the advantages of low cost,safety,and continuous production.These technologies will be the development trend of spherical ADN granulation in the future.
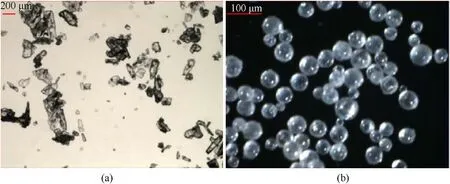
Fig.23.Morphology of the (a) original ADN and (b) ADN produced by micro-reaction technology.
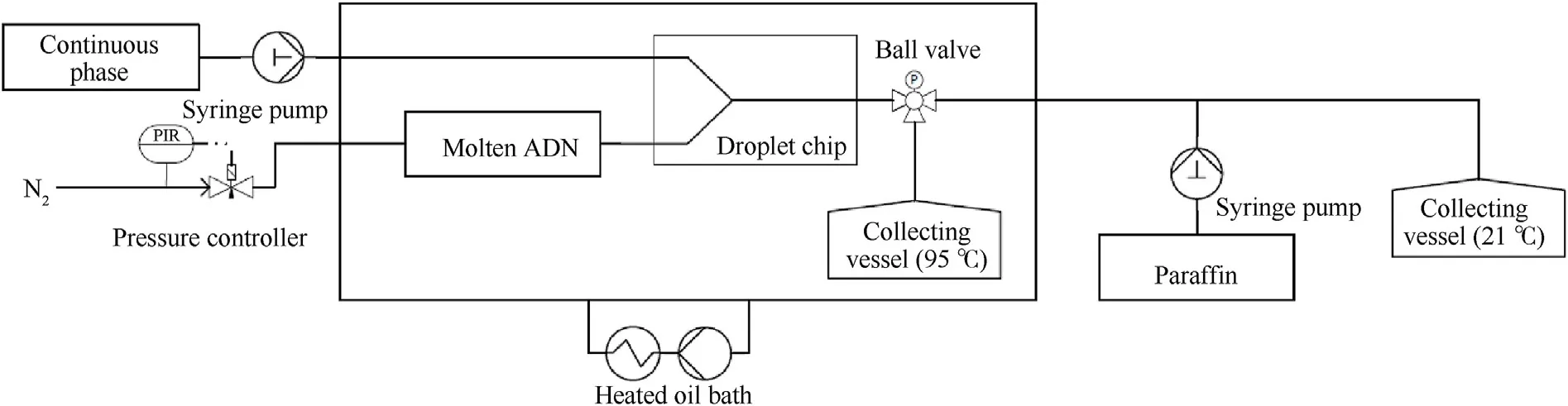
Fig.24.Experimental setup of micro-reaction prilling technology.

Fig.25.Experimental setup in oil bath and syringe pump.
5.2.Research on surface coating of ADN
Although prilling technology can reduce the hygroscopicity of ADN,it does not fundamentally change its surface chemical structure,nor does it introduce new chemicals.Therefore,the decrease of the hygroscopicity of ADN is limited.Surface coating is a method to reduce the hygroscopicity of ADN by coating its surface with a layer of hydrophobic material,which has the advantages of remarkable anti-hygroscopic effect and little influence on the performance of ADN.
Teipel et al.[89,113]precoated the surface of ADN particles with a layer of wax and then coated them again with amino resin to reduce their hygroscopicity(Fig.29).It is found that the amino resin is closely adsorbed on the surface of ADN,which can play a good hydrophobic role.The microencapsulated ADN particles are loosely stacked on each other and are finally collected and packaged after separation and drying.
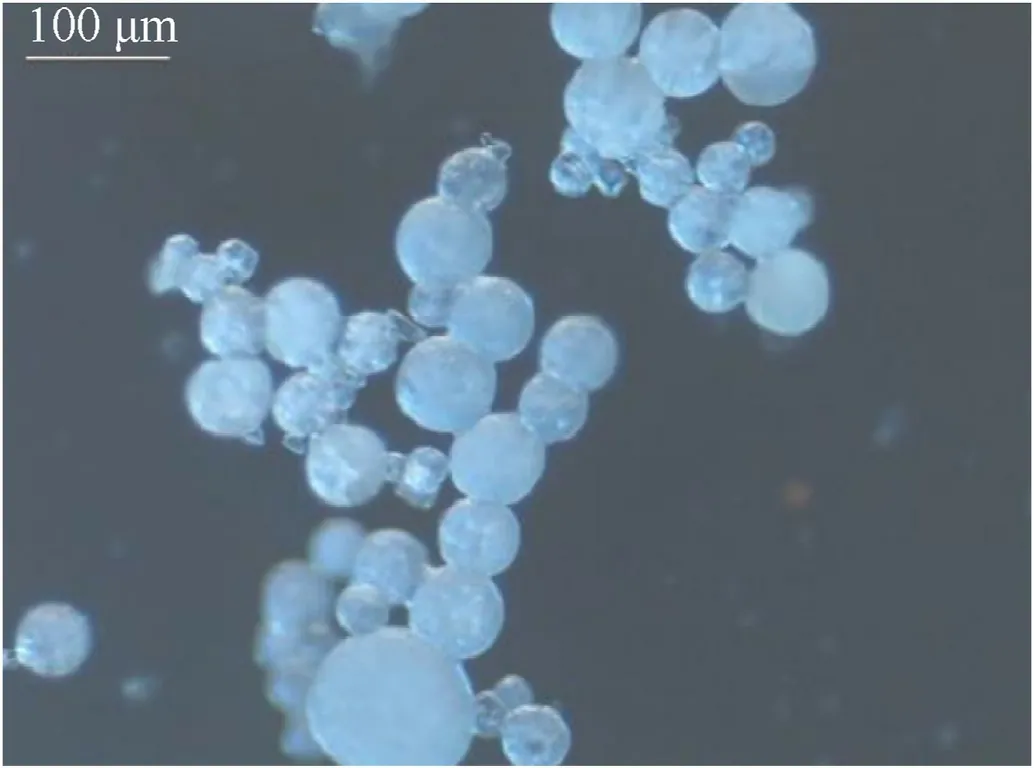
Fig.26.Light microscope images of prilled ADN.
Teipel et al.[20] also microencapsulated ADN particles with ethyl cellulose(EC)by dissolving ADN particles and ethyl cellulose in a cyclohexane solution and adding appropriate protective colloid to the mixture to inhibit the agglomeration of ADN particles.It is found that the macromolecular polymer EC is well aggregated on the surface of ADN particles to form microcapsules,which can prevent ADN particles from external effects.Performance test results show that the thermal stability of ADN is improved and its hygroscopicity is reduced.
The Fraunhofer ICT applied fluidized bed technology to the prilling and coating process of energetic materials,and a variety of composite energetic materials can be prepared through this technology (Fig.30).To solve the problem of incompatibility between ADN and isocyanate [114,115],Heintz et al.[116-118] adopted fluidized bed technology for coating prilled ADN with glycidyl azide polymer(GAP).In this process,an UV-curing polymer is used as the coating material to improve the mechanical stability of ADN,and nitrogen is used as a protective gas to reduce the viscosity of the coating material and improve safety when processing reactive substances.SEM results show that the morphology of the coated ADN is more uniform with a particle size of 96 μm (Fig.31).In addition,compared with the initial sample,the coated ADN has improved compatibility with other propellant components.
Fig.28.Microscope image of prilled ADN prepared by the Roxel company.
Heintz et al.[119] also used the method to microencapsulate prilled ADN with HTPB/isocyanate and polyacrylate/silane(Fig.32).Compared with uncoated prilled ADN,the resulting samples have a significantly improved compatibility with binder systems due to the coating materials(HTPB and polyacrylate).The average particle size of coated prilled ADN is 101-136 μm and with a single batch yield of 5 kg.
Santhosh et al.[120] coated ADN particles with EC and polymethyl methacrylate (PMMA) and tested their hygroscopicity under different relative humidity conditions.The results of hygroscopic experiments show that under an RH of 74%,ADN coated with 2% EC and 2% PMMA have better anti-hygroscopic effect,which can reduce the hygroscopic rate of ADN from 7.6 to 4.43%and 4.09%after exposure to air for 1 h.Fig.33 shows the plots on the extent of moisture uptake of ADN coated with EC and PMMA.
Silva et al.[121] used a simple coacervation method to microencapsulate spherical ADN,and the steps are as follows:
a.Spherical ADN is uniformly dispersed in n-hexane solution.
b.Then,HTPB is gradually added into the mixture,which is stirred until ADN is completely dissolved.

Fig.27.Membrane emulsification recrystallization technology.
c.Finally,methylene diphenyl diisocyanate and toluene diisocyanate are added as curing agents to improve the stability of microencapsulates.
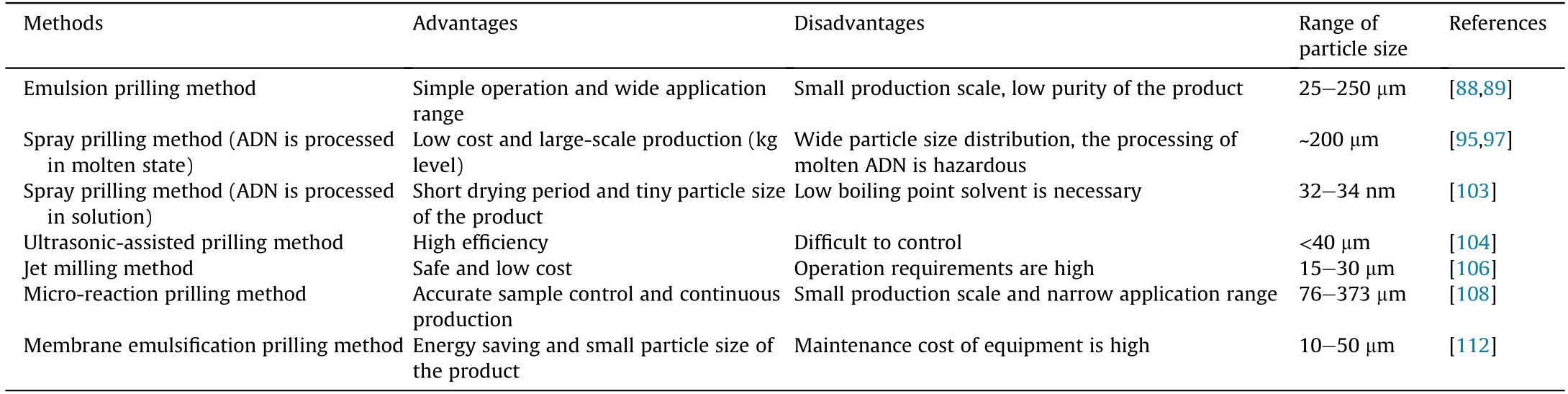
Table 6 Comparison of different prilling methods.
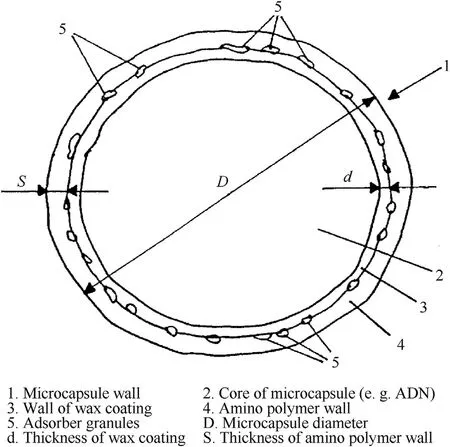
Fig.29.ADN microcapsule.

Fig.30.Laboratory-scale (left) and field-scale fluidized bed coating apparatus (right).
The researchers also explored the coating effect of the microcapsules by changing the type of curing agent,curing time,and curing temperature.The results of impermeability test show that even after 2 h,the microencapsulated ADN particles can still maintain a better morphology,and their hygroscopicity is significantly reduced (Fig.34).
Rahman et al.[122] microencapsulated ADN using ultrasound sonication technology with toluene mixture as a solvent and polystyrene(PS)and HTPB as coating polymers(Fig.35).The results of SEM show that compared with the pristine ADN (Fig.36),the surface of ADN coated with PS mixture and HTPB mixture is smoother,and the pores on the particle surface are smaller with a particle size in the range of 150-295 μm and 155-305 μm(Fig.37).It is found at a relative humidity of 65%,the hygroscopic rates of ADN coated with PS mixture and HTPB mixture reduced from 68 to 16% and 20% after being placed in a closed container for 4 h(Fig.38).The results of thermal analysis show that the thermal decomposition temperature and activation energy of coated ADN are obviously improved.It can be seen that coating ADN with PS mixture and HTPB mixture can significantly reduce its hygroscopicity and improve its stability.
Graphene oxide (GO) has the characteristics of high specific surface area,excellent lubricity,and stronger toughness.Thus,it can reduce the adsorption of water molecules in the air on the surface of solid particles [123,124].Therefore,it is feasible to coat prilled ADN with GO to reduce its hygroscopicity.Yan et al.[125]proposed a facile method to coat ADN by using a pickering emulsion as a soft template and alkylated graphene oxide nanosheets(AmGO) as a particle surfactant.The authors studied the antihygroscopic effect of coated ADN by controlling the concentration of AmGO,ratio of water to toluene,and other factors.The hygroscopicity results show that when the mass concentration of AmGO is 1%and the volume ratio of toluene to water is 2:1,the coated ADN has the best anti-hygroscopic effect,and the hygroscopicity is reduced from 1.1 to 0.42%.In addition,when the mass concentration of AmGO is 2%,the heat release of coated ADN can be improved by 60%.All these results show that ADN@AmGO composites have potential applications in high energy solid propellants.The preparation flow chart of ADN@AmGO composite material and the corresponding hygroscopic curve are shown in Fig.39 and Fig.40.
Xu et al.[126]coated spherical ADN with polyurethane binders.The process is summarized as follows:
a.As-prepared polyurethane binder prepolymer and hexamethylene diisocyanate biuret(N-100)as curing agents are added into tetrahydrofuran,and the mixture is stirred for 4 h.
b.Then,some ADN is added into the solution,and the mixture is uniformly stirred for 6 h.
c.Finally,the coated ADN is prepared by evaporating the solvent,filtration,washing,and drying.
Under a temperature of 10°C and a relative humidity condition of 50-60%,the hygroscopic rate of prilled ADN is 3.85%and that of coated prilled ADN is 0.193% after exposure to air for 24 h.The authors pointed out that a polyurethane binder consisting of a large number of weakly polar hydrocarbon chains forms a coating on the surface of ADN,which can play a very good hydrophobic role and thus inhibit the moisture absorption of ADN (Fig.41).

