Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis
2022-12-30ZhoDingYutingLiHngYngYngfnLuJunTnJinoLiQinLiYuChenLeonShwFushengPn
Zho Ding,Yuting Li,Hng Yng,Yngfn Lu,Jun Tn,Jino Li,Qin Li,Yu’n Chen,Leon L.Shw,Fusheng Pn
a College of Materials Science and Engineering,National Engineering Research Center for Magnesium Alloys,Chongqing University,Chongqing 400044,China
b The State Key Laboratory of Refractories and Metallurgy,Institute of Advanced Materials and Nanotechnology,Wuhan University of Science and Technology,Wuhan 430081,China
c Department of Mechanical,Materials and Aerospace Engineering,Illinois Institute of Technology,Chicago 60616,United States
Abstract Hydrogen energy has been recognized as “Ultimate Power Source” in the 21st century,which could be the best solution to the looming energy crisis and climate degeneration in the near future.Due to its high safety,low price,abundant resources and decent hydrogen storage density,magnesium based solid-state hydrogen storage materials are becoming the leading candidate for onboard hydrogen storage.However,the high operation temperature and slow reaction rate of MgH2,as a result of the large formation enthalpy and high reaction activation energy,respectively,are the firs and most difficul problems we need to face and overcome to realize its industrialization.Herein,a state-of-the-art review on tailoring the stable thermodynamics and sluggish kinetics of hydrogen storage in MgH2,particularly through nanoengnieering and catalysis is presented,aiming to provide references and solutions for its promotion and application.Promising methods to overcome the challenges faced by MgH2/Mg,such as bidirectional catalysts and nanoconfinemen with in-situ catalysis are compared and the required improvements are discussed to stimulate further discussions and ideas in the rational design of MgH2/Mg systems with ability for hydrogen release/uptake at lower temperatures and cycle stability in the near future.
Keywords: Hydrogen storage materials;MgH2;Nanoengineering;Catalysis;Hydrogen release;Hydrogen uptake.
1.Introduction
The world energy structure has experienced several adjustments,and every adjustment will bring a huge change to the human society [1].As exhibited in Fig.1,the wide-spread application of coal and oil have directly promoted the innovation of steam engines and internal combustion engines in the 18th and 19th century,respectively,which have been marked as the signals of the beginning of 1st Industrial Revolution and 2nd Industrial Revolution [2,3].It is worth noting that,modernization of the economy has been built upon the extensive use of traditional fossil fuels [4,5].According to current trends,the total world energy demand would double by 2050,if the current share of fossil fuels remains unchanged by then,it is very likely that the emissions will exceed the limit required to keep the average global warming below 2 °C/year[6,7].Moreover,the conventional resources to be exploited on the earth were not inexhaustible.Statistics show that coal,oil and gas would be nearly exhausted in the end of 21st century [8].Considering the current energy crunch and climate change concerns,hydrogen energy shown in Fig.1 is a boon because of its natural advantages,including high energy density,large calorifi value,abundant resource,zero pollution,zero carbon emission,storable and renewable [9,10].

Fig.1.The Evolution of the world energy structure with its carbon footprint.
Hydrogen is flammable explosive and highly diffusible as a gaseous substance under normal conditions,so guaranteeing safety,ensuring efficien y and preventing leakage during hydrogen storage and transportation have been given the top priorities in the practical applications [11–14].Currently,there are three major hydrogen storage technologies,compressed gaseous storage,cryogenic liquid storage and solid-state materials storage.Among these,the characteristics of good safety,convenient transportation,long service life,high volume storage density,low storage pressure and high purity of hydrogen release make the solid-state hydrogen storage technology perfect for hydrogen fuel cell vehicles [15].
Due to the development and expansion in the past 40 years,solid-state hydrogen storage has been divided into many subfields including metal/alloy hydrides,complex hydrides,amino/imino compounds,ammonia borane compounds and so on [16,17].MgH2with the superior mass hydrogen storage density (7.69 wt.%) and volume hydrogen storage density (106 kg·m−3) has been identifie as one of the most promising solid-state materials to meet the technical criteria of the on-board hydrogen storage system define by the U.S.Department of Energy [18–21],which requires hydrogen storage materials with gravimetric capacity of 6.5 wt.%H2and volumetric capacity of 70 kg·m−3at temperatures ranges from −40 to 85 °C.However,the practical applications are severely hindered by the unfavorable thermodynamics and sluggish kinetics of its decomposition and rehydrogenation: MgH2⇌Mg + H2,caused by its large formation enthalpies (ΔH = 76 kJ ·mol−1H2) and high reaction activation energies (ΔE = 160 kJ·mol−1) [22].In comparison to alloying and reactant destabilization,nanoengineering and catalysis have gradually become mainstream technological approaches to tailor the hydrogen storage performance of MgH2,through which it is much easier to achieve low-cost,high-efficien and large-scale materials preparation [23–25].
2.Nanoengineering
Nanoengineering has been recognized as the most simple and effective technology to modify the hydrogen storage performance of MgH2.Compared with bulk MgH2,nanostructured MgH2could not only shorten hydrogen diffusion path,but also provide more grain boundaries and larger specifi surface areas,offering more hydrogen diffusion channels and active reaction sites and thus improve the hydrogen desorption and adsorption kinetics of MgH2.Furthermore,the extra interfacial energy stored by nanostructured MgH2itself could reduce the thermodynamic stability of MgH2[26,27].So far,the nanostructured MgH2is mainly prepared by mechanical milling and nanoscaffold confinement
2.1.Mechanical milling
Mechanical milling,including vibratory mills,attritor mills,shaker mills and planetary mills are usually employed to prepare large scale quantities of nanostructured materials[28,29].Vibratory ball mills,such as SPEX 8000,are the most commonly used for experimental studies.As illustrated in Fig.2a,during milling,a cylindrical vial of about 0.1 L capacity undergoes a vibratory motion along the horizontal direction.During attritor milling shown in Fig.2b,both the materials and balls are agitated by the high-speed rotation of the shaft with impellers,triggering various forces such as impact,rotational,tumbling and shear on the on the materials,and thereby resulting in optimum size reduction and dispersion [30].Planetary mills with the vial capacity of 0.25–1 L,could process significantl larger volume of powder materials at one time.Fig.2c shows an example that how 2 or 4 such containers are rotated around two parallel axes (like the Earth’s rotation around the Sun) [31].A powder agglomerate’s deformation process can be inferred from Fig.2d–f[32] during the impact with milling balls.Powder acts like a porous material when it is struck by a ball firs (Fig.2d).During the impact,powder particles would slide and rotate,rearranging their positions due to the particle interactions.Once compressed to a critical height of h’,the powder’s porosity decreases,while some particles may escape from the contact area (Fig.2e).Noted this stage involves dissipating the fraction of the ball’s kinetic energy.The individual powder particles trapped in the contact area begin to deform elastically and plastically as the impact progresses (Fig.2f).In brief,bulk powders would be repeatedly extruded,deformed,fractured and welded,and accordingly lead to smaller grain size,larger surface area,more sorption sites and higher dispersing ability[33],which would greatly enhance the powder activity and may even trigger the multi-phases chemical reactions.Several parameters can be varied for the mechanical milling synthesis in accordance with various requirements,such as milling speed,milling time,milling temperature,milling atmosphere,ball-to-powder mass ratio,canister composition and diameter,and ball density and diameter [34,35].As exemplifie by TEM in Fig.2h&i,smaller MgH2grain could be obtained with longer ball milling time (to a certain range) [36].
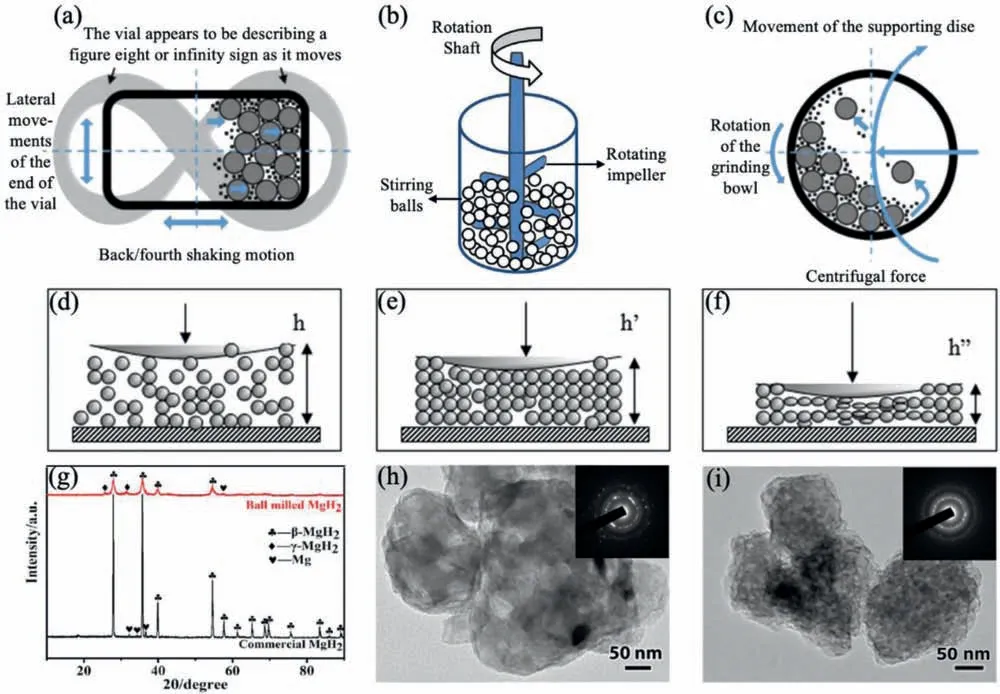
Fig.2.Working principles of (a) vibratory mills [31],(b) attritor mills [30] and (c) planetary mills [31];(d) (e) (f) Illustration of the deformation of powder agglomerate during the impact process [32];(g) XRD patterns of MgH2 before and after ball milling for 10 min [39];TEM micrographs and accompanying electron diffraction patterns of (h) 1 h and (i) 10 h milled MgH2 [36].
