钠离子电池正极材料V2O5研究进展
2023-01-14周娅时润娜郎笑石姚传刚蔡克迪
周娅,时润娜,郎笑石,姚传刚,蔡克迪,
钠离子电池正极材料V2O5研究进展
周娅1,时润娜1,郎笑石1,姚传刚2,蔡克迪1,2
(1. 渤海大学 化学与材料工程学院,辽宁 锦州 121013; 2. 辽宁省超级电容器工程技术研究中心,辽宁 锦州 121013)
钠离子电池(SIBs)具有资源丰富、成本低廉及供应风险低等优势,被认为是极具潜力的下一代电化学储能器件。目前,五氧化二钒(V2O5)正极材料因具有高工作电压及高理论容量等优点,逐渐成为SIBs正极材料的研究热点。然而,V2O5正极材料的低离子扩散系数、低电导率及反复的离子嵌入/脱嵌所致的结构不稳定等缺点,限制了其在SIBs中的应用。分析了V2O5正极材料的晶体结构和储钠机制,并通过形貌控制、晶体结构修饰、化学预插入以及与其他材料复合等改性方法,综述了V2O5正极材料在SIBs中的研究进展,最后对V2O5正极材料的发展趋势进行了展望。
V2O5; 形貌控制; 晶体结构; 化学预插入; 导电材料
近年来,随着清洁能源和可再生能源(太阳能、风能、潮汐能等)的不断探索与发展,高效储能系统受到了科研人员的关注。目前,锂离子电池(LIBs)因其能量密度高、循环寿命长、环境友好等特点,在国内外便携式电子设备、新能源汽车等大规模储能系统市场上占据了主导地位。但是,锂(Li)元素存在资源短缺、在地壳中分布较少的缺点,导致LIBs成本较高。钠(Na)是地壳中含量最多的元素之一,其质量约占总元素质量的2.74%,且钠离子电池(SIBs)的电荷存储机制与LIBs相似,因此SIBs逐渐成为科研工作者的研究热点[1⁃4]。
SIBs的研究始于20世纪80年代,但在发展过程中,关于SIBs的研究却停滞不前。这主要是由于:与Li+半径(0.076 nm)相比,Na+半径(0.102 nm)较大,充放电过程中体积变化大,容易引起电极材料结构的崩塌;Na有较高的电负性(-2.71 V),使SIBs的能量密度低于LIBs;Na的低熔点、枝晶生长和界面老化等缺点带来了使用安全问题[5⁃7]。因此,寻找和优化优异的电极材料是开发SIBs的关键。

针对V2O5正极材料出现的问题,目前采取的措施是通过形貌控制,从而增大比表面积,缩短Na+的扩散距离;通过晶体结构修饰和化学预插入方法来增大V2O5层间距,提高结构稳定性;通过与导电材料复合以增加V2O5的电导率等。本文综述了V2O5作为SIBs正极材料的研究进展,分析了V2O5的储钠机制,归纳了V2O5正极材料改性的研究成果,并对其未来发展方向进行了展望。
1 V2O5的结构
V2O5是正交晶型,其晶格常数为:=1.151 nm,=0.356 nm,=0.437 nm[15]。V2O5具有典型的层状结构,层内各原子以共价键相连,层与层之间以微弱的范德华力结合,整个结构由金字塔形[VO5]结构单元以上上下下方式连接而成。在每个金字塔中,1个V原子被5个O原子嵌入,5个V-O将V原子与O原子连接起来。在同一层的金字塔中,每个O原子都由2个或3个V原子结合,其中有1个长的V-O(0.21~0.27 nm)和1个短的V=O(0.15~0.17 nm)。此外,金字塔顶端的O原子与相邻层内的V原子之间存在微弱的V-O相互作用,键长约为0.28 nm,这种结构非常利于离子的嵌入和脱出。正交相V2O5的晶体结构如图1所示[16⁃18]。
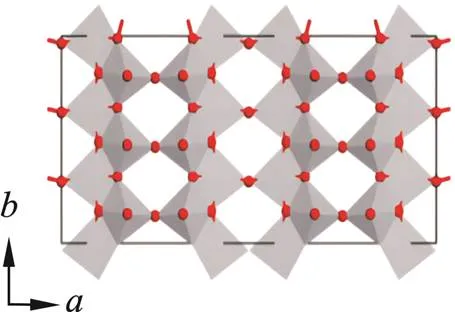
图1 正交相V2O5的晶体结构
2 V2O5的储钠机制
V2O5作为SIBs正极材料,当Na+嵌入时,会被存储到与平面平行的V2O5四棱锥层间,V2O5在SIBs中可逆充放电过程为:V2O5+Na++e-→NaV2O5(0<<2)[19]。
但是,在首次充放电过程中,V2O5发生不可逆相变,有一小部分的Na+被嵌入晶格中无法脱出,这会导致容量的衰减。W.Y.Li等[19]对V2O5正极材料在充放电过程中的结构变化进行了考察,结果如图2所示。