Fig.31.SEM images of (a) prilled ADN without coating and (b) prilled ADN coated with the difunctional GAP.

Fig.32.SEM images of (a) prilled ADN coated with 5% polyacrylate/isocyanate N75 and (b) prilled ADN coated with 5% polyacrylate/silane.
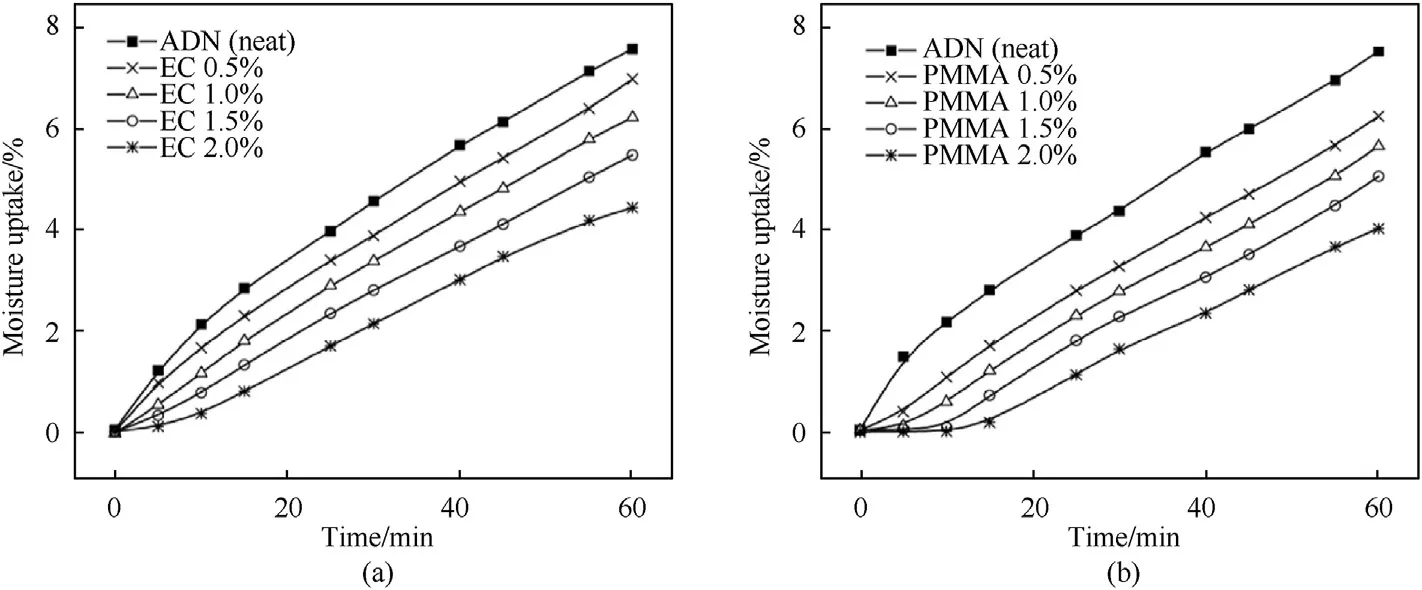
Fig.33.Moisture absorption of ADN coated with EC and PMMA at a 74% RH.
He et al.[127]used high-nitrogen compounds to coat ADN and test the hygroscopicity of uncoated ADN and coated ADN at a temperature of 25°C and a relative humidity of 76%.Results show that uncoated ADN completely deliquesces into a solution after 24 h,whereas the hygroscopic rate of coated ADN is only about 5%after 3 weeks.
Studies have shown that the anti-hygroscopic effect of the powder surface coating largely depends on the type of coating agent,experimental temperature,and relative humidity of air,among others.Wang [79] adopted the liquid coating method to treat spherical ADN with stearic acid,polyvinyl butyral,methenamine,and 2,4,6-trinitrotoluene (TNT) to achieve the antihygroscopic effect on the ADN surface.Untreated ADN and several kinds of coated ADN particles are exposed to the air for 96 h at a temperature of 25-30°C and a relative humidity of 60%.It is found that spherical ADN coated with stearic acid has the best antihygroscopic effect,which can reduce the hygroscopic rate of ADN from 19.04 to 9.52% and from 23.2 to 12.84%,respectively.The authors also pointed out that because stearic acid is a surfactant,one end of the molecule is an oleophilic group and the other end is a hydrophilic group.This special structure makes the hydrophilic group of stearic acid closely combines with ADN with its oleophilic group facing outwards,thus enhancing the hydrophobic ability of ADN.
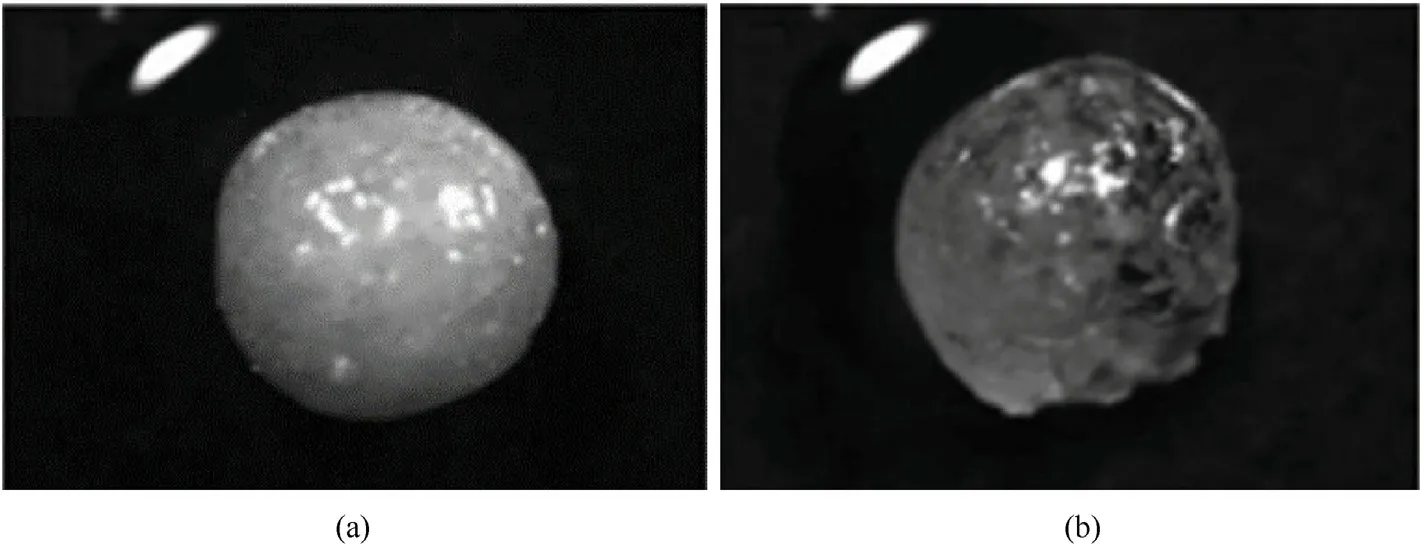
Fig.34.Micrographs of (a) microcapsuled ADN and (b) microcapsuled ADN after undergoing the impermeability test (2 h).
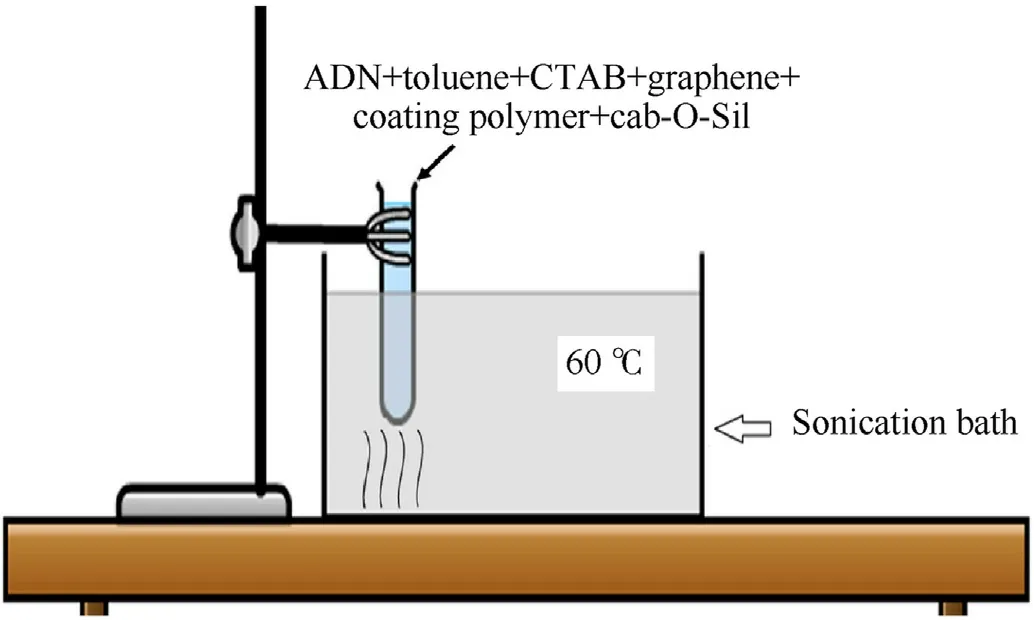
Fig.35.Experimental setup of ADN sonication.