After 640 min/920 min’s high-energy ball milling,the obtained nanostructured MgH2could release 1.20 wt.%/1.32 wt.% H2in 12 h at 265 °C,while bulk MgH2could only release 0.21 wt.% H2at the same temperature [37,38].This has been attributed to the isomerization of MgH2induced by high-energy ball milling.Parts of the stable tetragonalα-MgH2has already transformed to the metastable rhombicγ-MgH2(Fig.2g),which could greatly reduce the hydrogen release temperature and rate [39–41].But unfortunately,the formation ofγ-MgH2is irreversible,it has completely disappeared after the firs dehydrogenation and cannot be formed if the hydrogen pressure is lower than 10,000 bar,which is almost impossible [42,43].
The traditional method of preparing MgH2involves reacting Mg powder with H2at the temperature of about 427 °C and under 75 bar hydrogen pressure for several hours [44].The surface of micrometer-sized Mg grains may form a shell of MgH2during its hydrogenation,which would prevent further reaction of the remaining Mg core [45,46].Therefore,bulk Mg powder has a rather slow hydrogenation rate and the presence of Mg is always discovered using XRD since the reaction is hardly completed.Chen and Williams[47]conducted the firs attempts to produce MgH2by mechanical milling Mg under 3.4 bar hydrogen pressure,and it took 25 h for the complete hydrogenation.Similar experiments were conducted under 5–10 bar hydrogen pressure by different groups[48–50],but none could achieve MgH2yield above 50 wt.%,due to the sluggish kinetics.Finally,complete hydrogenation within 1 h becomes possible with the addition of graphite and heating the milling canister to 300 °C simultaneously[51].Noted that graphite is able to help reduce agglomeration of MgH2/Mg particles during the repeated cold-welding caused by mechanical milling [52].This function of graphite can result in the formation of nano-MgH2/Mg particles with high specifi surface area for rapid hydrogeneration and dehydrogenation.In addition,the hydrogenation time of ball milled Mg was proved to gradually decrease with increasing hydrogen pressure [53].For example,the hydrogenation process was longer than 24 h under 10 bar hydrogen pressure,but when the pressure increased to 40 bar,the process only needs less than 8 h.The same trend has been discovered for the nucleation time of MgH2: it would cost more than 2 h under 10 bar hydrogen pressure,while several seconds was enough under 90 bar hydrogen pressure.
2.2.Nanoscaffold confinemen
For the nanostructured materials prepared by ball milling,particles coarsening often occur,which would result in the gradual decay in the reversible hydrogen storage capacity[37].This is attributed to the agglomeration and sintering of the ball milled particles during the hydrogen release and uptake cycles at relatively higher temperatures.Hence,if we could confin MgH2/Mg particles in the nanoporous materials,both the dehydrogenation and rehydrogenation would happen in the given frames.This would effectively inhibit the crystal growth and particle agglomeration,allowing MgH2/Mg to maintain its nanostructure with high activity during cycles of hydrogen desorption and absorption.
For the firs time,de Jongh et al.[54] successfully prepared ultra-fin Mg particles with diameters of 2–5 nm and smaller than 2 nm in 2007 by impregnating nanoporous carbon materials with molten Mg melt.Since then,the "critical loading" has been noticed for scaffold materials,which is determined by the pore volumes and pore size distributions.Excessive materials would remain as bulk outside the pores.The system of scaffold materials for MgH2/Mg has been flouishing in the last decade,which can be classifie into several groups,such as ordered mesoporous silica,porous MOFs,and various porous carbon materials including graphene,ordered mesoporous carbon,carbon fibe,carbon nanotubes and carbon aerogel.
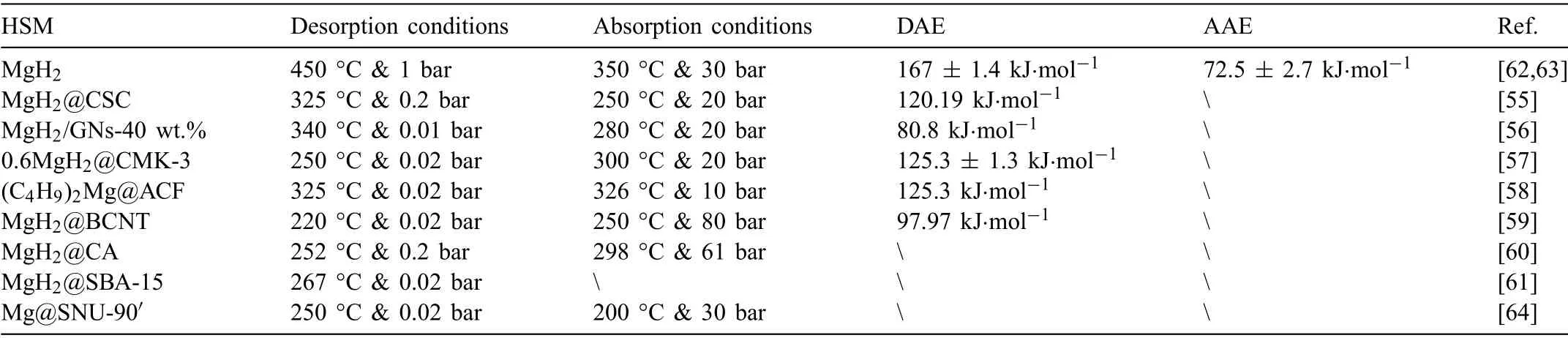
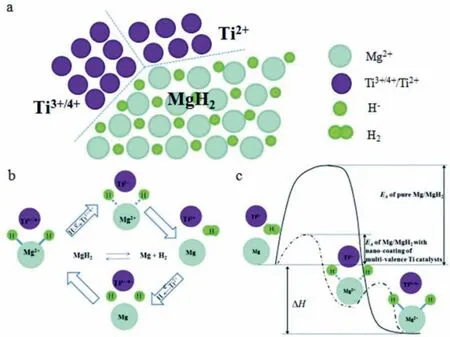
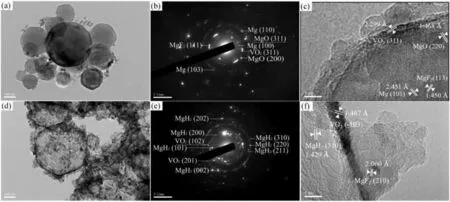
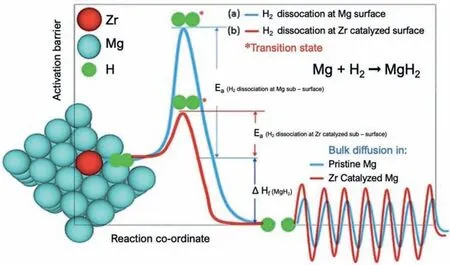
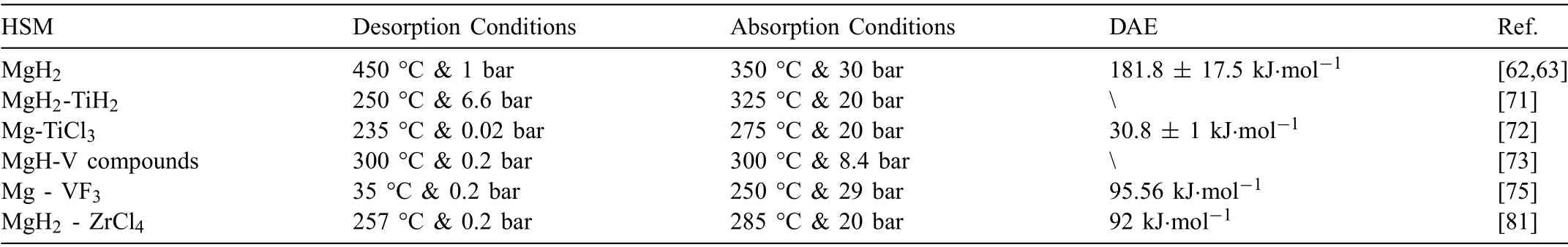
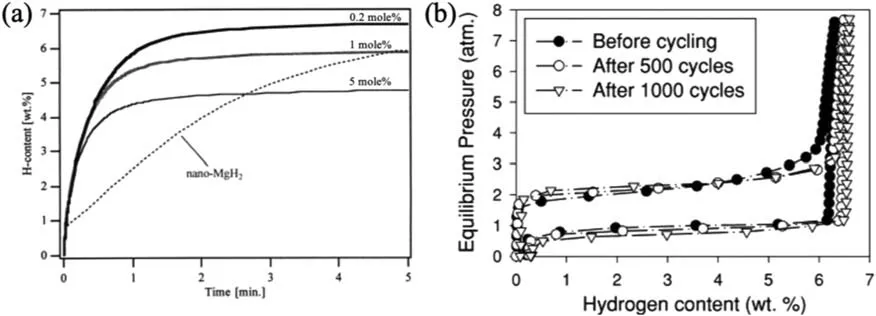
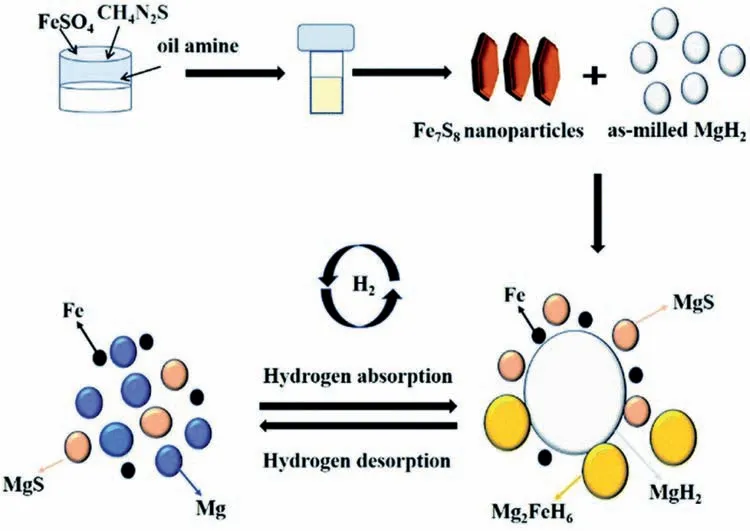
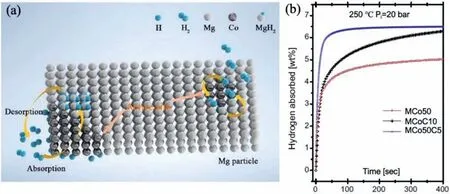
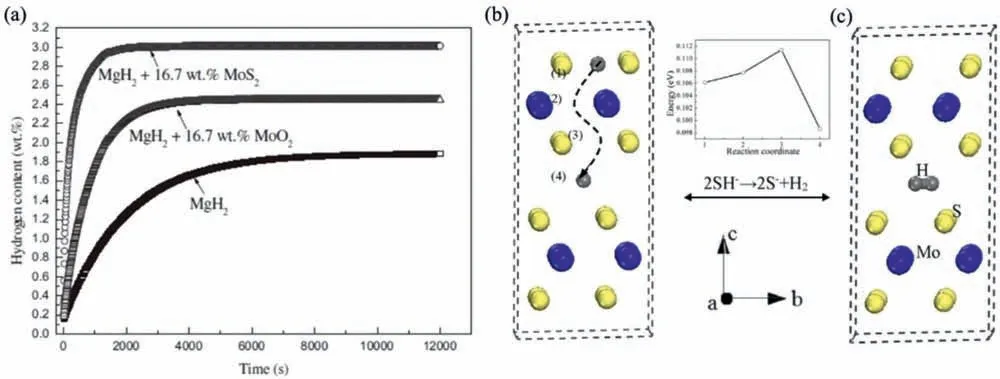
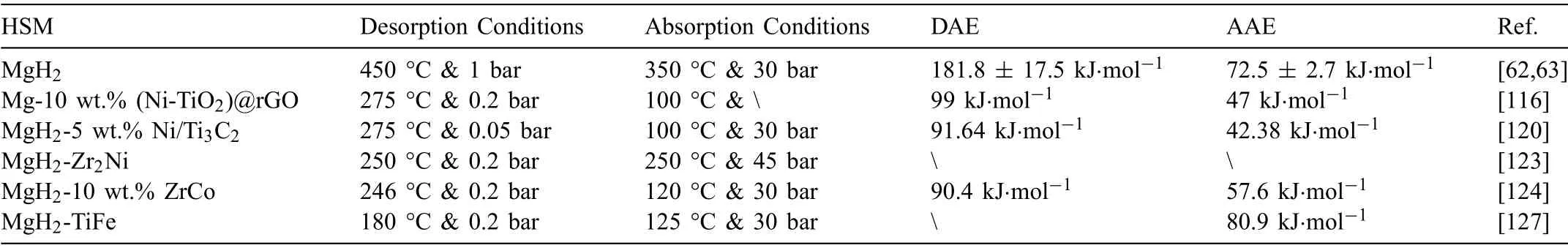
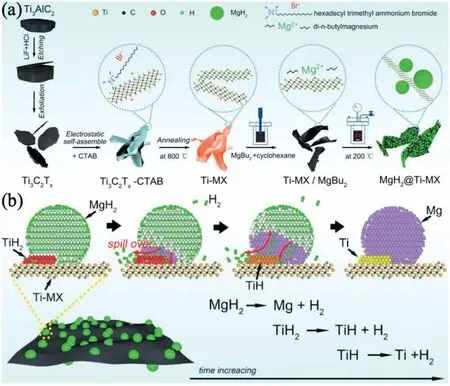
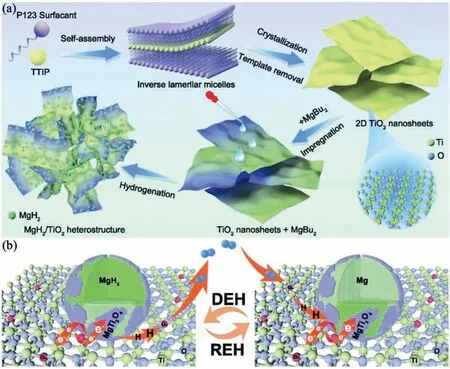
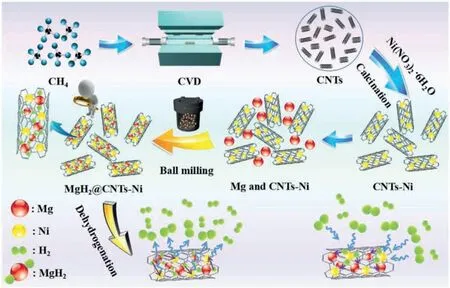
Due to the unique features of lightweight,high specifi surface area,porous volume,and thermal stability,carbon materials become the most popular scaffold materials.Through nano-confinin MgH2in the coconut shell charcoal(CSC) [55],multi-walled carbon nanotube (CNT),graphite(G) and activated carbon (AC),the onset dehydrogenation temperatures of all the composites have been decreased:MgH2@CSC(245°C) Fig.3.(a) Schematic illustration of the synthesis process of MgH2 NPs on GNs [56];(b-d) Representative SEM,TEM and HRTEM images BCNTs [59];(e)Schematic illustration of the loading process of MgH2 on SBA-15 [61]. Table 1 Desorption conditions,absorption conditions,desorption activation energies (DAE) and absorption activation energies (AAE) of the nanoscaffold confine Mg-based hydrogen storage materials (HSM). Doping of transition metals and their compounds is an important method to improve the reaction kinetics,which could accelerate the dissociation and reconstruction of H2on the MgH2/Mg surfaces,thereby lowering the reaction activation energy and increasing the hydrogen release and uptake rate[65–68].However,MgH2/Mg have strong selectivities on the catalysts for dehydrogenation and rehydrogenation because of the chemical bonding between the constituent atoms.According to the formation entrophy of binary metal hydrides,the elements in the periodic table have been categorized into two types,as shown in Fig.4 [69]. Fig.4.Periodic table with relative subdivision of the A- and B-type elements,taking into account the grouping according to the enthalpies of formation of the binary metal hydrides [69].A-type elements are represented in the shades of red,whereas B-type elements in shades of blue. The dopants of transition metals and their compounds having strong affinit with hydrogen,such as Ti,V,Zr and Nb,are able to reduce the dehydrogenation activation energy of MgH2,while their catalytic effects on the rehydrogenation of Mg is not so ideal.We term this group of transition metals and their compounds as "hydrogenphilic" catalysts in this article. Via reactive ball milling with Ti,the dehydrogenation activation energy of MgH2-25% Ti composites has been decreased from 135 kJ·mol−1to 53.6 ± 9.3 kJ·mol−1H2[70].For the Mg/MgH2system,TiH2does not benefi the thermodynamic properties,but can modify the reaction kinetics[71].The existence of TiH2phase with stable crystallite size would help prevent the grain coarsening of MgH2and ensure the nucleation of MgH2phase over the whole Mg crystallites before the formation of the blocking MgH2layer.Moreover,TiH2can also serve as a gateway for hydrogen dissociation and diffusion.Therefore,the hydrogen release of 0.7MgH2-0.3TiH2nanocomposite is completed within 100 s at 300 °C,which is nearly 20 times faster than that of ball milled MgH2.Through the chemical reaction between Mg with TiCl3in the tetrahydrofuran solution [72]: Mg + TiCl3→MgCl2+ Ti,the thin fil of Ti-based compounds (TiH2,TiCl3and TiO2)with 10 nm thickness has been synthesized and uniformly coated on Mg surface.As illustrated in Fig.5,the multivalence Ti (Ti0,Ti+2,Ti+3and Ti+4) could act as the electron intermediates and active catalysis sites for the electrons transfer between Mg2+and H−,which would favor the hydrogen recombination and thus improve the hydrogen desorption performance.The dehydrogenation entropy (ΔS) has increased to 136.1 J·K−1·mol−1for Mg-Ti composite from 130.5 J·K−1·mol−1for MgH2,which results in a much lower desorption peak temperature of 279 °C at 1 bar equilibrium pressure. Fig.5.Schematic diagram of the catalytic mechanism of Ti-based compounds during the hydrogenation and dehydrogenation of Mg/MgH2 system[72]. Core-shell nanostructured Mg-10 wt.