(a) V2O5正极材料的第1次和第2次充放电曲线(电流密度为100 mA/g) 第1次充放电 第2次充放电(b)不同状态下V2O5纳米线的原位XRD图 (c) Na+嵌入/脱嵌后V2O5的结构变化示意图
由图2可知,在电压为1~4 V的条件下,第1次放电时每个V2O5晶胞中可插入0.02 mol的Na+,可表示为:α⁃V2O5+0.02Na++0.02e-→α⁃Na0.02V2O5;然后,进一步嵌入Na+导致α⁃V2O5与α′⁃Na0.88V2O5不可逆相变,此时层间距由0.437 nm转变为0.478 nm,可表示为:α⁃Na0.02V2O5+0.86Na++0.86e-→α′⁃Na0.88V2O5;在接下来的充放电过程中,0.60 mol的Na+可逆嵌入,形成α′⁃Na1.48V2O5,此时层间距变化率只有0.42%,即:α′⁃Na0.88V2O5+0.60Na++0.60e-↔α′⁃Na1.48V2O5。
虽然V2O5(ɑ⁃V2O5)的层间距(0.437 nm)能保证Na+的嵌入/脱嵌,但是反复嵌入/脱嵌Na+导致的结构稳定性差、充放电过程中的不可逆相变以及电导率低等问题,使V2O5的电化学性能较差。通过对V2O5材料的形貌控制、晶体结构修饰、化学预插入及与其他材料复合的改性方法有望解决以上问题。
3 V2O5的改性研究
3.1 形貌控制





3.2 晶体结构修饰
V2O5根据其亚稳态多态性的不同,可分为α⁃V2O5、β⁃V2O5、γ′⁃V2O5、δ⁃V2O5、ε′⁃V2O5等。晶体结构的修饰使V2O5层间距变大,降低电荷/电子的迁移能垒,在充放电过程中抑制相变;因层间距可调节,还可以适应Na+快速嵌入/嵌出过程中的体积变化,从而促进电化学性能。


δ⁃V2O5相当于双层V2O5[29],双层V2O5的层间距在0.88~1.38 nm,是α⁃V2O5(0.44 nm)的两倍以上。双层V2O5包括干凝胶、气凝胶、薄膜、一维纳米结构等,其中干凝胶和气凝胶是水合五氧化二钒(V2O5·H2O)经过不同的干燥方式制得的。干凝胶由简单的溶剂干燥形成,具有较低的比表面积(小于10 cm2/g),而气凝胶通过超临界干燥形成,具有多孔结构和较高的比表面积。单层正交相V2O5和双层V2O5·H2O的晶体结构如图4所示。

(a)单层正交相V2O5 (b)双层V2O5·nH2O
V2O5·H2O中的水分子嵌入在V2O5层状结构间,其浓度可以通过温度进行调控。水分子可以在插入的阳离子与阴离子之间提供静电屏蔽,促进Na+的扩散。因此,V2O5·H2O具有较低的离子嵌入能和层间距可控的优点,在一定程度上可大大减少Na+的扩散阻碍,对提高材料的容量和倍率性能起一定作用。





图5 ε′⁃V2O5的结构特征
总之,通过对V2O5的晶体结构修饰不仅可以改善Na+扩散速率,而且可调控的层间距可缓解充放电过程中Na+嵌入/脱嵌引起的体积变化,提高V2O5的循环性能。
3.3 化学预插入
虽然修饰V2O5·H2O晶体结构可增大层间距,但是V2O5·H2O层与层之间相互作用较弱,易引起“晶格呼吸”(层间距的收缩和膨胀),从而使结构坍塌,导致V2O5在循环过程中容量快速衰减[40]。因此,可以通过预插入各种有机/无机材料的方法,发挥V2O5层间柱的作用来提高结构稳定性,抑制不可逆相变,同时减少容量损失。另外,该方法也可抑制V2O5在电解液中的溶解,提升循环性能。