Fig.36.SEM image of pristine ADN.
Gong et al.[128,129] used trimethyl aluminum and water as precursors to deposit alumina thin films on ADN by atomic layer deposition (ALD) method.Under a reaction chamber pressure and deposition temperature of 133 Pa and 55°C,respectively,the thickness of the ALD alumina film is controlled by changing the deposition cycle.The results of SEM and X-ray photoelectron spectroscopy show that the surface of ADN is completely coated with the alumina film.After placing uncoated ADN and the samples coated by 400 cycles of ALD in the air for 48 h at a temperature of 23°C and a relative humidity of 70%.It is found that the alumina film can maintain the morphology of spherical ADN with an insignificant decrease in its hygroscopicity.Fig.42 shows the photographs of uncoated ADN and the samples coated by 400 cycles of ADN after 48 h of exposure to the air.
In order to overcome the incompatibility between ADN and isocyanate curing agents,Lu et al.[130] coated spherical ADN particles with GAP/bis-propargyl-succinate (BPS) crosslinked polymers as a coating material via 1,3 dipolar cycloaddition reactions.Fig.43 shows the surface morphology of uncoated prilled ADN and prilled ADN coated with GAP/BPS crosslinked polymers.It can be seen that coated ADN is smoother with a smaller gap among particles on its surface.The compatibility of ADN with the coating system and hygroscopicity of coated prilled ADN are investigated by vacuum stability test and dynamic hygroscopic analytical method,respectively.Results show that compared with the GAP/N-100 crosslinked polymers,the GAP/BPS crosslinked polymers exhibited better compatibility and excellent coating effect with ADN,and the saturated hygroscopicity of coated ADN is only 0.78%under temperature and relative humidity conditions of 35°C and 50%,respectively.
Li et al.[131,132]proposed a method for preparing coated prilled ADN by ultrasonication and in situ coating technology(Fig.44).The main steps are as follows:
a.Through calculation of interface energy and adhesion,coating materials(P1,P2,and P3;the author did not point out the names of these substances)that have a strong binding force with ADN are screened out.
b.Then,a saturated solution of ADN is prepared in propanol.Surfactants and as-prepared coating precursors(P1,P2,and P3)are dissolved in the ADN solution.
c.Under ultrasonication,the above mixture is slowly added into an anti-solvent (such as toluene and n-hexane) of ADN so that the recrystallization and surface coating process of ADN can be carried out simultaneously.Ultrasonication time and power are controlled to promote the preparation of coated prilled ADN particles.
Experimental results show that the crystalline structure of coated ADN has not changed.The thermal decomposition temperature of ADN has increased by 11°C,its initial decomposition temperature on a thermogravimetric plot has been delayed by 15°C,and its impact sensitivity (I50) has increased by 3.6 J.All the evidences show that the stability of ADN has improved.Under temperature and relative humidity conditions of 30°C and 75%,respectively,the saturated hygroscopic rate of coated prilled ADN decreases from 55% to less than 2.5%.In addition,the method has the advantages of mild experimental conditions and simple process,which makes it very suitable for the industrial production of ADN.
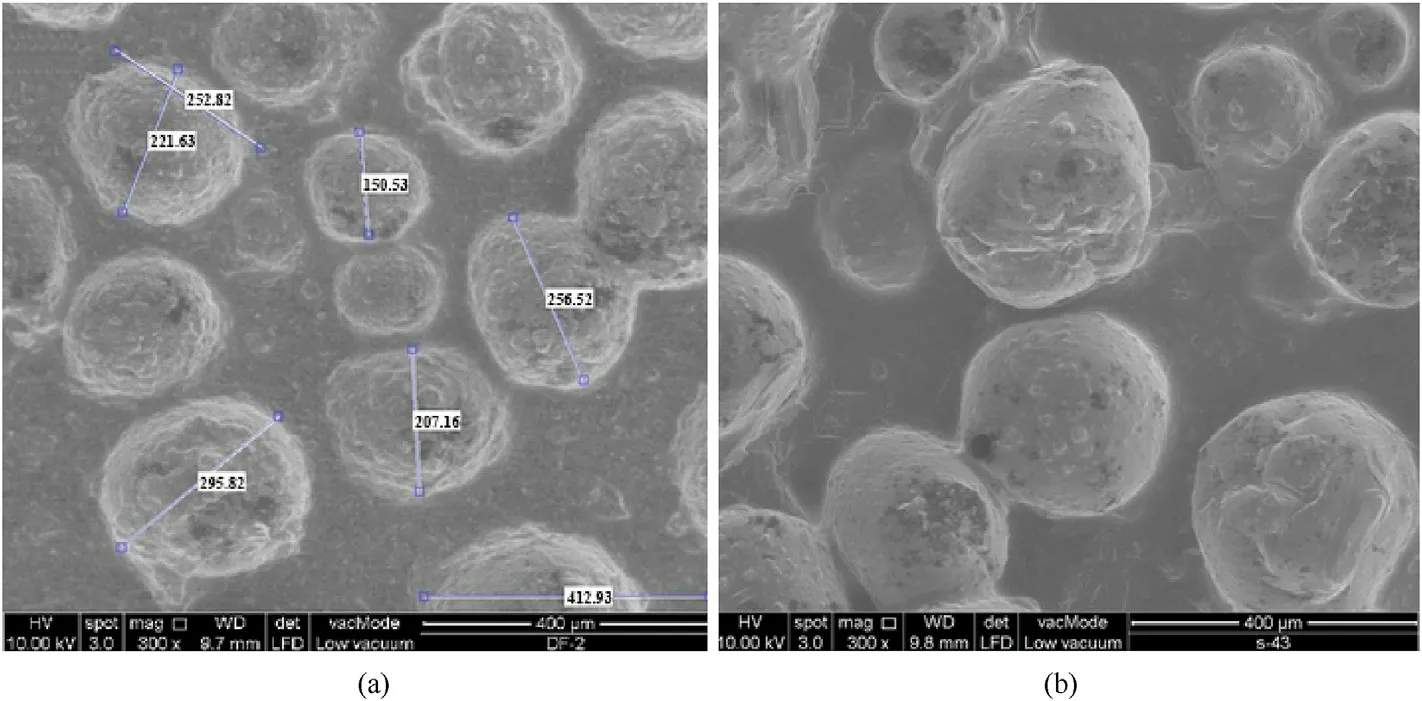
Fig.37.SEM images of (a) ADN coated with 5% PS and (b) ADN coated with 5% HTPB.
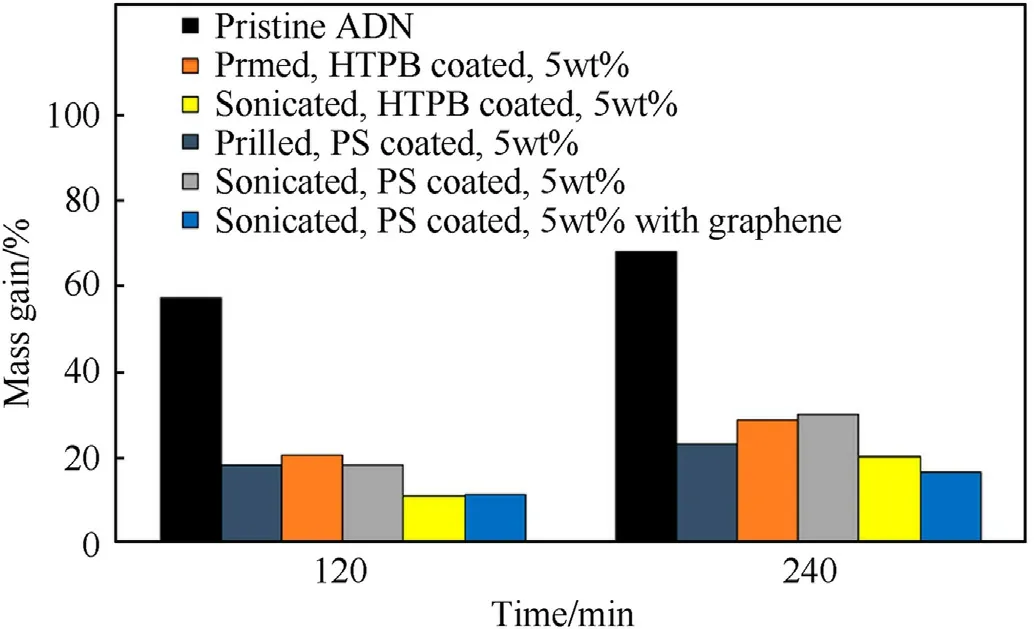
Fig.38.Mass gain in various types of ADN.
The coating materials used in the discussed coating methods and the particle size range of the final product are shown in Table 7.
5.3.Research on crystal modification of ADN
Raw ADN grains generally are needle shaped with strong hygroscopicity and high mechanical sensitivity,which severely limits its application.Therefore,it is necessary to improve the crystal morphology of ADN particles.Crystal modification of ADN refers to optimizing the crystal structure and surface morphology of the grains by means of cocrystallization technology or adding crystal growth modifiers in the process of solution crystallization,so as to reduce the hygroscopicity of ADN and improve its mechanical properties.
5.3.1.Research on solution crystallization of ADN
In 1988,Nagao et al.[133] prepared prismatic ADN using cyclohexane as a solvent.Benazet et al.[134,135] proposed a solution crystallization process for improving the morphology of ADN by introducing crystal growth modifiers,and they successfully obtained ADN crystals with a low shape factor.The process is as follows:
a.Dried raw ADN crystals and a crystal growth modifier (calcium or magnesium salt) are added into a mixture of ethanol and methanol.
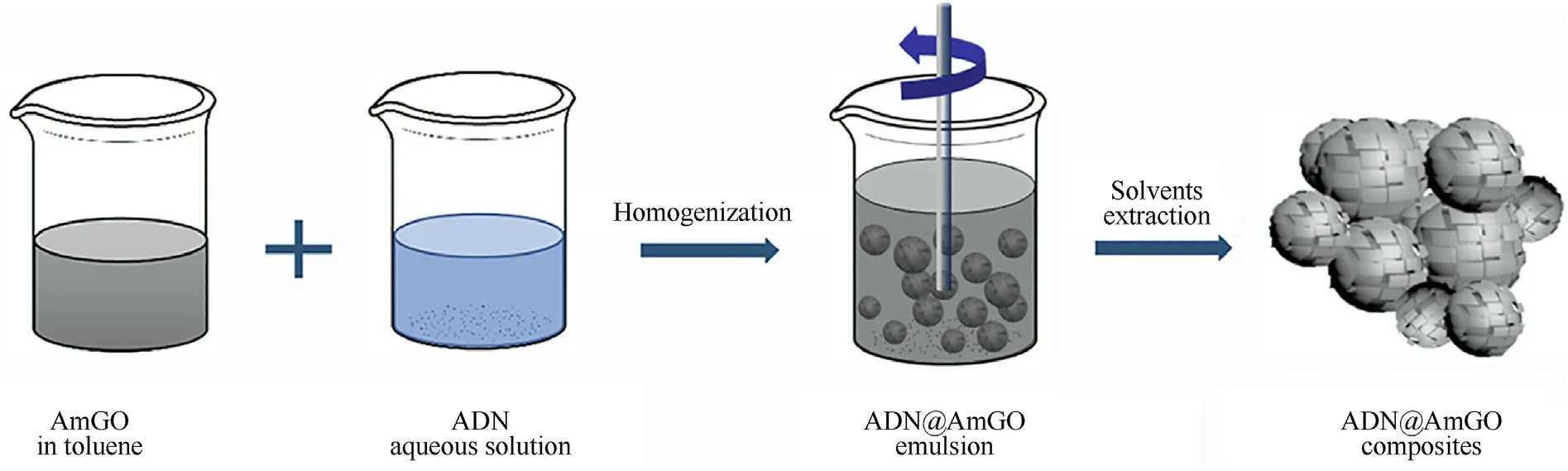
Fig.39.Preparation of the ADN@AmGO emulsions and their corresponding composites.
b.ADN grains are prepared as white crystallized sugar after stirring,cooling,and drying.
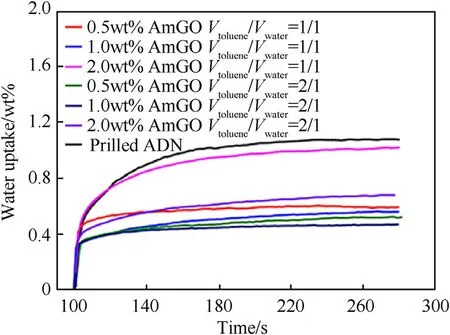
Fig.40.Water adsorption of prilled ADN and ADN@AmGO composites as a function of time.
Compared with the traditional needle-shaped ADN,the modified ADN prepared by this method has a more regular morphology,and the shape factor of the crystal is 1.5-5.The SEM images of the needle-shaped ADN and modified ADN are shown in Fig.45.
Muscatelli [136] introduced a simple crystallization method to prepare quasi-spherical ADN in a viscous medium.Crude ADN and nitrate(generally between 0.1%and 0.5%by mass)are added into a mixture of 1,4-butanediol and glycerol (volume ratio 1:1).The solution is stirred to adjust the nucleation and growth of ADN crystals,thus preventing the precipitation of particles.Quasi-spherical ADN with a shape factor of 1-1.5 and a median particle size of 100-400 μm is prepared after cooling,crystallization,and filtration(Fig.46).The purpose of introducing nitrate in the crystallization process is to reduce the nucleation temperature of ADN and increase the range of the metastable zone of the solution.In addition,the application of thermal cycle in the crystallization process is beneficial to the particle size gradation of energetic materials.