% MFx(M=V,Ni,La,Ce) composites are prepared by arc plasma.Mg-NiF2composite presents the best hydrogenation kinetics,as a result of the formation of the Mg2Ni and MgF2shell on Mg core during the arc plasma evaporation through 3Mg + NiF2→ MgF2+ Mg2Ni,while Mg-VF3composite shows the slowest hydrogenation kinetics(2Mg + VF3→MgF2+ MgF + V) [73].However,VF3exhibit the best catalytic effect on the dehydrogenation among the hydrogenated composites.The desorption peak temperature of MgH2-VF3has been reduced by 42 °C when compared to nanostructured MgH2,and the hydrogen release rate has been promoted to 1000 ml·g−1H2in 15 min and 1478.26 ml·g−1H2in 60 min.This has been attributed to the VO2shell(oxidation of V occurs during cycles)on MgH2surface,which is able to impede the Mg agglomeration,as shown in Fig.6.Mechanical milling would induce the reaction between MgH2and V,resulting in the formation of nanostructuredβ-MgH2+γ-MgH2+VH0.81composite,which is capable of releasing hydrogen at low temperatures with fast kinetics[74].The dehydrogenation activation energy of milled MgH2-5 at.% V composite has been reduced to 62 kJ·mol−1H2and the hydrogen desorption could be completed within 2000s at 235 °C.Further investigations demonstrate that V doping actually does not affect the dehydrogenation thermodynamics because the formation enthalpy and entropy of the composite are the same as those of pure Mg;instead,the hydrogen release reaction is controlled by the metal/hydride interface motion.Nanoparticles of Mg,VH2,and MgH2are prepared from Mg-10.2 at.% V nanoparticles by hydrogen plasma-metal reaction [75]: Mg + V + H2→Mg + MgH2+ V.It is proposed that V dopant has no effect on the thermodynamics of hydrogenation either.The hydride composites could release 4.0 wt.% H2via MgH2+ VH2→Mg + V + H2within 15 min at 300 °C.The hydrogen desorption energy of the composite is 119.4 kJ·mol−1H2,which is nearly 1/3 of 323 kJ·mol−1H2for nanostructured MgH2particles.Experimental analysis reveals that the catalytic effects of V-based compounds on the hydrogen desorption of MgH2is ordered as V2O5>VN>VC>V,and the mixture of MgH2-V2O5-VN-VC shows even more favorable kinetics after exposure to the air for a moment [73]. Density functional theory (DFT,VASP) is employed to study the effect of Zr-doped and vacancy-like defects on the hydrogen desorption from MgH2(110) surface [76–78].The occupation energy analysis reveals that Zr atoms prefer the interstitial sites,so the dopant of a Zr atom would create a Mg vacancy.It is the Mg vacancy that alters the surface geometry and weakens the Mg-H bonds,reducing the dehydrogenation activation energy and consequently making the hydrogen release from Zr-doped composite much easier [79].During the initial dehydrogenation,the doped ZrF2would react with MgH2to produce MgF2and ZrH4via 2MgH2+ ZrF4→ZrH2+ 2MgF2+ H2,which could help stabilize Mg and MgH2phases at their interfaces due to the quite similar lattices [78].The continuous refinemen of MgH2particles and obvious improvement of hydrogen diffusion by MgF2and ZrH4contribute to the modifie dehydrogenation kinetics.Remarkably,a new and stable [Mg,Ti]2TiH6phase [80] could be generated with extending cycling and annealing times.The dehydrogenation activation energy has been reduced to 92 kJ·mol−1from 150 kJ·mol−1for MgH2-ZrCl4composites and pure MgH2,respectively [81].The catalytic mechanism of ZrCl4can be explained through 2 aspects,as shown in Fig.7.Firstly,the homogenous distribution of ZrCl3and Zr nanoparticles (obtained through the redox reaction of ZrCl4during milling) on the composite surfaces is able to provide extra nucleation sites for hydrogen release.In addition,ZrCl3and Zr nanoparticles are capable of refinin the crystallite size of MgH2,which could decrease the hydrogen diffusion distance. Fig.6.(a) TEM bright fiel image of Mg-VF3 powder,(b) the corresponding SAED pattern,and (c) its HRTEM image;(d) bright fiel micrograph of the hydrogenated Mg-VF3 powder,(e) the corresponding SAED pattern,and (f) its HRTEM image [73]. Fig.7.Schematic diagram of catalytic mechanism of Zr on the de-/hydrogenation of MgH2/Mg [81]. DFT calculations (VASP) discover that the substitution of Nb atom at Mg site is easier to occur than the formation of Mg vacancies,while both of them have been demonstrated effective in desorbing hydrogen at lower temperatures from MgH2[82–84].A likely mechanism is the clustering of H around Nb atoms after substitution,which could provide express channels for hydrogen release,as shown in Fig.8.The transient and metastable NbHx(x≈0.6) phase during dehydrogenation detected by synchrotron radiation,could act as a gateway for hydrogen release [85].The dehydrogenation activation energy of MgH2-5 at.% Nb is 62 kJ·mol−1,which is much lower than 120–142 kJ·mol−1for MgH2[86–88].The dopants of Nb2O5and Nb would react with MgH2via 5MgH2+ Nb2O5→5MgO + 2NbH2+ 3H2and MgH2+ Nb →2NbH + Mg during ball-milling,respectively.Both NbH2and NbH would decompose into Nb during dehydrogenation,and then be hydrogenated to NbH during rehydrogenation,via which Nb catalyzes the hydrogen storage of MgH2.Because of the hard and brittle characteristic of Nb2O5,the Nb2O5doped and milled samples have smaller sizes of MgH2(10–20 nm).As such,MgH2-Nb2O5composite have lower hydrogen desorption peak temperature of 207 °C than 254 °C for MgH2-Nb composite.The cubic perovskite phase composed of Nb(Mg0.333Nb0.667)O3has been discovered for the firs time during the initial dehydrogenation of MgH2-Nb samples [89].This perovskite compound is catalytically active and proton conductive,which could also promote the hydrogen diffusion and migration,thereby catalyzing the hydrogen release from MgH2.The hydrogen storage properties of the “hydrogenphilic” catalysts doped Mg-based materials have been listed in Table 2 for direct comparisons. Fig.8.