C.Z.Liu等[45]采用溶胶⁃凝胶和冷冻干燥方法,将Y3+预插入V2O5·H2O中。结果表明,与纯V2O5相比,Y0.02V2O5的循环稳定性更强,Na+扩散系数更大,电化学反应电阻更低。第一性原理计算结果表明,预插入的Y3+形成了[YO6]层间柱,将V2O5双层牢固地结合在一起,产生了额外的V4+,引入了氧空位,有效提高了V2O5·H2O的结构稳定性和电导率,同时抑制了V2O5·H2O在反复充放电循环过程中的不可逆相变,从而提供了优异的循环稳定性。然而,预插入的金属离子在循环过程中会重新平衡晶格电荷分布,且低价态客体离子与宿主的氧端基团之间形成不稳定的键,并不能完全抑制“晶格呼吸”,仍然会导致比容量的快速衰减。所以,可以预插入有机分子,使不同官能团与双层V2O5发生反应,从而产生结构缺陷,或者是利用强化学键扩大层间距和稳定层状结构来提高电化学性能。

由图7可知,在电压为1.5~3.0 V时可插入1 mol Na+,此时V2O5(001)晶面间距为0.478 nm,比原来的晶面间距(0.437 nm)增加了9.4%。PVO材料(001)晶面间距(1.42 nm)没有发生变化,这说明PVO材料有零应变行为,可提供丰富的Na+存储位点,并增强Na+扩散率。这种特性不仅使该材料保持高储钠容量,而且还显著增强材料优异的循环稳定性和高倍率性能。

图6 YxV2O5(x=0、0.02、0.06)样品的制造工艺示意图

图7 聚苯胺/V2O5复合材料(001)晶面的分析
总之,预插入V2O5的电化学性能高度依赖于插入介质的种类或数量,不管是插入金属离子还是有机、无机材料,不仅可提高材料的容量和循环稳定性,而且在一定程度上可以提高材料的电导率。
3.4 与其他材料的复合