Li et al.[137]used n-propanol and chloroform as crystal growth controlling agents to prepare flake-like ADN crystals with uniform appearance.Yang et al.[138] introduced a method of preparing quasi-spherical ADN crystals (Fig.47) by solvent-nonsolvent recrystallization technology.The steps are as follows:
a.ADN is uniformly added into a mixture of alcohols (such as ethanol or isopropanol)and esters(such as ethyl acetate or butyl acetate),and the ADN solution is obtained after heating and stirring.
b.Dichloromethane (nonsolvent of ADN) is dissolved in the ADN solution,and the mixture is stand for 2 h without disturbance.
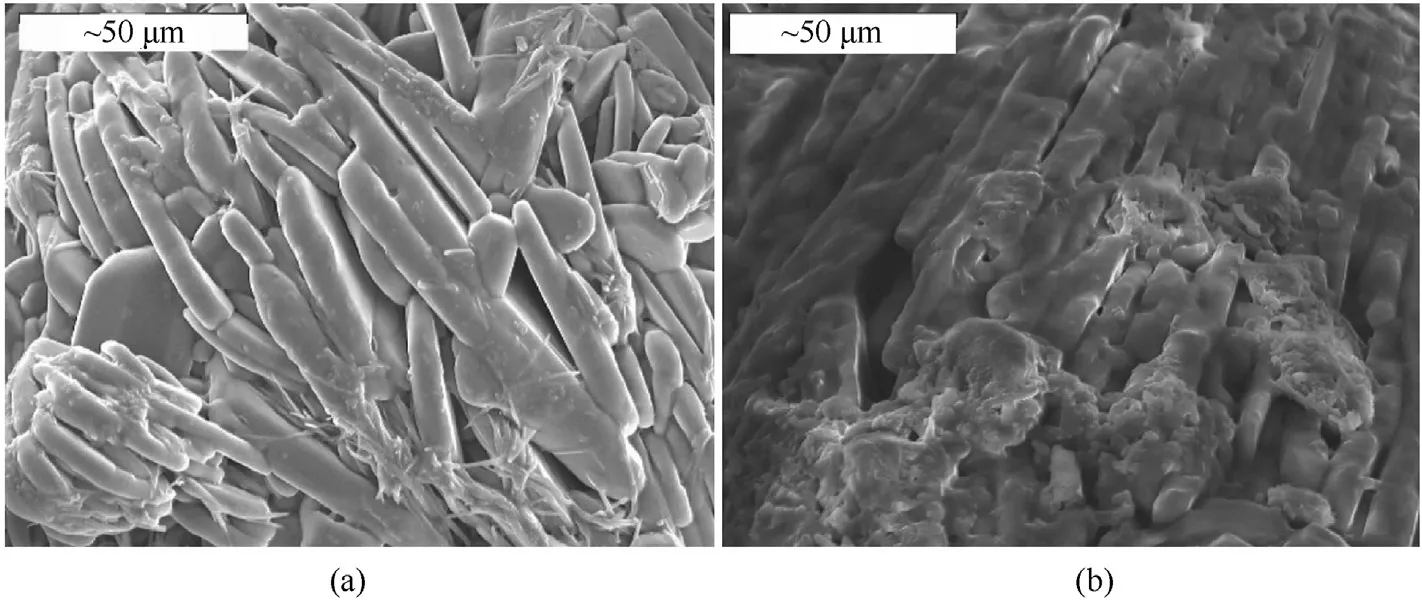
Fig.41.SEM images of (a) ADN before coating and (b) ADN after coating.
Fig.42.Comparison between (a) uncoated ADN and (b) ADN coated with 400-cycle ALD Al2O3 after 48-h air exposure.
c.Quasi-spherical ADN crystals are obtained after filtration and drying without introducing inert substances.
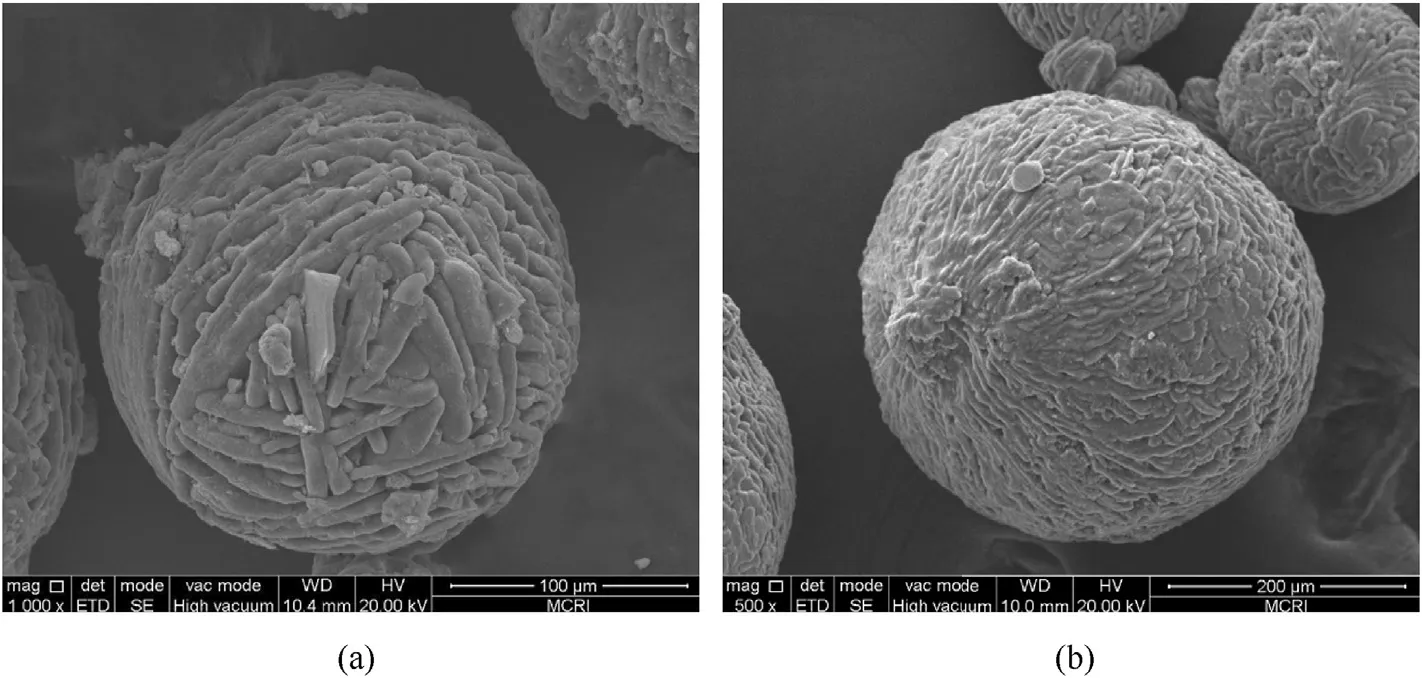
Fig.43.SEM images of (a) uncoated ADN-prills and (b) ADN-prills coated with GAP/BPS cross-linked polymers.
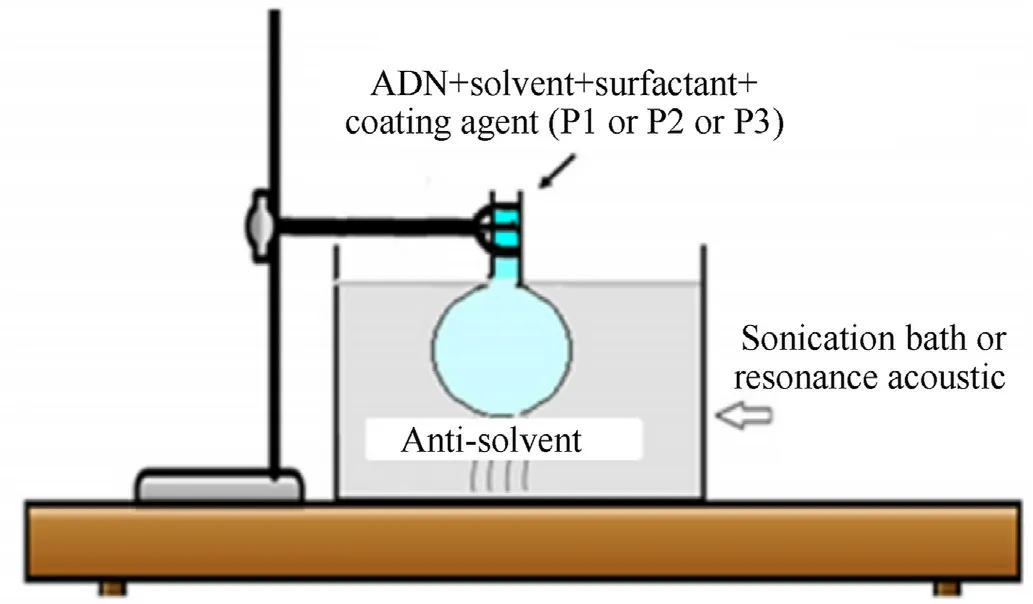
Fig.44.ADN prilling and coating process.
Compared with the ADN crystals obtained from other solvents,the quasi-spherical crystals produced by this method have low hygroscopicity and better mechanical sensitivity.
Wang et al.[139] used the solution crystallization process to prepare a nearly spherical ADN with a particle size of 210 μm by means of programmed temperature controlling and the introduction of crystal growth modifiers (Fig.48).Hygroscopic test results show that under temperature and relative humidity of 25°C and 75.3%,respectively,the hygroscopic rate of is only 3.5%after 8 days of air exposure (Fig.49).It is found that the integrity and compactness of the crystal structure are responsible for a significant decrease in the hygroscopicity of sub-spherical ADN.
Studies have shown that ultrasonic-assisted deposition technology can effectively change the morphology of crystals and inhibit crystal agglomeration.Ren et al.[140] combined the solvent-nonsolvent recrystallization method with ultrasonicassisted deposition technology to prepare rod-shaped ADN crystals with a smoother surface without edges.It can be seen from the SEM images in Fig.50 that the introduction of a crystal control agent during the recrystallization process can produce ADN crystals with a more uniform morphology and with a particle size distribution range of 33-142 μm.After exposure of the untreated ADN and rod-shaped ADN to the air for 48 h at a temperature of 20°C and relative humidity of 70%,their hygroscopic rates are 3.557%and 1.998%,respectively.Table 8 shows the hygroscopicity of untreated ADN and rod-shaped ADN crystals.
Comparison of the discussed methods indicated that the introduction of a crystal growth modifier such as a nitrate or a calcium salt in the solution crystallization process of ADN can effectively improve its surface morphology.Most of the crystals prepared by this method are short rods with smooth surfaces and no sharp edges.This is because in the process of solution crystallization,adding an appropriate amount of crystal growth control agent can reduce the growth rate difference between the fast-growing surfaces and the slow-growing surface of ADN,making the crystal growth more regular and finally achieving the purpose of adjusting the crystal morphology of ADN.
5.3.2.Research on anti-hygroscopic cocrystals of ADN
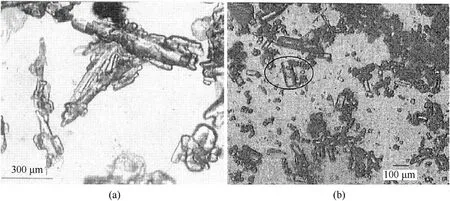
Fig.45.(a) Needle-shaped ADN and (b) ADN prepared by the solution crystallization process.
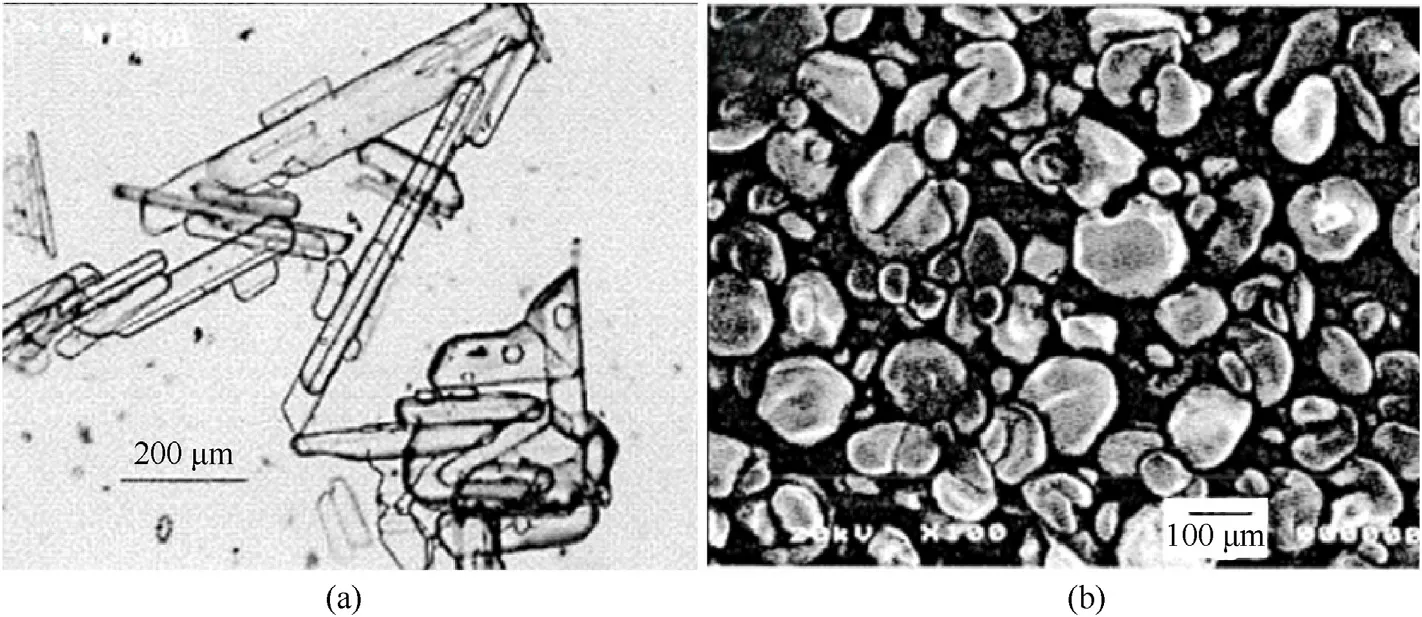
Fig.46.(a) Flake ADN and (b) quasi-spherical ADN prepared by the solution crystallization method.
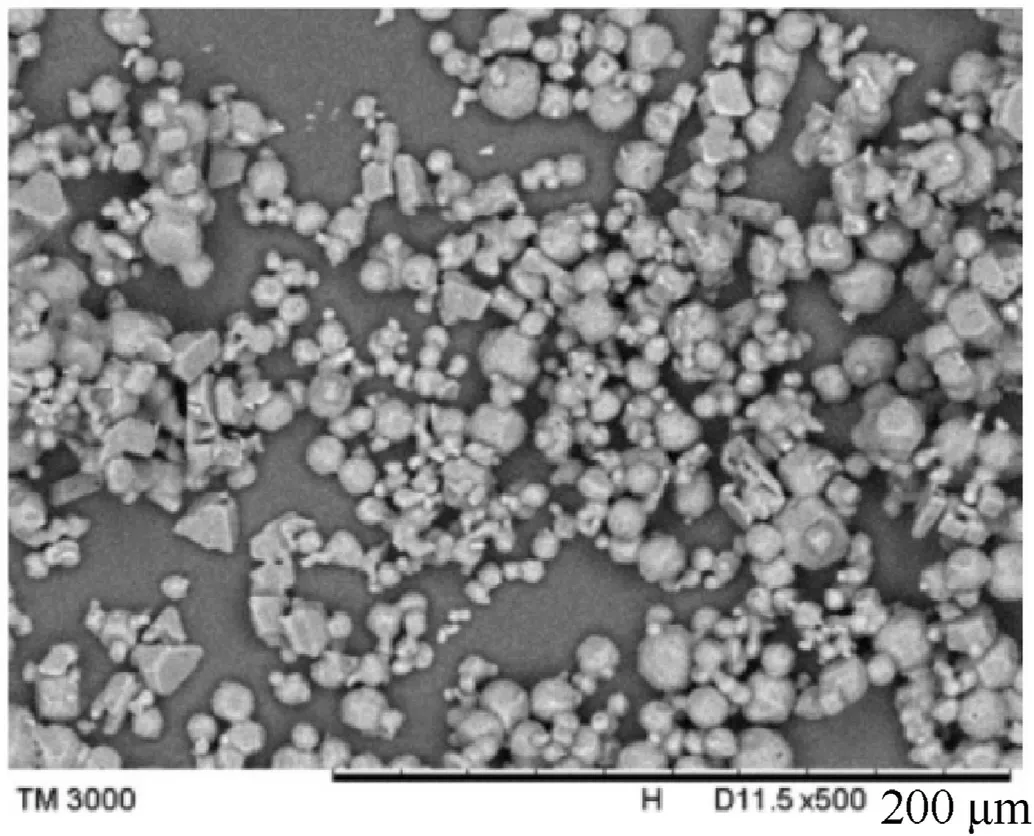
Fig.47.SEM image of quasi-spherical ADN crystals.
Cocrystallization technology is an effective method of combining two or more components(which are solid at 25°C)in a defined stoichiometric ratio to form a new single-phase material[141-146].In the field of energetic materials,cocrystallization technology can optimize the physicochemical properties of the materials,including their hygroscopicity,melting point,and thermal stability [147].Studies have shown that the physicochemical properties of cocrystals are greatly affected by those of their ligands.Therefore,it is necessary to predict the physicochemical properties of cocrystals through theoretical calculations,so as to provide guidance for their preparation [148,149].
To decrease the strong hygroscopicity of ADN,Guo [80] predicted the possibility of preparing CL-20/ADN crystals by the molecular simulation software Materials Studio and further studied the crystal structure and hygroscopicity of CL-20/ADN cocrystal(Fig.51).The author screened out a large number of CL-20/ADN cocrystals through theoretical calculation and performed hygroscopic kinetic simulations on the selected CL-20/ADN cocrystals.Results show that CL-20/ADN cocrystals can inhibit the adsorption of water molecules on the surface of ADN.Guo [80] also prepared CL-20/ADN cocrystals by solvent evaporation method on the basis of theoretical calculation and tested the hygroscopicity of ADN and CL-20/ADN cocrystals.For a relative humidity of 75%,the hygroscopic coefficient (η) of ADN is 49.87;in these conditions ADN quickly deliquesces and an aqueous solution is formed.On the contrary,the morphology of CL-20/ADN cocrystals has a minimal change,and their hygroscopic coefficient is only 7.89 (Fig.52).
In order to achieve the balance of the oxidizer and fuel ratio in energetic materials,Bellas et al.[150] successfully prepared ADN/pyrazine-1,4-dioxide(PDO)cocrystals(molar ratio 2:1)by solution method or liquid-assisted grinding method.Cocrystallization directly addresses the affinity of ADN for water by introducing intermolecular/ionic hydrogen bonding interactions,thereby improving the stability of ADN in air.Experimental hygroscopicity results show that the critical relative humidity of ADN is 53.5% at 25°C,whereas the critical relative humidity of ADN/PDO cocrystals is 79.5% under the same condition (Fig.53).It can be seen that cocrystallization is an effective way to reduce the hygroscopicity of ADN.