(a) The geometries of MgH6 in Mg16H32 supercell;(b) MgH5 in Mg16H31 supercell following the removal of the H atom marked A;(c) same as in(b) except the H atom marked B is removed;(d),(e),and (f) the same legend as in (a),(b),and (c) except Mg is replaced by the Nb atom [84]. Table 2 Desorption conditions,absorption conditions and desorption activation energies (DAE) of the “hydrogenphilic” catalysts doped Mg-based hydrogen storage materials (HSM). The dopants of transition metals and their compounds having weak affinit with hydrogen such as Cr,Fe,Co,Ni and Mo have been able to reduce the hydrogenation activation energy of Mg,while their catalytic effect on the dehydrogenation of MgH2is not as good as expected.We term this group of transition metals and their compounds as "hydrogenphobic" catalysts in this article. Under 8.4 bar hydrogen pressure,nanostructured Mg-0.2 mol.% Cr2O3,Mg-1 mol.% Cr2O3and Mg-5 mol.%Cr2O3composites have the capability of storing 6.7 wt.%,6.0 wt.% and 4.7 wt.% H2within 2 min at 300 °C,respectively,while only 3.9 wt.% H2can be stored by pure Mg at the same conditions,as shown in Fig.9a [90].It is interesting to note that,the absorption kinetics does not alter with the different doping amount of Cr2O3(in a certain range).The reaction kinetic simulation analysis concludes that the optimum doping amount of Cr2O3is 0.2 mol.%,and the rate-controlling step for hydrogen absorption is the hydrogen diffusion and migration in the solid MgH2and MgH2-Cr2O3[91].During ball milling,Cr2O3would be heterogeneously accommodated in the nanocrystalline MgH2matrix,leading to the formation of brittle chains.The dehydrogenated MgH2-10 wt.% Cr2O3composite exhibits excellent hydrogenation kinetics and is able to absorb 6.0 wt.% H2within 1 min under 10 bar hydrogen pressure at 300 °C [92].But the microstructure investigation reveals that Cr2O3doping does not alter the crystalline size of MgH2.Moreover,the specifi surface area of the composite has been decreased to 9.0 m2·g−1from 43.9 m2·g−1for the milled pure MgH2.These finding indicate that the distribution of Cr2O3nanoparticles does not play the vital role in the hydrogen sorption properties of Mg.Fig.9b presents that the hydrogen storage capacity of the MgH2-0.2 mol.%Cr2O3composite has increased by 8% after 1000 cycles[93].Structure relaxations and crystallite growth occurring during the cycling have been identifie to be responsible for the increased cyclic hydrogen storage capacity from 5.9 wt.% to 6.4 wt.% H2. Fig.9.(a) Hydrogen absorption curves of MgH2/Cr2O3-composites with different oxide contents at 300 °C under a hydrogen pressure of 8.4 bar [90];(b)Pressure-composition isotherms at 300 °C for MgH2 with 0.2 mol.% Cr2O3 catalyst addition in the as-milled state and after 500 and 1000 cycles [93]. FeF3would react with MgH2during mechanical milling to generate Fe nanoparticles and protective MgF2via the reaction of 3MgH2+ 2FeF3→3MgF2+ 2Fe + 3H2[94].Dehydrogenated MgH2-2 mol% FeF3composite could absorb 6.0 wt.% H2within 600 s under a hydrogen pressure of 32 bar at 200 °C,which is the fastest hydrogen absorption rate among the Mg-based hydrogen storage materials reported at this temperature.The mechanism has been explained from thermodynamic and kinetic perspectives.Thermodynamic analysis has predicted the process of hydrogen absorption at the Mg/Fe interface is thermodynamically favorable due to the reduction of the total interfacial energy.Kinetic simulation has revealed that Mg/Fe interfaces could act as fast diffusion paths and diffusion short circuits,which could notably accelerate the kinetics of rehydrogenation.Mg15Fe alloy prepared by 150 h’s low-energy ball milling of the mixture of Mg and Fe powders under 5 bar hydrogen pressure exhibits excellent hydrogen absorption kinetics and could still uptake 4.85 wt.% within 10 min under 24 bar hydrogen pressure at 350 °C even after 100 cycles [95].Subsequent experiments and simulations indicate that the hydrogenation of Mg-Fe compounds is controlled by the diffusion of hydrogen,regardless of the Mg–Fe proportions or temperatures.DFT calculations [96] (VASP) reveal that Fe doping would remarkably weaken or even break Mg-H bonds,which would be liable for the lower reaction temperature and faster reaction rate.The onset temperatures of hydrogen release and uptake for ball-milled MgH2-5 wt.% Fe nanosheets (NS) composite have been lowered to 182.1 °C and 75 °C,respectively[97].Particularly,the dehydrogenated composite is able to absorb 6.0 wt.% H2within 10 min under 32 bar hydrogen pressure at 200 °C,while at the same conditions,the dehydrogenated ball-milled MgH2could only absorb 2.3 wt.%H2.Ball milling with Fe7S8would introduce the reaction:8MgH2+ Fe7S8→MgS + 7Fe + 8H2,and the newly produce Fe would react with MgH2to form the intermediate phase of Mg2FeH6through 2MgH2+ Fe + H2→Mg2FeH6during hydrogenation,which is the key reason of the superior hydrogen sorption kinetics of dehydrogenated MgH2-Fe7S8composite [98].Furthermore,Mg2FeH6is reversible and would take part in the subsequent dehydrogenation and rehydrogenation via Mg2FeH6⇌2MgH2+ Fe + H2.The schematic diagram of the catalytic mechanism is illustrated in Fig.10.The dehydrogenated MgH2-16.7 wt.%Fe7S8composite is able to absorb 4.0 wt.% H2within 1800s under 30 bar hydrogen pressure at 200 °C,which is much better than the 1.85 wt.% H2absorbed by the dehydrogenated MgH2under the same conditions.The same mechanism can be applied to justify the outstanding hydrogen sorption kinetics by the dopant of Fe3S4because of the similar reaction during ball milling: 4MgH2+ Fe3S4→4MgS + 3Fe + 4H2[99]. According to the heat of formation,the compounds stability are ordered as MgH2>MgH2-Ti>MgH2-Co,therefore,the eases of hydrogen release and uptake (lower temperate,faster rate) are deducted and ordered as MgH2-Co>MgH2-Ti>MgH2[100].The BCC lattice parameters,which play the essential roles in determining the equilibrium hydrogen pressure,are discovered to increase with increasing Co amount ranging from 37 at.% to 50 at.%,and reaches its maximum value of 0.3199 nm at 50 at.% Co.Beyond that,it would decrease with the further increasing amount of Co (until 80 at.%).BCC Mg50Co50and Mg60Co40alloys prepared by 100 h and 200 h’s ball milling could absorb 2.1 wt.% and 2.7 wt.% H2,respectively and transfer to tetragonal MgCoH2hydride under 60 bar hydrogen at 100 °C [101].Nevertheless,none of these hydrogenated alloys could release hydrogen at a low temperature of 100 °C.DFT calculations (Materials Studio) [102,103] have predicted that the dopant of Co could weaken the Mg-H bond,leading to a lower activation energy and reaction temperature.Besides,the uniform distribution of Co particles on MgH2surface after ball milling could not only provide active sites for the hydrogen discharging/charging,but also act as express channels for the hydrogen diffusion.All of these contribute to the modifie hydrogen storage performance of MgH2.The activation energies of dehydrogenation and rehydrogenation for ball milled MgH2-7 wt.% (FCC) Co composite have been decreased to 76.6 ± 9.3 kJ·mol−1and 68.3 ± 6.0 kJ·mol−1,respectively.Moreover,the phase transformation between Mg2Co and Mg2CoH5during cycles could serve as a hydrogen pump to promote the hydrogen sorption kinetics,as manifested in Fig.11a.The dehydrogenated MgH2-7 wt.%(FCC)Co composite is able to uptake 6.0 wt.