将V2O5与其他结构稳定的金属氧化物复合也是提高其电化学性能的一种方法。图8为三维TiO2⁃V2O5⁃C纳米结构的制备过程及其作为SIBs正极材料的应用示意图。


图8 三维TiO2⁃V2O5⁃C纳米结构的制备过程及其作为SIBs正极材料的应用示意图
4 结论与展望
V2O5是一种很有潜力的高容量正极材料,由于其独特的晶体结构在SIBs中发挥了重要的作用。但是,V2O5的电导率低、结构稳定性差和离子扩散系数小等问题限制了其在高效储能系统中的应用。研究者采用不同的改性方法,例如利用形貌控制增大了比表面积,缩短了Na+扩散距离;通过晶体结构修饰和化学预插入方法,增大了层间距,提高了Na+的扩散系数;与其他导电材料复合,提高了电导率和结构稳定性,有效地提高了V2O5正极材料的电化学性能。
虽然V2O5正极材料在SIBs应用方面取得了巨大的研究进展,但是由于V2O5的固有缺点,与实际应用还有很大的差距。因此,未来可以从以下几个方面进行研究:(1)将形貌控制、化学预插入和其他导电纳米材料相结合,充分利用不同改性方法的优点提高V2O5正极材料的电化学性能;(2)利用理论计算或原位表征技术等手段合理设计并优化材料结构,同时探究新型材料的储钠机理与其电化学性能之间的关系;(3)选择合适的V2O5正极材料和负极材料以及匹配的电解液体系,对未来发展的高性能Na+全电池应用提供一种新的研究思路,可以在一定程度上发挥V2O5正极材料的优势。相信随着科研水平的不断提高,V2O5有望在SIBs中大规模应用。
[1]Wang Q H, Xu J T, Zhang W C, et al. Research progress on vanadium⁃based cathode materials for sodium ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(19): 8815⁃8838.
[2]Liu Z, Yu Q, Zhao Y L, et al. Silicon oxides:A promising family of anode materials for lithium⁃ion batteries[J]. Chemical Society Review, 2019, 48(1): 285⁃309.
[3]Yan B, Zhong S K, Liu X J, et al. Growth of large⁃area petal⁃like V2O5thin nanosheets for superior lithium storage[J]. Journal of Alloys and Compounds, 2021, 875: 159899.
[4]Goikolea E, Palomares V, Wang S J, et al. Na⁃ion batteries⁃approaching old and new challenges[J]. Advanced Energy Materials, 2020, 10(44): 2002055.
[5]Ghimbeu C M,Gorka J,Simone V,et al.Insights on the Na+ion storage mechanism in hard carbon:Discrimination between the porosity,surface functional groups and defects[J].Nano Energy,2018,44:327⁃35.
[6]Chen M Z, Liu Q N, Hu Z, et al. Designing advanced vanadium⁃based materials to achieve electrochemically active Multielectron reactions in sodium/potassium⁃ion batteries[J]. Advanced Energy Materials, 2020, 10(42): 2002244.
[7]Wang X H, Li Q, Wang H Y. Anatase TiO2as a Na+⁃storage anode active material for dual⁃ion batteries[J]. ACS Applied Materials Interfaces, 2019, 11(33): 30453⁃30459.
[8]Zhang W Y, Jin H X, Chen G W, et al. Sandwich⁃like N⁃doped carbon nanotube@Nb2C MXene composite for high performance alkali ion batteries[J]. Ceramics International, 2021, 47(14):20610⁃20616.
[9]Tomboc G M, Wang Y T, Wang H, et al. Sn⁃based metal oxides and sulfides anode materials for Na ion battery[J]. Energy Storage Materials, 2021, 39: 21⁃44.
[10] Qin M S, Ren W H, Jiang R X, et al. Highly crystallized prussian blue with enhanced kinetics for highly efficient sodium storage[J]. ACS Applied Materials Interfaces, 2021, 13: 3999⁃4007.
[11] Guo W B, She Z Y, Xue H T, et al. Density functional theory study on the Ti3CN and Ti3CNT2(T = O, S and F) as high capacity anode material for Na ion batteries[J]. Applied Surface Science, 2020, 529: 147180.
[12] 卢虹宇,赵景新,彭琪贺,等.正极材料五氧化二钒常规改性的研究进展[J].化学世界, 2020, 61(3):153⁃164.
Lu H Y,Zhao J X,Peng Q H,et al.Progress in conventional modification of vanadium pentoxideas cathode material[J]. Chemical World,2020,61(3):153⁃164.
[13] West K, Christiansen Z B, Jacobsen T, et al. Sodium insertion in vanadium oxides[J]. Solid State Ionics, 1988, 28⁃30: 1128⁃1131.
[14] Lu J S, Nguyen M N, Liu W R, et al. Electrochemical properties of novel FeV2O4as an anode for Na⁃ion batteries[J]. Scientific Reports, 2018, 8: 8839.
[15] Tan H, Yu X Z, Huang K, et al. Large⁃scale carambola⁃like V2O5nanoflowers arrays on microporous reed carbon as improved electrochemical performances lithium⁃ion batteries cathode[J]. Journal of Energy Chemistry, 2020, 51: 388⁃395.
[16] Geng H B, Cheng M, Wang B, et al. Electronic structure regulation of layered vanadium oxide via interlayer doping strategy toward superior high⁃rate and low⁃temperature zinc⁃ion batteries[J].Advanced Function Materials,2019,30(6):1907684.
[17] Chernova N A, Roppolo M, Dillon A C, et al. Layered vanadium and molybdenum oxides: Batteries and electrochromics[J]. Journal of Materials Chemistry, 2009, 19(17): 2526⁃2552.
[18] Ghulam A, Lee J H, Si H O, et al. Investigation of the Na intercalation mechanism into nano⁃sized V2O5/C composite cathode material for Na⁃ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(9): 6032⁃6039.