Fig.48.Microscope image of (a) needle-shaped ADN and (b) sub-spherical ADN.

Fig.49.Hygroscopicity of quasi-spherical ADN at 25 °C.
Yangetal.[151,152]preparedADN/1,4,7,10,13,16-hexaoxacyclooctadecane [18-crown-6,18C6] cocrystal by solvent evaporation method(Fig.54),which comprises the following steps:
a.ADN is added into methanol or ethanol solvent,and a saturated solution of ADN is obtained after heating,dissolving,and filtration.
b.A saturated solution of ADN/18C6 cocrystal is prepared by dissolving 18C6 into the ADN solution.Then,the solution is filtered,and the filtrate is placed in an incubator at a constant temperature.
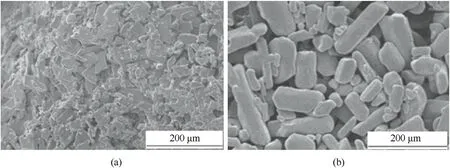
Fig.50.SEM images of (a) untreated ADN and (b) ADN prepared by recrystallization with a crystal control agent.

Table 8 Hygroscopic rate of untreated ADN and rod-shaped ADN.
c.Finally,the solvent is evaporated to reduce supersaturation,and ADN/18C6 is obtained after nucleation growth,filtration,and drying.

Fig.51.Morphological change of ADN and CL-20/ADN cocrystal.
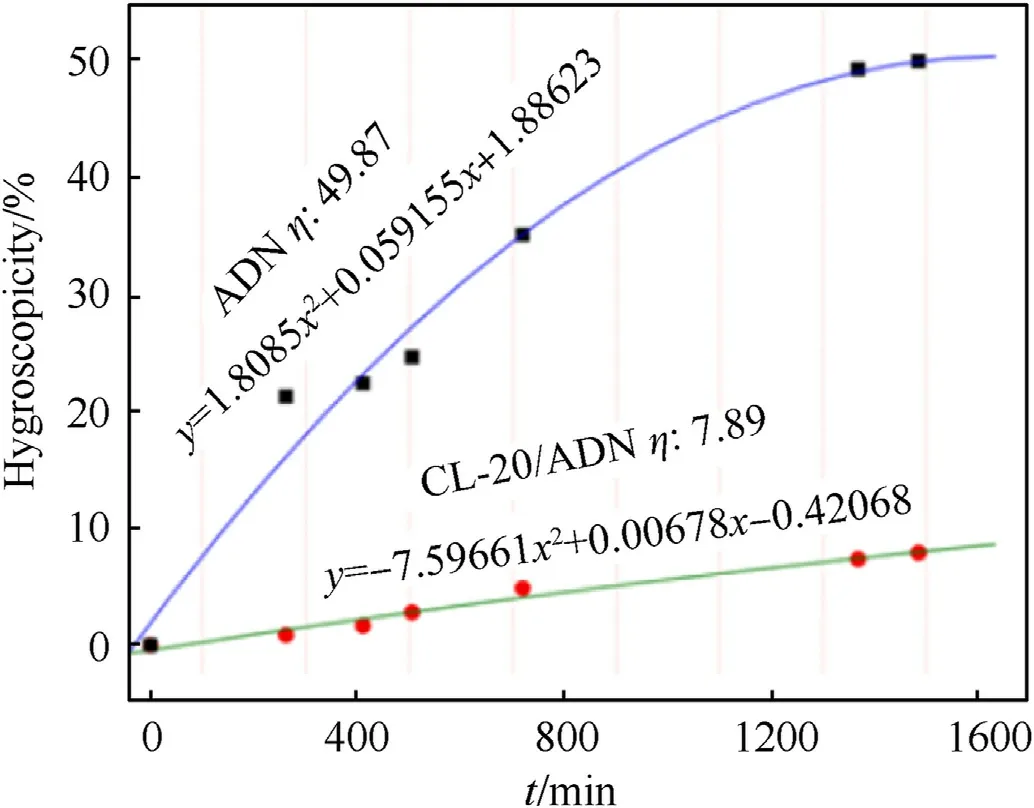
Fig.52.Hygroscopic curves of ADN and CL-20/ADN cocrystals.
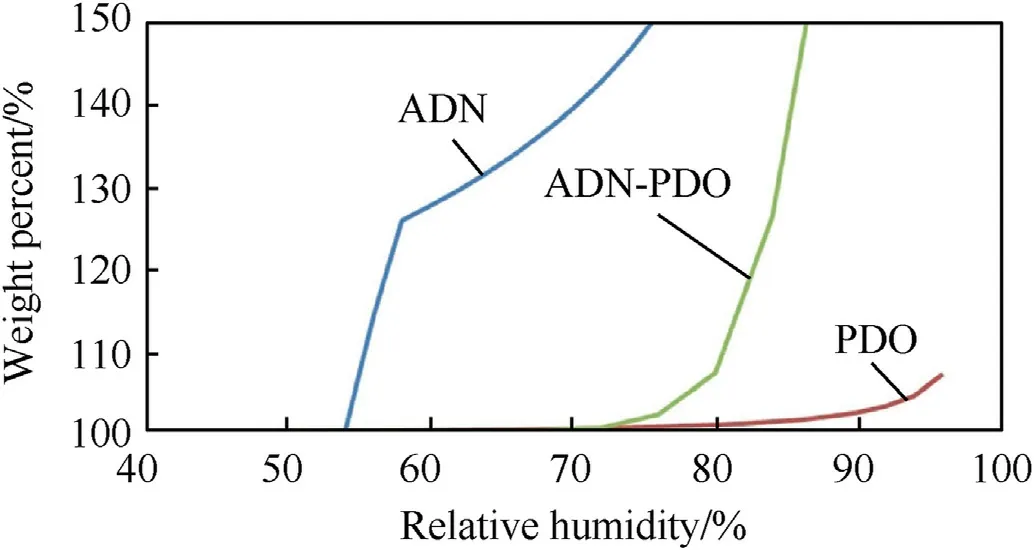
Fig.53.Dynamic vapor sorption traces for ADN (blue curve),PDO (red curve),and ADN-PDO (green curve).
After ADN and ADN/18C6 cocrystals are exposed to the air for 12 h,it is found that ADN is eventually dissolved into an aqueous solution,and its hygroscopic rate is as high as 18%,whereas the surface morphology of ADN/18C6 cocrystal did not change,and the hygroscopic rate is only 1.2% (Fig.55 and Fig.56).
In order to design the anti-hygroscopic cocrystal of ADN with better performance,Ren et al.[153,154] selected nine commonly used energetic materials as cocrystal ligands of ADN,namely,TNT,RDX,HMX,CL-20,TNAZ,benzotrifuroxan,butane-1,2,3,4-tetrayl tetranitrate,1,3,5-trinitrobenzene (TNB),and 2,4,6-trinitro-Nmethylaniline.Through prediction of the cocrystal structure,it is found that CL-20,RDX,HMX,and TNAZ are most likely to form cocrystals with ADN among the nine ligands.The predicated results of hygroscopicity show that the saturated hygroscopic rate of ADN is 6.82-22.30%,whereas the saturated hygroscopic rate of ADN/CL-20 and ADN/HMX cocrystals is 1.33-3.00% and 0.14-2.67%,respectively.These results indicate that CL-20 and HMX as cocrystal ligands can greatly reduce the hygroscopicity of ADN.
6.Application of ADN in powder and explosives
In recent decades,ADN has attracted the attention of many researchers because of its high energy density,low characteristic signal,and halogen-free characteristics.As early as the 1980s,Russia had already developed ADN-based propellant formulations(HTPB/AP/ADN/Al) and used them in the second-stage engine of the SS-24 strategic missile.The maximum load of each engine is approximately 34,746 kg [22].In the 1980s,the USA also had incorporated ADN-based propellants as low characteristic signal propellants into its long-term development plan of national defense technology.The Fraunhofer ICT of Germany and the Bofors AB company of Sweden also worked on the development and application of ADN.Sections 6.1-6.6 are brief introductions to the application of ADN in propellants,gunpowder,and explosives in recent decades.