%H2in 40 min under 32.5 bar hydrogen pressure at a temperature as low as 70°C.In spite of this superior property,doping of multi-walled carbon nanotubes (MWCNTs) to the MgH2-5 wt.% Co composite would further accelerate the hydrogen sorption by promoting the formation of Mg2CoH5[104].With the further catalytic effect of MWCNTs,the dehydrogenated MgH2-5 wt.%Co-5 wt.%MWCNTs composite has the ability to absorb 6.5 wt.% H2within 100 s under 20 bar hydrogen pressure at 250 °C (Fig.11b) [105]. Fig.10.Schematic diagram of the catalytic mechanism of Fe7S8 during the hydrogenation/dehydrogenation processes on MgH2 [98]. Ball milling of Mg nanoparticles with the fl werlike NiS particles prepared by solvothermal reaction method would form a core-shell Mg-NiS composite[106].During the firs hydrogenation and dehydrogenation cycle,NiS shell would react with MgH2through 5MgH2+2NiS+3H2→MgH2+Mg2NiH4+2MgS+Ni,resulting in the evenly distribution of Ni,MgS and Mg2Ni products on the Mg surfaces,as illustrated in Fig.12a.The Mg-MgS-Mg2Ni-Ni composite is able to absorb 3.5 wt.% H2within 10 min under 40 bar hydrogen pressure at 150 °C(Fig.12c) and desorb 3.1 wt.% H2in 10 min at 300 °C.The apparent activation energies of hydrogenation and dehydrogenation have been decreased to 45.45 kJ·mol−1and 64.71 kJ·mol−1,respectively.As illustrated in Fig.12b,the outstanding hydrogen sorption kinetics have been attributed to the following three factors,(i) the multi-phases catalytic effect of thein situformed Ni,MgS and Mg2Ni,(ii)the porthole effect from the volume expansion and microstrain during the phase transformation between Mg2Ni and Mg2NiH4(MgH2+ Mg2NiH4→Mg + Mg2Ni + 3H2),and (iii) the nanosize effect of Mg/MgH2particles in reducing the distance of hydrogen diffusion.The dope of 10 wt.%nano-dispersed Ni particles over mesoporous carbon material (Ni@CMK-3) prepared by impregnation-reduction,has decreased the activation energies for hydrogen release and uptake to 43.4 kJ·mol−1and 37.4 kJ·mol−1,respectively [107].The onset desorption temperature has been reduced to 180 °C from 350 °C for pure MgH2.It is encouraging that the dehydrogenated MgH2-10 wt.% Ni@CMK-3 composite has the ability of absorbing 3.9 wt.% H2under 30 bar hydrogen pressure even at the temperature as low as 55 °C.When the concentration of Ni is low,its main role is to facilitate the dissociative chemisorption of H2.However,when the concentration of Ni is high,it would be responsible for producing Mg-Ni alloys.The onset temperature for hydrogen adsorption has been reduced to 175 °C after doping of 5 at.%Ni from 275 °C for pure MgH2[108].Moreover,the hydrogen storage capacity has been increased by nearly 50%to 6.13 wt.% from 4.08 wt.% H2for pure MgH2.MgH2-16.7 wt.% MgNi2composite is prepared by the hydrogen combustion of the ball milled Mg powder and MgNi2alloy through 5Mg + MgNi2+ 6H2→2MgH2+ 2Mg2NiH4,and MgNi2would disappear and Mg2Ni would appear after dehydrogenation via MgH2+ Mg2NiH4→Mg + Mg2Ni + 3H2[109].Hence,MgNi2acts not only as a catalyst,but also participates in reactions to generate Mg2NiH4.Therefore,the dehydrogenated composite can absorb 4.7 wt.% H2within 500 s under 30 bar hydrogen pressure at 200 °C,while only 1.0 wt.% H2could be absorbed by pure Mg at the same conditions. Fig.11.(a) Schematic summary on Co catalyzed Mg particle in hydrogenation process [104];(b) Hydrogen absorption curves at 250 °C for MCo50C5,MCoC10 and MCo50 composites [105]. Fig.12.(a)Schematic diagram of the fabrication of the Mg-MgS-Mg2Ni-Ni nanocomposite and its firs activization process at 673 K;(b)Schematic diagram of the catalytic mechanism of Ni,Mg2Ni,and MgS catalysts during the hydrogenation/dehydrogenation processes of the nanocomposite;(c) Hydrogen absorption curves under 4 MPa hydrogen pressure [106]. Fig.13.(a) Hydriding curves of MgH2 - 16.7 wt.% MoX2 (X = S,O) composites and as-milled MgH2 [113];The hydrogen diffusion mechanism in MoS2:(b) reaction path of H atom and (c) hydrogenation model [110]. Ball milling would induce the reaction between MoX2(X = S and O) and MgH2[110]:2MgH2+ MoS2→ 2MgS + Mo + 2H2and 2MgH2+ MoO2→ 2MgO + Mo + 2H2.The improved hydrogen storage performance of MgH2-MoX2has been ascribed to (i) more active sites for hydrogen diffusion caused by thein situformed MgS and MgO phases,and (ii) Mo’s ability to promote the dissociation and reconstruction of hydrogen and its uniform dispersion after ball milling.As shown in Fig.13a,the hydrogen storage capacities (at 150 °C) have been increased to 2.45 wt.%for MgH2-16.7 wt.% MoS2composite and 3.01 wt.% for MgH2–16.7 wt.% MoO2composite from 1.88 wt.% for pure MoH2,and their corresponding hydrogen desorption temperature has been decreased to 377 °C and 367 °C from 389 °C for pure MoH2,respectively.Other than only a litter improvement on hydrogen desorption,the catalytic effect on hydrogen absorption is much more obvious.The uptake time for milled MgH2to reach 90% of its maximum storage capacity at 150 °C is 72 min,whereas this time has been shortened by 57% (31 min) and 82% (13 min) for MoO2and MoS2doped samples,respectively.MoS2is more preferred than MoO2because of the higher activity of S2−than O2−[110].Similar improvement has been reported [111,112],showing that MgH2-5 wt.% MoS2composite could release 1.3 wt.% H2in 10 min at 300 °C and the dehydrogenated composite could absorb 3.5 wt.% H2within 10 min under 30 bar hydrogen pressure at 200 °C.The first-principl calculations (CASTEP) has been employed to study the hydrogen diffusion mechanism and hydrogenation process of MgS2with S-S interlayer [113].Theoretical results shown in Fig.13b,c suggest that hydrogen inside the MgS2tend to diffuse into the S-S interlayer along the interstitial site(path: IT-IT).H2molecule is stable in the interlayer since the charge interaction between H atom and H atom is stronger than that between H atom and S atom.The hydrogen storage capacity of MgS2phase is calculated to be 0.83 at.%.Herein,we have summed up the hydrogen storage properties of Mg-based materials after doping the “hydrogenphobic”catalysts in Table 3 for straight comparisons. Table 3 Desorption conditions,absorption conditions and absorption activation energies (AAE) of the “hydrogenphobic” catalysts doped Mg-based hydrogen storage materials (HSM). The preparation of bimetal AB catalysts has brought about widespread attentions,where A represents the transition metal element having strong affinit with hydrogen and B represents transition metal element having weak affinit with hydrogen[114,115].Bimetal nanoparticles usually have higher activity and selectivity than single metal nanoparticles.The introduction of a second metal could change the structure of active components and stabilize the active center because of the interaction between the doped metal and carrier as well as the interaction between these two metals.As a result,the catalytic efficien y of bimetals is “bidirectional” and far superior to single metals.Note that bidirectional catalysts are not limited to bimetals only but can include their compounds as discussed below. The Ni and TiO2co-doped reduced graphene oxide [(Ni-TiO2)@rGO] nanocomposite exhibits splendid catalytic performance both on the hydrogen absorption and desorption from Mg/MgH2system [116].The apparent activation energies of hydrogenation and dehydrogenation have been decreased to 47.0 kJ·mol−1and 99.3 kJ·mol−1for Mg-10 wt.