[19] Li W Y, Ji J C, Yao J H, et al. Sodium ion storage performance and mechanism in orthorhombic V2O5single⁃crystalline nanowires[J]. Science China Materials, 2021, 64: 557⁃570.
[20] Rui X H, Tang Y, Malyi O I, et al. Ambient dissolution⁃recrystallization towards large⁃scale preparation of V2O5nanobelts for high⁃energy battery applications[J]. Nano Energy, 2016, 22: 583⁃593.
[21] Su D W, Dou X, Wang G X.Hierarchical orthorhombic V2O5hollow nanospheres as high performance cathode materials for sodium⁃ion batteries[J]. Journal of Materials Chemistry A, 2014, 2(29): 11185⁃11194.
[22] Zhao Z J, Li D L, Wang C Z, et al. Hierarchical V2O5microspheres: A pseudocapacitive cathode material for enhanced sodium ion storage[J]. Journal of Alloys and Compounds, 2022, 895: 162617.
[23] Wang L, Wang Y C, Cao A M, et al. Electrochemically anodized V2O5as an efficient sodium cathode[J]. Energy Fuels, 2021, 35(9): 8358⁃8364.
[24] Kulish V V, Manzhos S. Comparison of Li, Na, Mg and Al⁃ion insertion in vanadium pentoxides and vanadium dioxides[J]. RSC Advances, 2017, 7(30): 18643⁃18649.
[25] Córdobaa R, Kuhna A, Pérez J C F, et al. Sodium insertion in high pressure β⁃V2O5: A new high capacity cathode material for sodium ion batteries[J]. Journal of Power Sources, 2019, 422: 42⁃48.
[26] Baddour H R, Renard M S, Emery N, et al. The richness of V2O5polymorphs as superior cathode materials for sodium insertion[J]. Electrochimica Acta, 2018, 270: 129⁃137.
[27] Renard M S, Baddour H R, Ramos J P. Kinetic insight into the electrochemical sodium insertion⁃extraction mechanism of the puckered γ′⁃V2O5polymorph[J]. Electrochimica Acta, 2019, 322: 134670.
[28] Baddour H R, Renard M S, Ramos J P. Enhanced electrochemical properties of ball⁃milled γ′⁃V2O5as cathode material for Na⁃ion batteries: A structural and kinetic investigation[J]. Journal of Power Sources, 2021, 482: 229017.
[29] Moretti A, Passerini S. Bilayered nanostructured V2O5·H2O for metal batteries[J]. Advanced Energy Materials, 2016, 6(23): 1600868.
[30] Tepavcevic S, Xiong H, Stamenkovic V R, et al. Nanostructured bilayered vanadium oxide electrodes for rechargeable sodium⁃ion batteries[J]. ACS Nano, 2012, 6(1): 530⁃538.
[31] Su D W, Wang G X. Single⁃crystalline bilayered V2O5nanobelts for high⁃capacity sodium⁃ion batteries[J]. ACS Nano, 2013, 7(12): 11218⁃11226.
[32] Wei Q L, Liu J, Feng W, et al. Hydrated vanadium pentoxide with superior sodium storage capacity[J]. Journal of Materials Chemistry A, 2015, 3(15): 8070⁃8075.
[33] Wang H, Bi X X, Bai Y, et al. Open⁃structured V2O5·H2O nanoflakes as highly reversible cathode material for monovalent and multivalent intercalation batteries[J]. Advanced Energy Materials, 2017, 7(14): 1602720.
[34] Moretti A, Secchiaroli M, Buchholz D, et al. Exploring the low voltage behavior of V2O5aerogel as intercalation host for sodium ion battery[J]. Journal of the Electrochemical Society, 2015, 162(14): 2723⁃2728.
[35] Moretti A, Maroni F, Osada I, et al. V2O5aerogel as a versatile cathode material for lithium and sodium batteries[J]. ChemElectroChem, 2015, 2(4):529⁃537.
[36] Li Y W, Liu C Z, Xie Z P, et al. Superior sodium storage performance of additive⁃free V2O5thin film electrode[J]. Journal of Materials Chemistry A,2017, 5(32): 16590⁃16594.
[37] Li S Y, Li X F, Li Y W, et al. Superior sodium storage of vanadium pentoxide cathode with controllable interlamellar spacing[J]. Electrochimica Acta, 2017, 244: 77⁃85.
[38] Tolhurst T M, Leedahl B, Andrews J L, et al. Electronic structure of ε′⁃V2O5: An expanded band gap in a double⁃layered polymorph with increased interlayer separation[J]. Journal of Materials Chemistry A, 2017, 5(45): 23694⁃23703.
[39] Hadjean R B, Renard M S, Emery N, et al. The richness of V2O5polymorphs as superior cathode materials for sodium insertion[J]. Electrochimica Acta, 2018, 270: 129⁃137.
[40] Ji J C, Li Y W, Zhang Z L, et al. PEG intercalated vanadium pentoxide xerogel for enhanced sodium storage[J]. Materials Letters, 2020, 270: 127752.
[41] Clites M, Pomerantseva E. Bilayered vanadium oxides by chemical pre⁃intercalation of alkali and alkali⁃earth ions as battery electrodes[J]. Energy Storage Materials, 2018, 11: 30⁃37.