Fig.54.Preparation process of ADN/18C6 cocrystal.
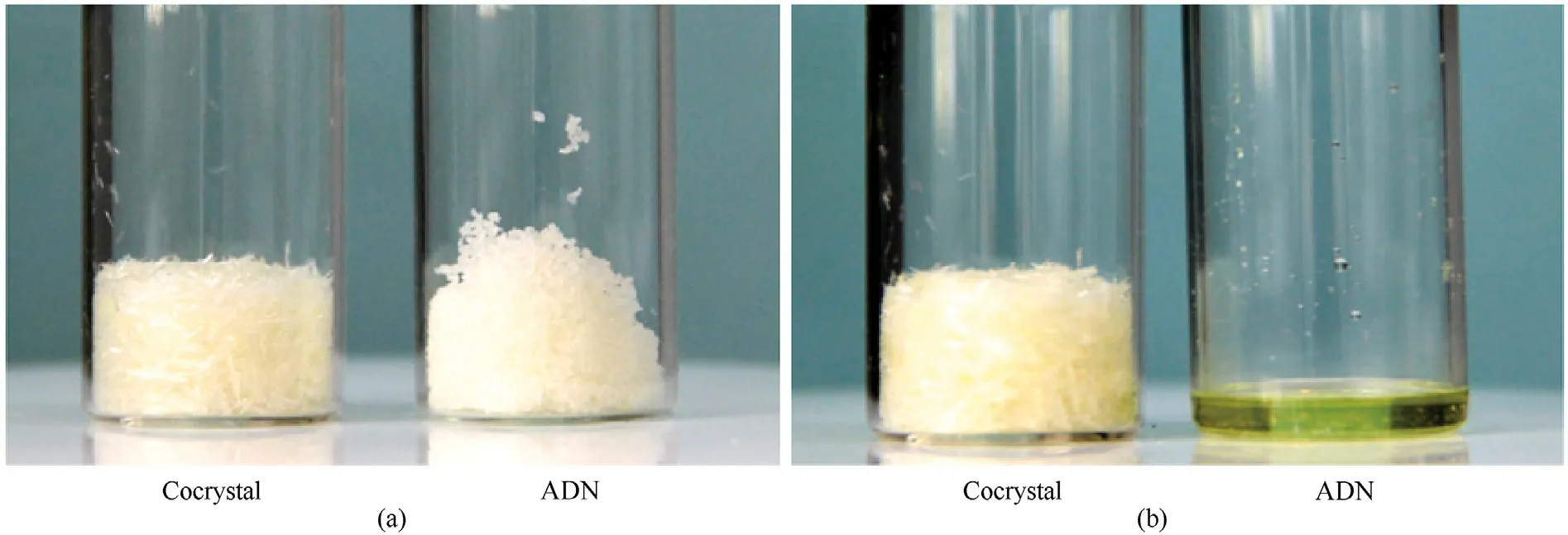
Fig.55.Status of ADN/18C6 cocrystal and pure ADN (a) before and (b) after hygroscopic test.
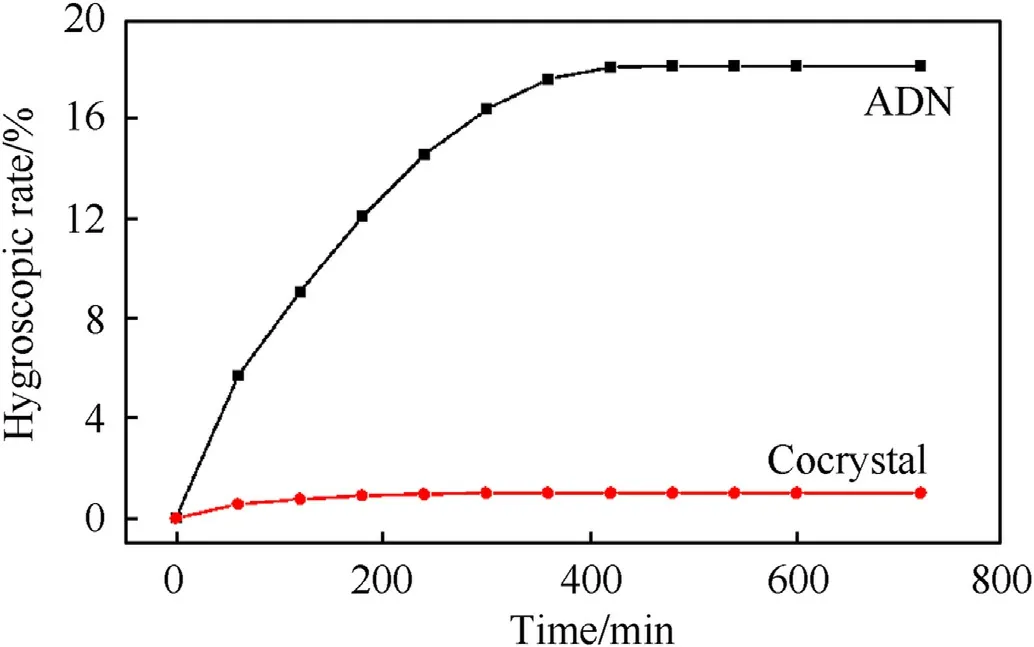
Fig.56.Hygroscopic rate curves of ADN/18C6 cocrystal and ADN.
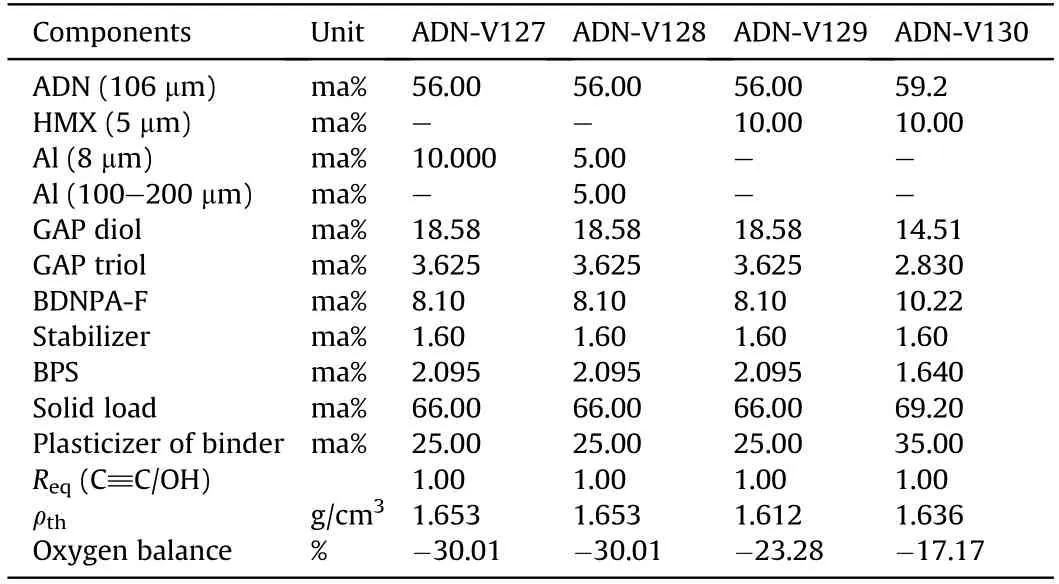
Table 9 Composition of ADN-GAPxx formulations.
6.1.Application of ADN in high energy propellants
In 2011,Cerri et al.[155,156]published two types of ADN-based propellant formulations.One is ADN-GAPxx propellant based on the GAP binder,including ADN-V127,ADN-V128,ADNV129,and ADNV130,and the other is ADN-D2200xx propellant based on the Desmophen®D2200 binder,containing ADN-V142 and ADN-V144.The authors also compared the performance of these two types of propellant formulations with that of AP-GAPxx and AP-D2200xx propellants.It is found that the strain capacity of ADN-based propellant is higher than that of AP-based propellant under the same loading condition.The results of accelerated aging test show that the performance of ADN-D2200xx propellant significantly changes,and the peak width of the loss factor increases,which may be caused by chain fracture or dehumidification.The composition ofseveral propellant formulations is shown in Table 9-Table 11.
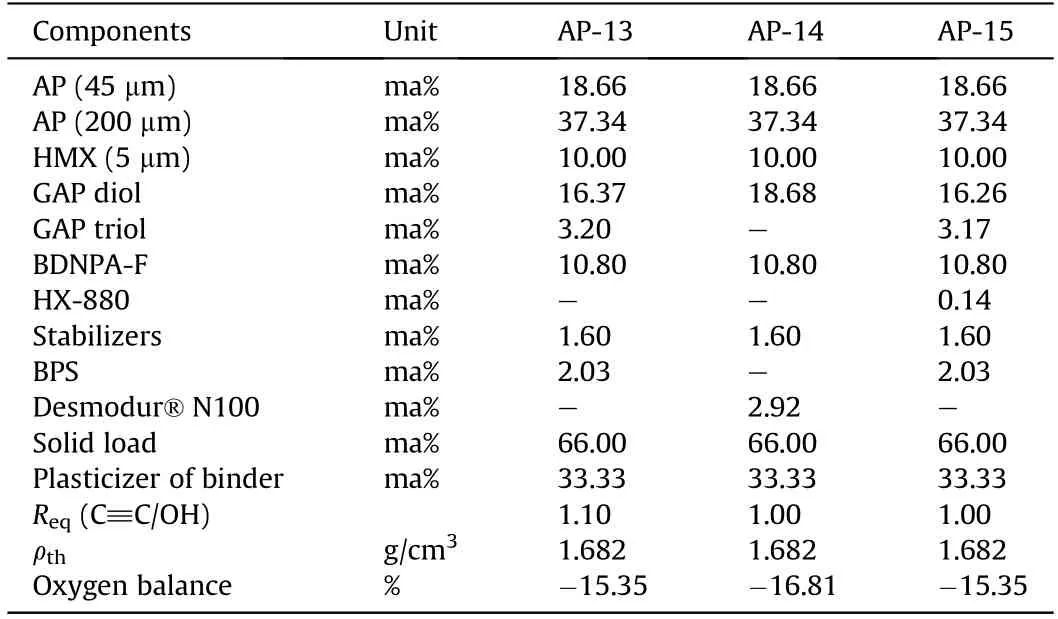
Table 10 Composition of AP-GAPxx formulations.
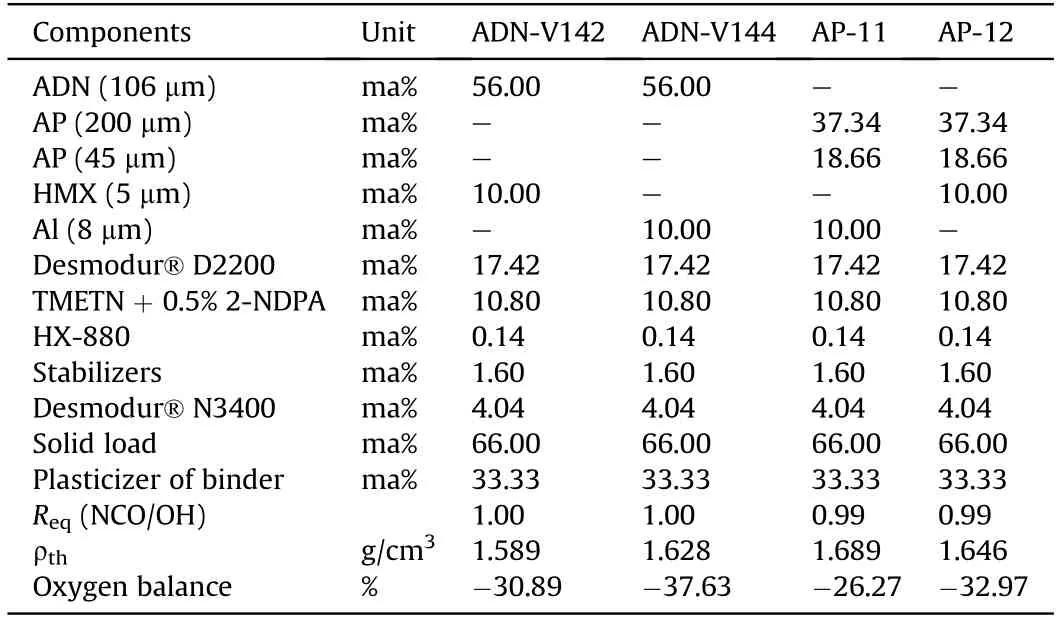
Table 11 Composition of ADN/AP-D2200xx formulations.
In 2013,the Fraunhofer ICT developed two new formulations of high-energy solid propellants [157].The main components of the propellants are ADN,GAP,and aluminum powder.One propellant is a GAP system cured by hexamethylene diisocyanate,the molar ratio of NCO to hydroxyl is 0.4,the content of ADN is 60%,and the content of aluminum powder is 16%.The other propellant is a GAP system cured by BPS,the molar ratio of BPS to hydroxyl is 0.8,the ADN content is 62%,and the aluminum powder content is 14%.It is shown that the elastic modulus of the two solid propellants both increased with an increase of the total solid content and slightly decreased with an increase of the aluminum powder content.When the mass fraction of solids in the propellant is more than 76%,the viscosity of the slurry is higher,which is more than 800 Pa s at 40°C.The maximum specific impulse of the propellant can reach 2685 N s/kg when the formulation is composed of 62% ADN,20%aluminum powder,and 18% GAP.
In 2017,the FOI proposed ADN/FOX-12/GAP insensitive propellant by forming cocrystals between ADN and FOX-12.The main components of the propellant are 30% GAP and 70% ADN/FOX-12 cocrystal.It is found that when the mass fraction of FOX-12 is 25%,the friction sensitivity of ADN/FOX-12/GAP propellant is equivalent to that of AP composite propellant,and its specific impulse is higher than that of traditional double-base propellant.Under a pressure of 7 MPa,the burning rate of ADN/FOX-12/GAP propellant is 15 mm/s,and the pressure index is 0.55.
In 2017,the Fraunhofer ICT had also carried out research on high energy green ADN/phase-stable ammonium nitrate (PSAN) solid propellant and evaluated its ballistic performance [158].Test results show that the combustion performance of ADN/PSAN propellant can be improved by adding benzoyl ferrocene into the formulation,and a stable combustion pressure can be controlled atabout 4 MPa.When the pressure is in the range of 4-13 MPa,the pressure index of ADN/PSAN propellant decreases,and the burning rate and specific impulse are also lower.It is found that properly increasing the ratio of ADN/PSAN is beneficial to improve the specific impulse and burning rate of the propellant.

Table 12 Formulations of ADN/AP-based propellants.
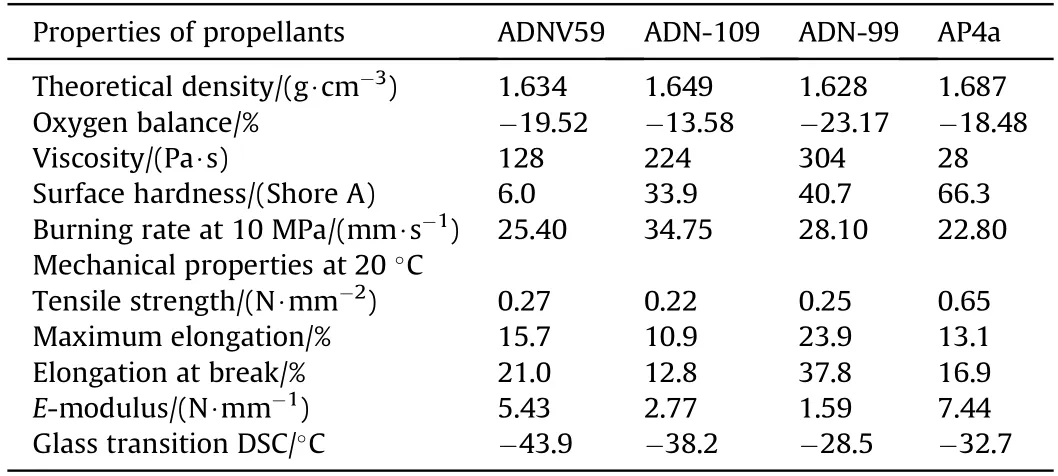
Table 13 Performances of ADN/AP-based propellants.
6.2.Application of ADN in low characteristic signal propellants
In 2008,Menke et al.[159,160] used BPS and N-100 as curing agents for GAP/trimethylolethanetrinitrate (TMETN) and developed three kinds of ADN/GAP propellants.They also compared the performances (processability,burning behavior,etc.) of the ADN/GAP propellant formulations with those of the corresponding AP propellant formulation.The formulations and performance of these propellants are shown in Table 12 and Table 13.
It can be seen from the data in Table 13 that the casting of ADNV59,AP4a is relatively easy due to the low viscosity of the slurry,whereas the casting of ADN109 and ADN99 is difficult.The surface hardness of ADN-based propellant containing BPS is slightly lower than that of AP-based propellant.In terms of burning rate,BPS cross-linked ADN-based propellant has higher burning rate than N100 cross-linked ADN-based propellant.These new ADNbased propellants all have the advantages of low characteristic signal and high burning rate.However,due to their high thermal sensitivity and easy detonation,it is currently difficult to use them in practical applications.
In 2009,the FOI[96]conducted a research based on a spherical ADN/GAP low signature propellant that is composed of 68% ADN and 32%GAP.Performance test results show that this propellant has a higher burning rate and a lower pressure index.Under a pressure of 7 and 10 MPa,the theoretical specific impulse is 2437 and 2499 N s/kg,respectively.The burning rate is 27 mm/s and the pressure index is 0.49 when the pressure is 10 MPa.In thecombustion process of the propellant,only a small amount of smoke is produced,and the solid residue content is very low.
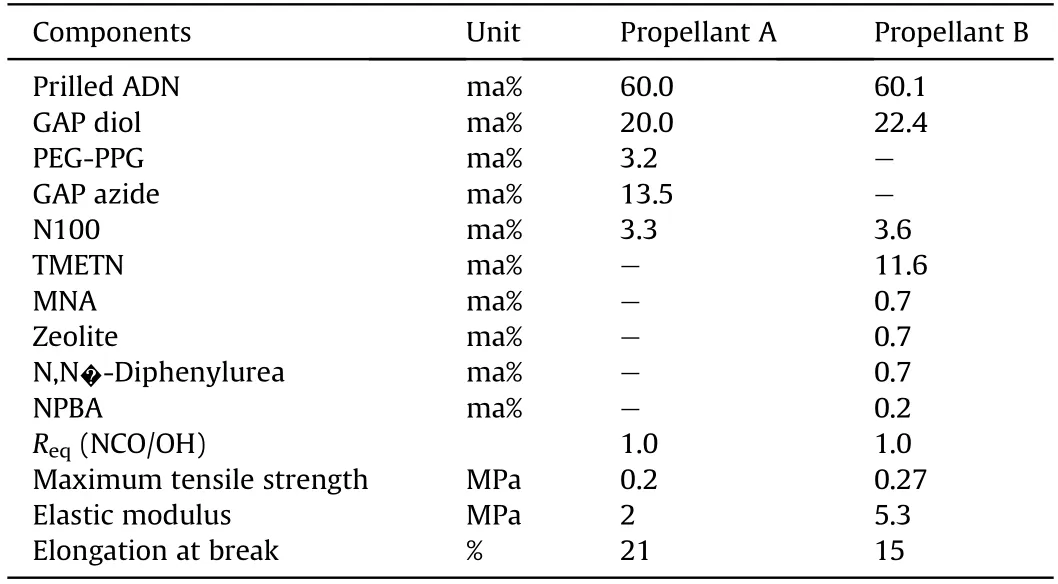
Table 14 Composition of ADN-based propellants and some test parameters.
In 2012,Landsem et al.[161]developed a smokeless composite propellant based on the spherical ADN/GAP binder system and studied its performance.The maximum tensile strength of this propellant is 0.2 MPa,the modulus of elasticity is 2 MPa,and the elongation at break is 21%.The researchers also mixed zeolite,Nmethyl-4-nitroaniline (MNA),and other additives into the ADN/GAP-based propellant to develop another smokeless composite propellant [119].The maximum tensile strength of the resulting propellant is about 0.27 MPa,the modulus of elasticity is 5.3 MPa,and the elongation at break is about 15%.It is found that the mechanical and curing properties of ADN/GAP-based propellants are poor,but their mechanical properties and durability can be improved by adding zeolite,HMX,and neutral polymer binder.The compositions and some physical properties of the two propellants are shown in Table 14.
In 2015,Gettwert et al.[162,163] proposed three propellant formulations based on ADN/GAP components,namely,Al/ADN/GAP,ADN/FOX12/GAP,and ADN/HMX/GAP propellants,and compared their performance with that of Al/AP/HTPB-based propellant.Under a pressure of 7 MPa,the specific impulse of Al/ADN/GAP,ADN/FOX12/GAP,and ADN/HMX/GAP propellants is 2247,2099,and 2237 N s/kg,respectively,which are all higher than the specific impulse of Al/AP/HTPB (2070 N s/kg).It is found that the total gas volume of the combustion products of ADN-based propellant is higher than that of AP-based propellant,which is the main reason for the higher specific impulse of ADN-based propellant.In addition,the researchers also tested the friction sensitivity,impact sensitivity,and chemical stability of the four different propellant formulations.Results indicate that these ADN-based propellant formulations are expected to replace Al/AP/HTPB composite propellant formulations in solid propellants.The composition and test results of the four propellants are shown in Tables 15 and 16.
6.3.Application of ADN in monopropellants
Since the1960s,hydrazine has become the material of choice for the liquid monopropellant and hydrazine-based propulsion systems have been widely used in space propulsion applications.The obvious advantage of hydrazine propellants is the high specific impulse with low adiabatic decomposition temperature.However,scientists are committed to finding new high performance nontoxic monopropellants for replacing hydrazine monopropellants due to the high toxicity and carcinogenicity of hydrazine.ADNbased monopropellant is a new type of green propellant developed in the past decades with the characteristics of high specific impulse,environmental friendliness,safety and low maintenance cost,which is especially suitable for low pollution space shuttle propulsion systems and space transportation power systems.
As early as 1997,the Swedish Space Company(SSC)and FOI had been studying the formulation of ADN monopropellants.They screened glycerin,glycine,and methanol from more than 100 kinds of materials as fuels for ADN-based propellants,and published a series of propellant formulations.The formulation performance of these monopropellants is shown in Table 17 [164-166].
It can be seen from the data in Table 16 that the specific impulse and density of ADN monopropellants are all higher than those of hydrazine and HAN propellants,and the freezing point of several ADN-based propellant is less than 0°C.In addition,ADN monopropellants can be stably stored for several hours at -20°C under the condition of no crystal nucleation,which means that they can replace hydrazine propellants (freezing point is 2°C) for some spacecraft operating systems.According to the standard of new monopropellant in the US Air Force Research Laboratory(AFRL),the researchers further screened the above formulations,and the test results are shown in Table 18.
It can be found that LMP-101 has excellent explosive performance,but poor thermal stability.FLP-106 has good explosive performance and thermal stability,and has been listed as a candidate formulation of monopropellants by the FOI.The thermal stability of LMP-103 is not very good,but it can be improved by adding 3-5% ammonia.
The SCC chose the improved LMP-103(called LMP-103S)as the candidate formulation of monopropellant,and successfully applied it to the Prisma satellites in 2010 [167-169].The formulation composition and properties of LMP-103 s are shown in Table 19.
In order to improve the energy level and compensate for the preheating temperature and vapor pressure of LMP-103s propellant,Kim et al.[170]developed two new monopropellants(KMP-4 and KMP-9),which are mainly composed of ADN,water,and tetraethylene glycol dimethyl ether (Tetraglyme) fuel.It can be seen from the data in Table 20 that the adiabatic decomposition temperature of KMP-9 propellant is significantly lower than that of LMP-103s propellant.However,the specific impulse of KMP-9 propellant is lower than that of LMP-103s propellant.The effective solution of the above problem will make the KMP-9 propellant the most promising candidate in space satellite systems.
6.4.Application of ADN in gel propellants
ADN has the characteristics of high energy,nontoxicity,and high solubility in water,so it is very suitable for use as a high-energy oxidizer in hydrogel propellants.In 2007,the Fraunhofer ICT proposed ADN-water gel propellants(ADN/H2O(3.5:1)+A200,ADN/H2O (3:1)+A200) with excellent performance [171].The main components of these propellants are aqueous solution of ADN and Aerosil® 200 gel.In the research of the thermal stability of the propellants,Arrhenius parameters of ADN-water gel propellants in different decomposition processes are determined by measuring the heat generation rate of the gelling agent and the stabilizer at different temperatures.It is found that the volume specific impulseof ADN/H2O(3.5:1)+A200 propellant can reach 2421 N s/dm3,and the oxygen balance is 18%-19%.The results of accelerated aging test show that the service life of this propellant can reach 15 years at a temperature of 30°C,and it is expected to be used in controllable thrust engines.
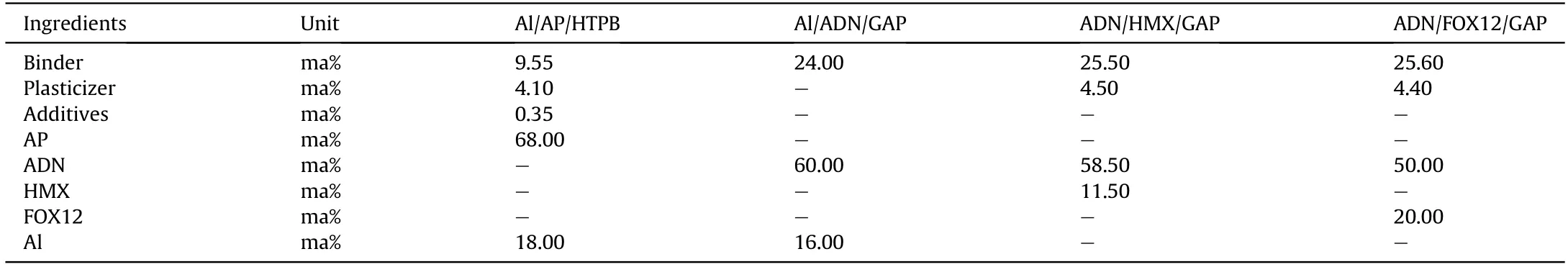
Table 15 Composition of selected propellant formulations.