%(Ni-TiO2)@rGO composite and its hydrogenated composite from 115–122 kJ·mol−1and 126–160 kJ·mol−1for nanostructured Mg and MgH2[117,118],respectively.This is reflecte in the 75 °C and 84 °C reduction on the initial hydrogen uptake and release temperature when compared to the additive-free sample.The obvious advantages of bimetallic catalyst have been further confirme with the fact that the hydrogen storage capacity of Mg-10 wt.% (Ni-TiO2)@rGO composite is 3.55 wt.% at 100 °C,which is much higher than 2.73 wt.% and 0 for Mg-10 wt.% Ni@rGO [119] and Mg-10 wt.%TiO2@rGO composites,respectively,at the same hydrogenation conditions. The sandwich like Ni/Ti3C2bimetal catalyst fabricated via modifie wet chemical method also shows excellent catalytic effect [120].MgH2-5 wt.% Ni/Ti3C2composite is able to release 6.25 wt.% H2within 10 min at 275 °C and uptake 4.55 wt.% H2within 20 min under 30 bar hydrogen pressure at 100 °C.The detailed mechanism analysis has been described as follows.The doped Ni is beneficia for the dissociation of H2,which would promote the hydrogen absorption[66].The doped Ti3C2is favorable for the break of Mg-H bonds,which would aid the hydrogen desorption.Moreover,the electrons transfer among Ti with multiple-valence (Ti0,Ti2+,Ti3+,Ti4+) would make the conversion between Mg2+and Mg and between H−and H much easier[72].In addition,the abundant heterogeneous interfaces generated by the coexistence of Ni nanoparticles and Ti3C2matrix [121] and the electrons transfer between them at the interface [122] would work together to further accelerate the hydrogen desorption and absorption kinetics.However,it should be pointed out that 5%decay of the total hydrogen storage capacity has been noted with only 10 cycles. The doping of hard and big cube Zr2Ni alloy powder[123] prepared via 150 h’s ball milling results in a remarkable reduction in the grain and particle size of MgH2and Mg,which has shortened the hydrogen diffusion path and thus modifie the hydrogen storage kinetics.As demonstrated in Fig.14a,b,it would take only 116 s and 613 s to uptake(under 8 bar hydrogen pressure) and release 5.1 wt.% H2for MgH2-10 wt.% Zr2Ni composite at 250 °C,while 500 s and 2712s are required for the Zr2Ni-free sample,respectively.However,due to the long time exposure to the high temperature,the Zr2Ni grain starts to grow gradually after holding at 250 °C for 300 h;therefore,the distinct degradation for the hydrogen storage capacity of the composite has been noticed,as displayed in Fig.15a. ZrCo nanosheets could uniformly disperse on the surface of MgH2particles after ball milling [124],and transform into ZrCoH3when exposed to hydrogen atmosphere.Note that the interfaces between ZrCoH3and Mg could act as express channels for hydrogen diffusion [125] and the volumetric expansion caused by the formation of MgH2would aid the exposure of inner Mg core [126],both of which have contributed to the enhanced hydrogen absorption kinetics.Similar phenomenon would occur at the interface between ZrCo and MgH2,which could expedite the hydrogen desorption kinetics as well.The formation of ZrCoH3is reversible: 2ZrCoH3⇌2ZrCo + 3H2,which could act as a hydrogen pump during hydrogenation.In addition,the density of states in Fig.14d indicates that thess-orbital overlap of Mg-H bond is considerably weakened with the main distribution of s electrons of Mg and H at −5∼0 eV and −5∼−7 eV,respectively.The activation energies of dehydrogenation and rehydrogenation for MgH2-10 wt.% ZrCo composite have been decreased to 90.4 ± 1.6 kJ·mol−1and 75.2 ± 2.7 kJ·mol−1from 181.8 ± 17.5 kJ·mol−1and 75.2 ± 2.7 kJ·mol−1for as-prepared MgH2and Mg,respectively.6.3 wt.% H2is released from MgH2-10 wt.% ZrCo composite within 5 min at 300°C,while 4.4 wt.%H2is absorbed by the dehydrogenated composite within 10 min under 30 bar hydrogen pressure at a low temperature of 120 °C.However,it is pity that serious decay of hydrogen storage ability has been noted with the increasing service time,as shown in Fig.15b,only 89% of the initial hydrogen storage capacity could be maintained in the 10th cycle. TiFe prepared via sintering [127],mechanical ball milling and wet chemical ball milling could act as hydrogen pumps and effectively facilitate the hydrogen diffusion along the interface between Mg and MgH2,which has been termed as a superior catalyst for the hydrogen storage of MgH2/Mg system.With doping of 10 wt.% TiFe,the activation energies of dehydrogenation and rehydrogenation have been decreased to 80.9 ± 2.5 kJ·mol−1and 60.7 ± 8.0 kJ·mol−1from 154.6 ± 16.3 kJ·mol−1and 72.5 ± 2.7 kJ·mol−1for asprepared MgH2and Mg,respectively.MgH2–10 wt.% TiFe composite has the capability of desorbing 6.5 wt.% H2within 10 min at 300 °C and the dehydrogenated composite is able to absorb 5.3 wt.% H2within 60 min under 30 bar hydrogen pressure at a low temperature of 125 °C.However,the performance of this composite is not stable.As exhibited in Fig.15c,in the firs cycle (300 °C),the composite could desorb 6.6 wt.% H2within 8 min,and then finis the absorption in a few seconds under 30 bar hydrogen pressure,but only 5.8 wt.% H2storage capacity is maintained in the 10th cycle.Table 4 has listed the hydrogen storage properties of the above-mentioned bidirectional catalysts doped Mg-based materials for simple comparisons. Fig.14.Effect of nanocrystalline big-cube Zr2Ni additives on the (a) hydrogenation and (b) dehydrogenation kinetics of MgH2 powders [123];The MgH2 absorption on ZrCo (110): (c) the adsorption configuratio and (d) the corresponding Density of States [124]. Table 4 Desorption conditions,absorption conditions,desorption activation energies (DAE) and absorption activation energies (AAE) of bidirectional catalysts doped Mg-based hydrogen storage materials (HSM). By integrating nanoengnieering and catalysis,the novel process called “nanoconfinemen within-situcatalysis” has been proposed lately. MgH2crystals with an average crystallite size of 3 nm were successfully fabricated and confine in the mesoporous structure of Ni-MOF scaffold via solution infiltratio of(C4H9)2Mg/heptane and subsequent hydrogenation [128].The schematic fl w diagram for the synthesis of the MgH2@Ni-MOF composite is illustrated in Fig.16.The “nano-size effect” is responsible for the thermodynamic destabilization of MgH2,which has made reaction enthalpies of desorption and absorption decrease to 69.7 ± 2.7 and −65.7 ± 2.1 kJ mol−1H2from 71.67 ± 1.08 and −75.7 ± 1.1 kJ mol−1H2for nanostructured MgH2[129] and Mg [130],respectively.It should be noted that the exposure to the hydrogen atmosphere would trigger the reaction between (C4H9)2Mg and Ni-MOF:2(C4H9)2Mg + Ni(Ni-MOF) +4H2→Mg2NiH4+ 4C4H10,and thein-situformed Mg2NiH4could act as “hydrogen pump” in this system,which could decompose into Mg2Ni during the dehydrogenation and then transfer to Mg2NiH4during hydrogenation: Mg2NiH4⇌Mg2Ni +2H2.The activation energies for dehydrogenation and rehydrogenation have been reduced to 144.7 and 41.5 kJ mol−1H2,respectively.The composite is able to release 1.5 wt.