[42] Cai Y S, Zhou J, Fang G Z, et al. Na0.282V2O5: A high⁃performance cathode material for rechargeable lithium batteries and sodium batteries[J]. Journal of Power Sources, 2016, 328: 241⁃249.
[43] Pan H, Zhang G B, Liao X B, et al. Sodium ion stabilized vanadium oxide nanowire cathode for high⁃performance zinc⁃ion batteries[J]. Advanced Energy Materials, 2018, 8(10): 1702463.
[44] Parmar R, Matsubara E Y, Gunnella R, et al. Electro⁃insertion of Mn2+ions into V2O5·H2O on MWCNTs coated carbon felt for binder⁃free Na+ion battery electrodes[J]. Sustainable Energy & Fuels, 2020, 4(8): 3951⁃3962.
[45] Liu C Z, Yao J H, Zou Z G, et al. Boosting the cycling stability of hydrated vanadium pentoxide by Y3+pillaring for sodium⁃ion batteries[J]. Materials Today Energy, 2019, 11: 218⁃227.
[46] Dong J, Jiang Y L, Wei Q L, et al. Strongly coupled pyridine⁃V2O5·H2O nanowires with intercalation pseudocapacitance and stabilized layer for high energy sodium ion capacitors[J]. Small, 2019, 15(22): 1900379.
[47] Wang Z Q, Tang X Y, Yuan S, et al. Engineering vanadium pentoxide cathode for the zero⁃strain cation storage via a scalable intercalation⁃polymerization approach[J]. Advanced Energy Materials, 2021, 31(22): 2100164.
[48] Zhao M S, Wang J, He X H, et al. Graphene modified vanadium oxide composite materials used in aqueous Na⁃ion batteries[J]. Energy & Fuels, 2021, 35(24): 20394⁃20399.
[49] Tian S, Cai T H, Wang D D, et al. A superior cathode of sodium⁃ion battery: A V2O5nanosheets anchored on carbon nanofibers[J]. IOP Conference Series: Earth and Environmental Science, 2021, 692: 022042.
[50] Etman A S, Sun J L, Younesi R. V2O5·H2O nanosheets and multi⁃walled carbon nanotube composite as a negative electrode for sodium⁃ion batteries[J]. Journal of Energy Chemistry, 2019, 30: 145⁃151.
[51] Shang C Q, Hua L, Lin Q, et al. Integration of NaV6O15·H2O nanowires and rGO as cathode materials for efficient sodium storages[J]. Applied Surface Science, 2019, 494: 458⁃464.
[52] Feng J J, Xiong Z M, Zhao L, et al. One⁃pot hydrothermal synthesis of NaV2O5·H2O/KB nanocomposite as a sodium⁃ion battery cathode for improved reversible capacity and rate performance[J]. Journal of Power Sources, 2018, 396: 230⁃237.
[53] Liu F S, Sun X X, Liu Y T, et al. TiO2nanorods confined in porous V2O5nanobelts and interconnected carbon channels for sodium ion batteries[J]. Applied Surface Science, 2019, 473: 873⁃884.
Research Progress of V2O5Cathode Material for Sodium⁃Ion Batteries
Zhou Ya1, Shi Runna1, Lang Xiaoshi1, Yao Chuangang2, Cai Kedi1,2
(1.College of Chemistry and Materials Engineering,Bohai University,Jinzhou Liaoning 121013,China;2.Liaoning Engineering Technology Research Center of Supercapacitor, Jinzhou Liaoning 121013,China)
Sodium⁃ion batteries (SIBs) have the advantages of rich resources and low cost and supply risk.Therefore,they are regarded as a new generation of electrochemical energy storage devices with great potential.At present,vanadium pentoxide (V2O5) has gradually become a research hotspot of cathode materials for SIBs because of its high working voltage and theoretical capacity. However,the disadvantages of V2O5cathode material,including low ion diffusion coefficient,low conductivity,and structural instability caused by repeated ion intercalation/deintercalation,have limited its application in SIBs.This paper analyzed the crystal structure and sodium storage mechanism of V2O5and summarized the research progress of V2O5cathode material in SIBs through modification methods such as morphology control,crystal structure modification,chemical pre⁃insertion,and composition with other materials.Finally,the paper prospected the development trend of V2O5electrode material.
V2O5; Morphology control; Crystal structure; Chemical pre⁃insertion; Conductive material
TQ150;TM911
A
10.3969/j.issn.1006⁃396X.2022.06.005
1006⁃396X(2022)06⁃0038⁃10
http://journal.lnpu.edu.cn
2022⁃05⁃16
2022⁃07⁃06
国家自然科学基金项目(22075030)。
周娅(1997⁃),女,硕士研究生,从事锂离子电池、钠离子电池研究;E⁃mail:zhouya1011@163.com。
蔡克迪(1982⁃),男,博士,教授,博士生导师,从事能源化学、能源材料研究;E⁃mail:caikedihit@bhu.edu.cn。
(编辑 喻育红)