Table 16 Test performance of propellants(under the condition of 7 MPa).
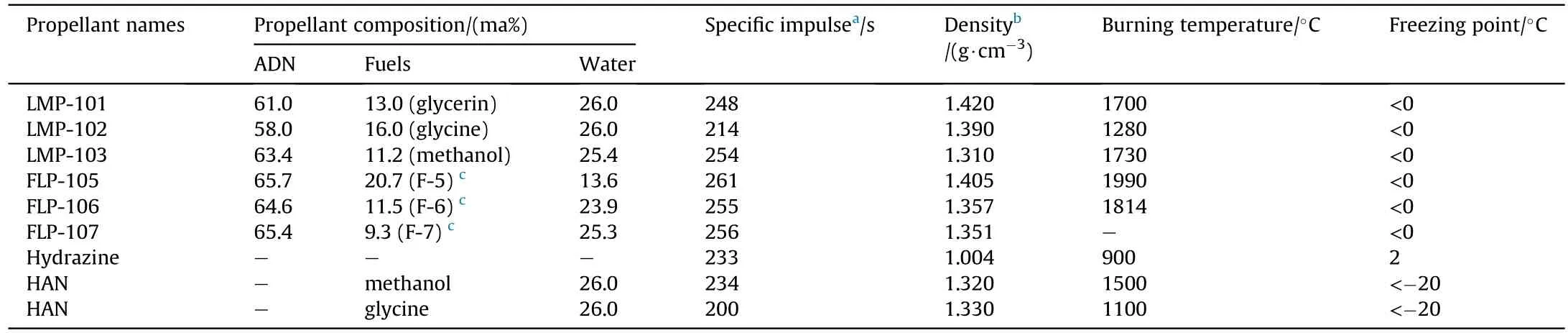
Table 17 Formulation performance of ADN-based monopropellants and comparison of them with hydrazine and hydroxylamine nitrate (HAN).
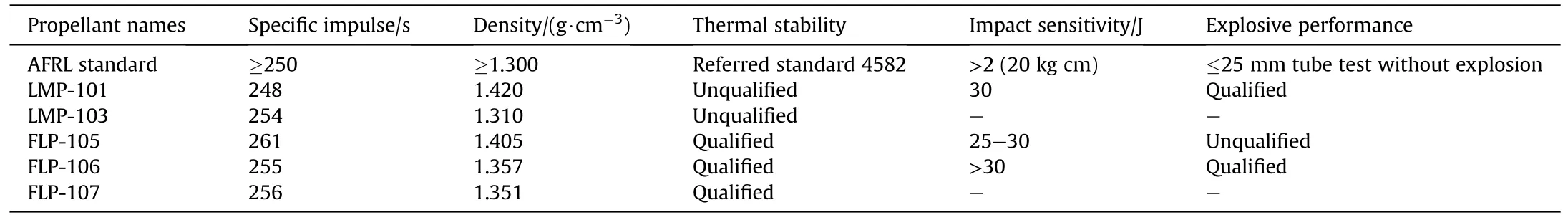
Table 18 Test results of several ADN-based monopropellant formulations.
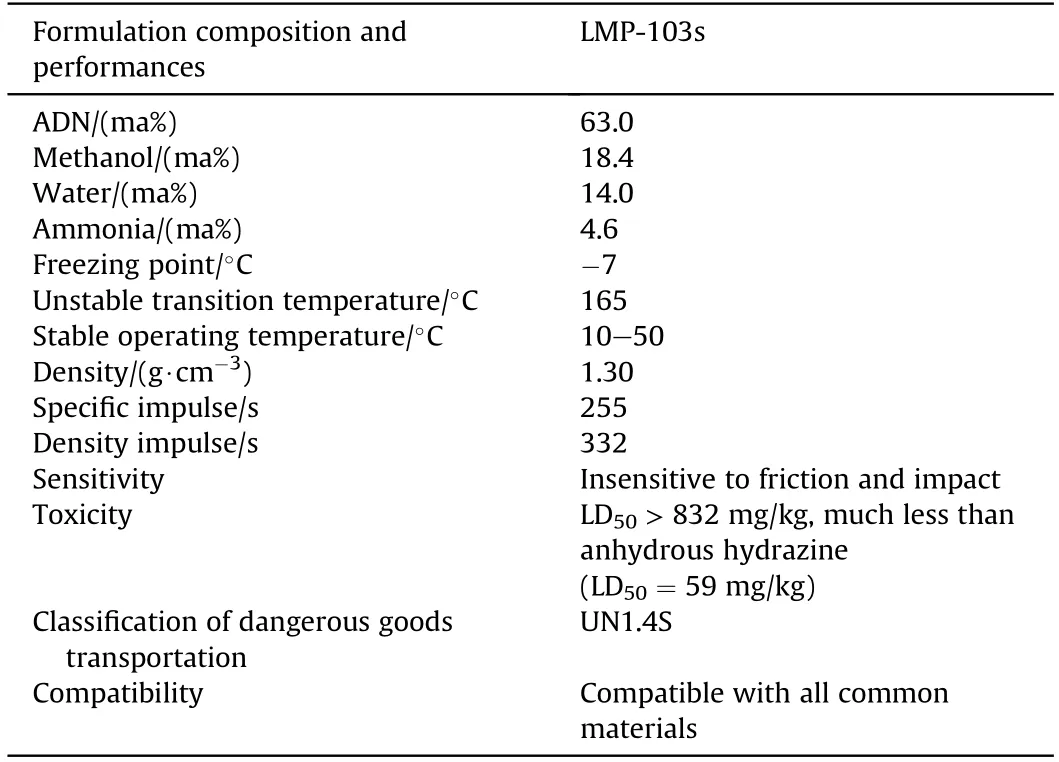
Table 19 Formulation composition and performance of LMP-103s.
6.5.Application of ADN in gun propellants
It is reported that ADN can be used as a component in lowvulnerability gun propellants [172].Wingborg et al.[173] found that ADN can be used in high performance gun propellants through thermodynamic calculation.Under a flame temperature of lower than 3500 K,the power of the gun propellant can be increased to 1310 kJ/kg,and the initial speed of the artillery can be increased by 2-5%.Therefore,ADN is one of the indispensable candidates in gun propellants.Zhang et al.[18] pointed out that the US Naval Weapons Research Center conducted numerous studies in order to find a gun propellant formulation with a gas molecular weight(MW) of ≤18 and a gunpowder force of ≥1400 J/g and finally developed several propellant formulations containing ADN.It is found that the formulation of ADN/TAGAZ has the highest gunpowder power,which can reach 1378 J/g.The formulations and performances of several kinds of gun propellant with fuel gas MW≤18 are shown in Table 21.
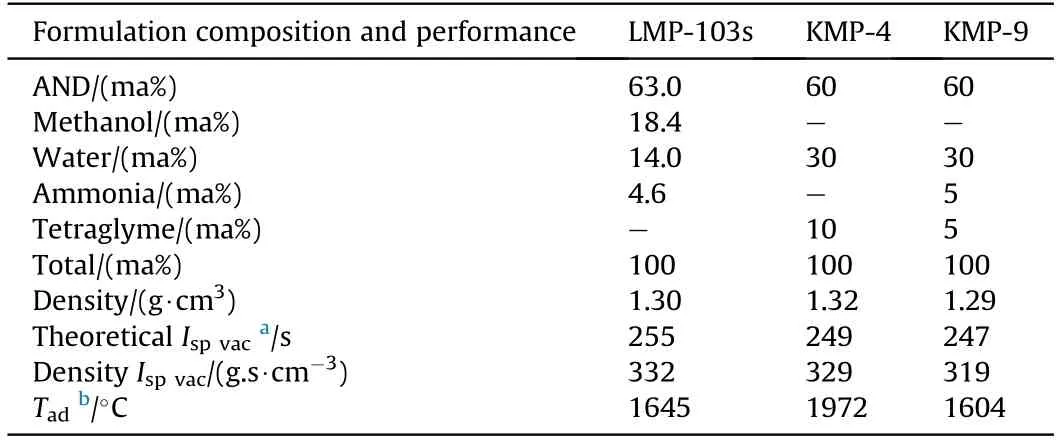
Table 20 Formulation composition and performance of ADN/Tetraglyme monopropellants and comparison of them with LMP-103s propellant.
6.6.Application of ADN in explosives
ADN is not only a high-energy oxidizer,but it is also an explosive with good performance,which is very suitable for the preparation of melt-cast explosives.Abraham et al.[174] used ADN instead of TNT as a matrix and prepared ADN/RDX,ADN/HMX,ADN/CL-20 melt-cast explosives using the same process used to produce TNT melt-cast explosives.It is found that the detonation performance of ADN melt-cast explosive is significantly better than that of TNT melt-cast explosive.When the mass ratio of ADN/HMX is 30:70,the detonation performance of the melt-cast explosive is equivalent to that of pure HMX,indicating that ADN is very suitable as a matrix for this type of molten-cast explosive.In addition,this technology is also suitable for the processing of hollow charges with a higher charge performance.The test results of various mixed melt-cast explosives are shown in Table 22.
In 2010,Hahma et al.[175]developed a melt-cast technology for preparing pure ADN,ADN/Al explosives.The researchers point out that molten ADN is unstable,which is due to the autocatalytic decomposition of ADN.Therefore,the temperature in the entire procedure must be strictly controlled below 100°C to avoid the risk caused by accidental ignition.It is found that MgO is an effective stabilizer to inhibit the thermal decomposition of molten ADN in the casting process.In addition,ADN contains a small amount of water (0.1-0.2% by mass),which can make it melt castable and mechanically stable.It can be seen from the data in Table 23 that cast ADN with aluminum (containing 1% MgO) can significantly increase the density of ADN charges,because aluminum functions as crystallizing kernels for ADN during the melt-cast process.
Due to the high oxygen balance of ADN,it can also be applied to high energy detonating compositions.In 2003,Gogulya et al.[176]prepared ADN/Al mixture by mixing ADN with different types of Alparticles,and investigated the effects of ultra-fine Al (100 nm,containing 83-87% pure metal) and commercial Al (15 μm,containing 98% pure metal) on the explosion heat and mechanical sensitivity of ADN.The results of calorimetric investigations indicate that the addition of aluminum powder significantly increases the explosion heat of ADN.But the mixtures of ADN with ultra-fine Al have no advantages in heat of explosion in comparison to ADN mixtures with commercial Al.This is because the value of explosion heat depends on aluminum content,rather than on Al particle size(specific surface).On the other hand,the addition of commercial Al to ADN slightly increases mechanical sensitivity,while mechanical sensitivity of ADN drastically increases (to the level of primary explosives) when ultra-fine Al is added.The high mechanical sensitivity of ADN/ultra-fine Al mixture can be explained by the chemical interaction between the thermal decomposition products of ADN and the fuel in the course of hot spot formation under explosive charge disintegration.The intensity of this interaction only depends on the reactivity of Al particles(related to the particle size of aluminum powder).
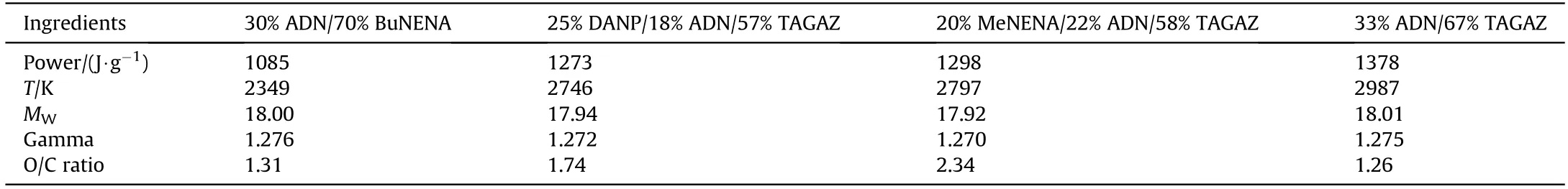
Table 21 Propellant formulations and properties of systems with gas Mw ≤18.