% H2in merely 150 s at 350 °C and absorb 1.15 wt.% H2within 3600 s under 32 bar hydrogen pressure at a temperature as low as 150 °C,whereas the nanostructured MgH2(derived from the hydrogenation of (C4H9)2Mg) and MgH2@SBA-15 sample could only release 0.1 wt.% and 0.4 wt.%H2in 1 h at 250 °C [61].Moreover,the hydrogen storage capacity is maintained at 1.45 wt.% at 350 °C and no apparent decay in the kinetics and storage capacity is observed in the firs 10 hydriding/dehydriding cycles.The enhanced hydrogen sorption kinetics and cycling stability of the MgH2@Ni-MOF composite have been attributed to the “nano-size” effect of MgH2and Mg crystals,“hydrogen pump” effects from transformation of MgNi2and Mg2NiH4,and “aggregation blocker” role of the Ni-MOF scaffold. Fig.15.(a) Hydrogenation and consequent dehydrogenation curves of 2546 complete cycles for MgH2/10 wt.% big-cube Zr2Ni nanocomposite powders obtained after 50 h of the ball milling time [123];(b) Isothermal dehydrogenation/hydrogenation curves of the MgH2–10 wt.% ZrCo composites and as a function of cycling [124];(c) Hydrogen absorption and desorption cycle curves of MgH2–10 wt.% TiFe at 300 °C [127]. Fig.16.Schematic illustration of the MgH2 confinemen process in a Ni-based metal-organic-framework (Ni-MOF) for the synthesis of MgH2@Ni-MOF composite [128]. Fig.17.Schematic illustration of (a) the fabrication of MgH2@Ti-MX and (b) the mechanism for the fast dehydrogenation [131]. Through the wet impregnation and confinemen of mesoporous CoS-nanoboxes (CoS-NBs),MgH2crystals with crystallite sizes ranging from 5 to 10 nm have been prepared.The much lower dehydrogenation and rehydrogenation enthalpies of the MgH2@CoS-NBs composite (68.1 ± 1.4 and−65.6 ± 1.1 kJ mol−1H2,respectively) are the reflectio of thermodynamics destabilization caused by the “nano-size” effect.Thein-situformed MgS layer from (C4H9)2Mg + CoS+H2→Co + MgS + 2C4H10during the initial hydrogenation would evenly coat on the Mg/MgH2crystals,which could not only act as active sites to facilitate the hydrogen absorption and desorption,but also serve as “aggregation preventer”to impede the agglomeration of Mg/MgH2particles during cycles.With the help of MgS layer and CoS scaffold,the composite exhibits an excellent cycling stability.Moreover,the scaffold has also been termed as “getaway” for hydrogen diffusion because of the mesoporous structure.The activation energies for dehydrogenation and rehydrogenation have been reduced to 120.8 ± 3.2 and 57.4 ± 2.2 kJ mol−1H2,respectively.The composite could release 1.76 wt.% H2in 2 h at 275 °C,while the nanostructured MgH2could only release 0.45 wt.% H2at the same conditions.Further investigations have revealed that the“nano-size”effect of MgH2and Mg crystals,the catalytic effect ofin-situformed MgS,and multifunctional role of the CoS-NBs scaffold jointly boost the hydrogen sorption performance and cyclic stability of MgH2@CoS-NBs composite. Fig.18.Synthesis process illustration of (a) the MgH2/TiO2 heterostructure and (b) the mechanism for the fast dehydrogenation [135]. Inspired by the phenomenon that structurally engineered 2D-MXene fla es in a 3D architecture can reduce the risk of restacking effectively [132],the delaminated Ti3C2Txwith a folded nanosheet morphology has been employed to anchor the ultradispersed MgH2/Mg nanoparticles [131].Fig.17a illustrates the preparation process of MgH2@Ti-MX (calcined Ti3C2).With a 60 wt.% loading ratio,60MgH2@Ti-MX composite is able to release 3.0 wt.% H2at 150 °C within 2.5 h and then uptake 4.1 wt.% H2at 175 °C within 20 min.Interestingly,the initial dehydrogenation temperature for the 60MgH2@Ti-MX composite has been lowered by 90 °C when compared to 250 °C for the blank MgH2nanoparticles without Ti-MX synthesized by the hydrogenation of MgBu2.The outstanding hydrogen desorption and absorption kinetics has been explained as follows.Besides the ability of weakening Mg-H bond through Ti doping [133],thein situformed TiH2from Ti + H2⇌TiH2during hydrogenation could serve as the nucleation and growth centers for Mg during hydrogen desorption.Because the microstructure evolution analysis reveals that the decomposition of TiH2occurs after the decomposition of MgH2,as depicted in Fig.17b,and nano-Mg grains prefer to nucleate at the interface between TiH2and MgH2[134].Furthermore,the grain/phase boundaries,dislocations,and vacancies from the multiphasic system of MgH2/Mg and Ti-MX,could also work as active sites and diffusion paths for facilitating hydrogen mobility at low operation temperature.Moreover,thanks to the confinemen of Ti-MX with the good chemical durability and physical stability,60MgH2@Ti-MX composite could still reversibly desorption/absorb of 4.0 wt.% H2even after 60 cycles at 200 °C,indicating 95% of its full capacity is maintained. Fig.19.Experimental steps of MgH2@Ni-CNTs synthesis and its H2 de- and ab-sorption mechanisms [140]. The idea of manufacturing MgH2(0D)/TiO2(2D) heterostructure utilizing graphene-like 2D TiO2nanosheets (NS)is triggered because it can not only promote the formation of nano-sized MgH2through the confinemen effect of TiO2NS but also possess catalytic effect for MgH2[135].Fig.18a presents the preparation procedure of the MgH2/TiO2heterostructures.Sufficien oxygen vacancies have been introduced into TiO2NS during the impregnation of MgBu2,which would obviously magnify the electrical conductivity and broaden the lattice space,thereby facilitating the electrons mobility and hydrogen diffusion,and producing more active sites for the nucleation sites for MgH2/Mg [136].Combining with the multi-valence Ti-based catalyst’s capability of weakening the Mg-H bond (Fig.18b),60MgH2@Ti-MX composite starts the hydrogen release from as low as 180 °C and exhibits impressive hydrogen desorption/sorption properties.The composite could release 2.116 wt.%·min−1H2at 300°C,which is 35 times faster than that of blank and nanostructured MgH2fabricated by the hydrogenation of MgBu2.The highly stable MgH2/TiO2heterostructures with a fl werlike structure [137],which is able to prevent the growth and agglomeration of MgH2during the cycles of hydrogen release and uptake at 300 °C,are responsible for the excellent hydrogen capacity retention (98.5%) even after 100 cycles. As one of the most important parameters for the nanoconfine materials,the loading rate (LR) determines directly the hydrogen storage ability of the composites.According to the BET and BJH analysis,the theoretical LR values of MgH2@Ni-MOF composite and MgH2@CoS-NBs composite[138] are estimated to be 50 wt.% and 60 wt.%,respectively.Nevertheless,the practical LR of the corresponding composites have been decreased to 35.5 wt.% and 42.5 wt.