Table 22 Detonation performance of different mixed explosives (with pure HMX as reference).
In 2021,Comet et al.[26]used ADN as an explosive oxidizer for preparing new detonating compositions,with red phosphorus(RP)and titanium hydride (TH),and measured mechanical sensitivity,explosion heat,and detonation properties of ADN-based compositions(ADN/RP and ADN/TH).It is found that ADN/RP is markedly more sensitive than ADN/TH to mechanical stress,especially to friction (Table 24).Interestingly,the melting and evaporation of ADN absorbs the heat released by the electrostatic discharge of thesample,which makes ADN-based compositions insensitive to electrostatic discharge.
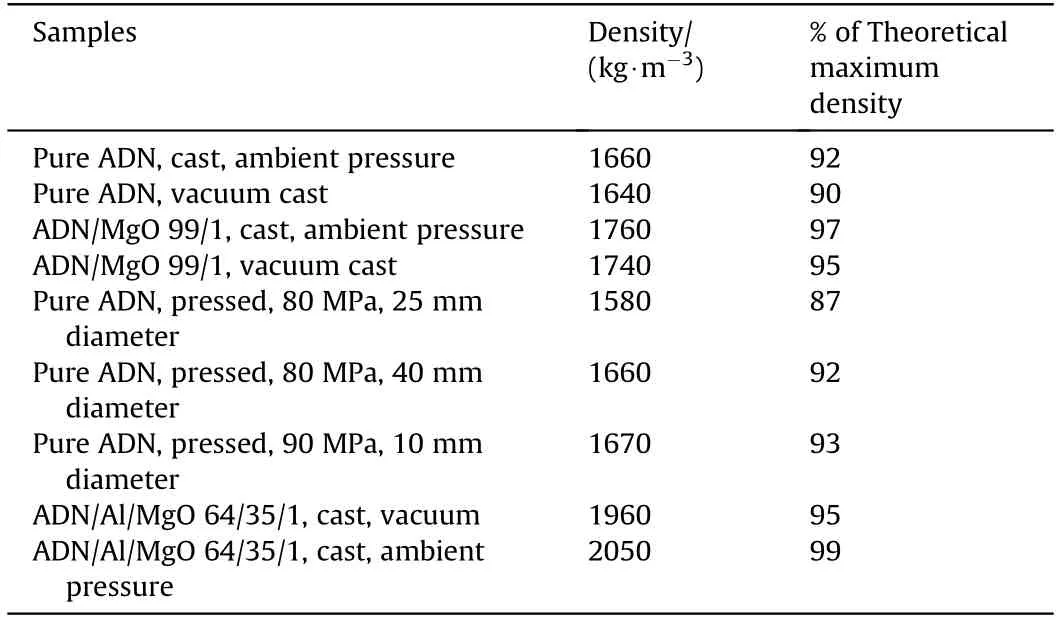
Table 23 Densities of pressed and cast ADN.

Table 24 Sensitivity thresholds to impact,friction and electrostatic discharge of ADN/RP and ADN/TH mixtures.
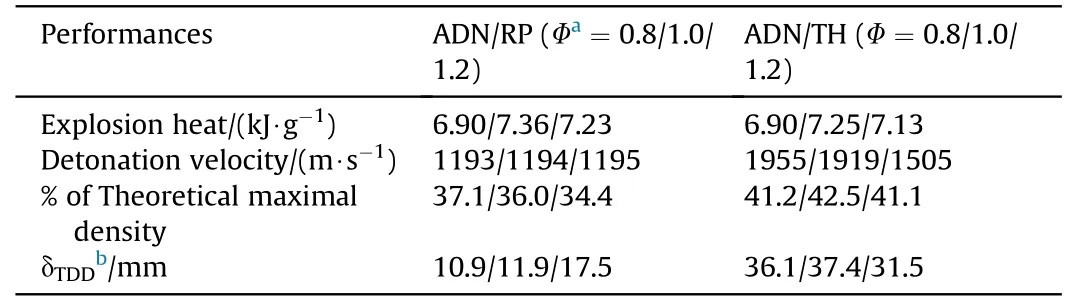
Table 25 Explosion heats and Detonation properties by of ADN/RP and ADN/TH compositions.
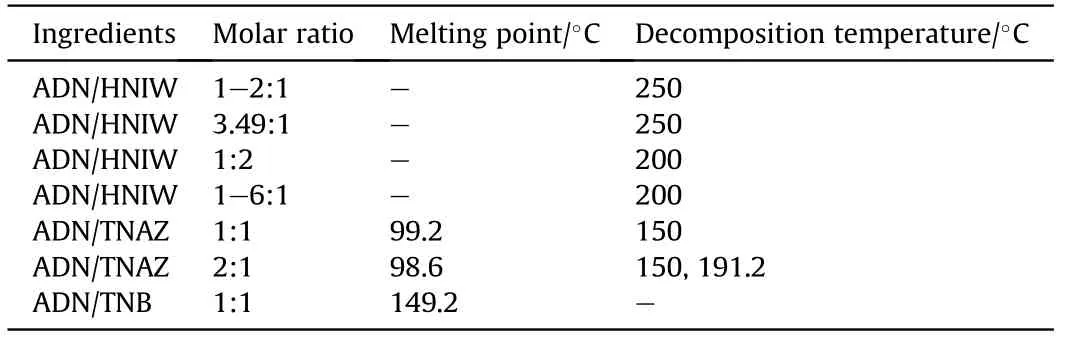
Table 26 Composition and some physical properties of cocrystal explosives.
The explosion heat value of pure ADN is only 3.92 kJ/g;it is almost doubled by the addition of the RP and TH (Table 25).From this perspective,ADN/RP and ADN/TH compositions outperform most energetic materials,such as sulphate-based nanothermites,intermetallic composites,and organic high explosives.It is found that the content of red phosphorus has little effect on the burning rate of ADN-based mixture,while titanium hydride has a significant effect on the burning rate of ADN-based mixture.This is because the combustion of hydrogen facilitates the transition to detonation of ADN.Less sensitive ADN-based compositions can be prepared by using phosphorus nitride instead of red phosphorus or titanium subhydrides instead of titanium hydride.
ADN can also form cocrystals with explosives such as CL-20,TNB,TNAZ,and other substances [18].Studies found that these explosives not only own an excellent explosive performance but also have a higher oxygen balance.At present,most explosive production technologies adopt melting and solvent methods to prepare such explosives.Table 26 lists the composition,melting point,and decomposition temperature of several ADN cocrystal explosives.
7.Conclusions
ADN,as a new type of high energy oxidizer,has attracted wide attention owing to its high enthalpy of formation and gas yield(the enthalpy of formation is-1207.4 kJ/kg,and the gas yield is 1084 L/kg).This paper reviews the research progress in the domains of ADN synthesis,thermal decomposition mechanism,hygroscopic mechanism,anti-hygroscopic technology,and its use in powders and explosives in past decades.At present,there are no less than twenty synthesis methods for ADN,such as the aminopropionitrile method,urea method,carbamate method,and sulfamate method.Among these methods,the sulfamate method is currently the most widely used due to its advantages: i.e.the use of cheap raw precursors,and the obtaining of a high yield.The thermal decomposition process of ADN is related to factors such as temperature,pressure,and experimental methods,which can be divided into the following two stages.In the first stage,ADN decomposes to generate NH3and HN(NO2)2and finally transforms into NH4NO3and N2O under the action of acid catalysis.In the second stage,the main decomposition product (NH4NO3) of ADN is further decomposed to produce N2O and H2O at a high temperature.
It is found that the moisture absorption by ADN largely depends on ambient temperature,relative humidity,sample purity,and other factors.The fundamental reason for the strong hygroscopicity of ADN is mainly related to its internal hydrogen bonds and its special molecular structure.The main methods to make ADN less hygroscopic include prilling,surface coating,and crystal modification technologies.Prilling technology is a way of changing the surface morphology of raw ADN into spheres to reduce its hygroscopicity,but this method does not change the surface chemical structure of ADN and does not introduce new chemical substances.Thus,the reduction of hygroscopicity of ADN is limited.Surface coating technology refers to coating the surface of ADN particles with a small amount of a hydrophobic material,so as to inhibit the contact between water molecules and ADN,thus reducing the hygroscopicity of ADN.The advantages of this method are that the amount of the coating agent is small,the anti-hygroscopic effect is significant,and it has limited effect on the performance of ADN.However,the surface coating method involves the screening of coating materials and an exploration process,which are more difficult and complicated.Crystal modification technology is a method to improve the structure and the morphology of ADN crystal by introducing a crystal control agent in the crystallization process or by using the cocrystallization technology.Compared with the traditional prilling method,the method of introducing a crystal control agent in the solution crystallization process not only can significantly decrease the hygroscopicity of ADN,but it also can improve the safety of the test process.However,this technology is closely affected by many factors,such as dispersion mode,solution impurities,and metastable region.Thus,it is necessary to further explore the growth parameters and crystallization process of ADN.Cocrystal technology can solve the problem of strong moisture absorption of ADN at the molecular level,but the theoretical design and preparation methods of the anti-hygroscopic cocrystal of ADN still need to be further studied.ADN has a wide application prospect in powder and explosives due to its high enthalpy of formation and positive oxygen balance.It is found that replacing AP with ADN in propellants can effectively improve the specific impulse and combustion rate of propellants.In addition,adding some ADN particles into explosives can significantly improve the detonation performance of explosives.
At present,the research on ADN has made prominent achievements,but there is still much work that is worthy of further study and exploration.Therefore,the author puts forward the following suggestions for future research reference:
a.Many researchers have done a lot of work on the synthesis process of ADN,aiming at reducing the production cost of ADN and decreasing environmental pollution.However,it is urgent to carry out an in-depth research on the recycling of nitration waste acid and the simplification and optimization of the synthesis process in order to realize the large-scale production of ADN.
b.The thermal decomposition mechanism and main decomposition products of ADN have been clarified.Studying the influence of common metal powder (aluminum powder and copper powder) on the thermal decomposition process of ADN will make ADN more widely used in energetic materials.
c.Currently,the anti-moisture absorption studies on ADN have made many significant achievements,but it is very difficult to solve the problem of strong hygroscopicity of ADN by using a single method.One of the future research directions in this field is to combine various methods to study the anti-hygroscopicity of ADN.
d.Further expand the research on ADN applications in powder and explosives,in order to improve the overall performance of ADNbased powder and explosives.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Project No.21805139,12102194,22005144 and 22005145),the Joint Funds of the National Natural Science Foundation of China (No.U2141202),Natural Science Foundation of Jiangsu Province (No.BK20200471) and the Fundamental Research Funds for the Central Universities (No.30920041106,30921011203).
杂志排行
Defence Technology的其它文章
- Blast wave characteristics of multi-layer composite charge:Theoretical analysis,numerical simulation,and experimental validation
- Sensitivity analysis of spacecraft in micrometeoroids and orbital debris environment based on panel method
- Dynamic mechanical characteristics of NdFeB in electromagnetic brake
- Quasi-static and low-velocity impact mechanical behaviors of entangled porous metallic wire material under different temperatures
- A small-spot deformation camouflage design algorithm based on background texture matching
- Deep learning-based LPI radar signals analysis and identification using a Nyquist Folding Receiver architecture