%,respectively.The values of LR are calculated by the maximum hydrogen storage capacity,since MgH2/Mg is the sole system that would be responsible for the hydrogen release and uptake of the composites.The reduced LR values are attributed to the elimination of excessive(C4H9)2Mg before hydrogenation,the formation of MgO for Ni-MOF confine composite,and the formation of MgO and MgS for CoS-NBs confine composite during hydrogenation.Experiences tell us that the nanoconfinemen of MgH2/Mg within the mesoporous scaffolds are rather difficul to achieve,which results in a quite low loading rate of MgH2in the fina composite and the inferior storage capacity.For example,the loading rates of synthesized MgH2@SBA-15 and Mg@CA composites are only 17 wt.%[61] and 22.5 wt.% [139],respectively.It has been proposed[130] that repeated infiltratio processes and increasing the impregnation time are effective to enhance the loading rate of the scaffold.There is no doubt that the structure modifi cation could alter the pore structures and thus lead to better loading rates. The CNTs-Ni nanoparticles with ultrafin Ni nanoparticles (10 nm) could be prepared via hydrothermal and followed by calcination process [140].Ball milling Mg with the CNTs-Ni would lead to the uniform distribution of Ni and Mg nanoparticles over the surface defects and loose structure of CNTs.This would aid to prevent the agglomeration of Ni and Mg/MgH2nanoparticles.Besides the catalytic effect of Ni on the dissociation and reconstruction of H2,it is discovered that doping of Ni nanoparticles would accelerate the hydrogen diffusion by altering the kinetic controlled step from nucleation/growth to phase boundary migration.All of these have contributed to the striking reaction kinetics and cycle stability of MgH2@5 wt.%Ni-CNTs composite.The experimental steps of MgH2@Ni-CNTs synthesis and its hydrogen desoroption and absorption mechanisms are illustrated in Fig.19.MgH2@5 wt.%Ni-CNTs composite is able to release 7.29 wt.% H2with 30 min at 250 °C and the dehydrogenated composite could absorb 7.20 wt.% H2with 30 min under 60 bar hydrogen pressure at 200 °C.The activation energy of hydrogen desorption has been reduced to 74.8 kJ·mol−1H2.Moreover,no degradation of hydrogen storage capacity and reaction kinetics have been discovered in the tested 10 cycles. The sustainable development of energy is essential and critical to social progress and human civilization.The promotion and application of hydrogen energy has been widely recognized as the path to “Carbon Neutrality”,which is a goal for more than 40 governments to achieve around 2060.Nevertheless,safe,reliable,and economic hydrogen storage is the bottleneck for large-scale hydrogen utilization.Therefore,how to tailor the stable thermodynamics and sluggish kinetics of hydrogen storage in the solid-state magnesium-based materials,which has been acknowledged as a promising hydrogen storage alternative for onboard energy carrier,has become a research hotspot in recent years.Based on the review of the latest advances,the following conclusions and perspectives can be drawn. (1) Mechanical milling is the most cost effective method to facilitate the reaction kinetics.However,nanostructured MgH2/Mg particles prepared by milling are peculiarly prone to coarsening due to the sintering and agglomeration with prolonged exposure to the high temperature atmosphere during hydrogen release and uptake,which would result in serious decay in hydrogen storage capacity with cycles.Additives in ball milling that can serve as catalysts and at the same time prevent agglomeration/particle coarsening of MgH2/Mg will offer a promising method to achieve superior hydrogen uptake and release kinetics and cycle stability simultaneously. (2) Through the nanoconfinemen of scaffold,MgH2/Mg nanoparticles exhibit good cyclic stability.However,the sluggish kinetics of hydrogen desorption and absorption make it still far from the practical requirements.Moreover,the application prospect of this technology largely depends on the preparation progress of the nanoporous scaffolds. (3) MgH2and Mg require different characteristics of catalysts to enhance dehydrogenation and hydrogenation,respectively.Doping of uni-transition-metal catalyst is able to improve either the hydrogen release from MgH2or the hydrogen absorption for Mg;however,up till now,there has been no uni-transition-metal catalyst that could achieve the bidirectional catalysis for MgH2/Mg system with high efficien y. (4) Doping bimetal catalysts has enabled the simultaneous catalysis of the hydrogen desorption and absorption for MgH2/Mg system.However,particles coarsening could still occur for the nanostructured composite due to the lack of confine framework,which would deteriorate its cycling stability. (5) The novel technological approach of nanoconfinemen within-situcatalysis,which employs nanoporous and bimetallic catalyst as the scaffold to confin MgH2/Mg particles and enhance reaction kinetics simultaneously,provides a promising strategy for the exploitation of advanced hydrogen storage materials.It may be the ultimate solution to achieve the practical application of magnesium-based hydrogen storage materials because 1○highly active MgH2/Mg nanoparticles confine in the independent nanopores are able to maintain the nanostrcutures during cycles,which would not only destabilize the thermodynamics but also shorten the hydrogen diffusion path;2○the bimetallic catalyst carrier has the capability of catalyzing hydrogen desorption and absorption as required;and 3○the adjustment of the nanoporous scaffold structures could improve the loading amount of MgH2/Mg,achieve the uniform distribution of MgH2/Mg particles,and prevent agglomeration and aggregation at the same time. (6) Many transition metal oxides and carbides have been found to possess catalytic effects for MgH2/Mg system.They deserve further investigation in the near future because oxides and carbides are typically more stable chemically and thermally than their transition metal elements and thus have the potential to provide catalytic functions and prevent particle coarsening for MgH2/Mg simultaneously,leading to fast hydrogenation/dehydrogenation kinetics and long-term cycle stability at the same time. Declaration of Competing Interest The authors declare that there is no conflic of interests regarding the publication of this article. Acknowledgments This work was funded by Chongqing Special Key Project of Technology Innovation and Application Development(Grant No.cstc2019jscx-dxwtBX0016),Guiding Project of Scientifi Research Program in Ministry of Education of Hubei Province (No.B2021025),and Fundamental Research Funds for the Central Universities (2022CDJXY-010 and 2022CDJQY-013).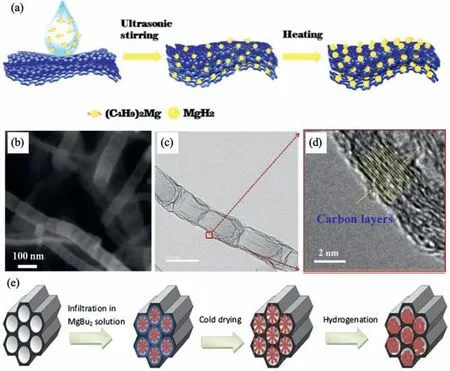
3.Catalysis

3.1.“Hydrogenphilic” catalysts

3.2.“Hydrogenphobic” catalysts


3.3.Bidirectional catalysts

4.Nanoconfinemen with in-situ catalysis


5.Conclusions and perspectives
杂志排行
Journal of Magnesium and Alloys的其它文章
- Grain refinemen of Mg-alloys by native MgO particles: An overview
- In silico studies of magnesium-based implants: A review of the current stage and challenges
- Investigating local corrosion processes of magnesium alloys with scanning probe electrochemical techniques: A review
- A tightly bonded reduced graphene oxide coating on magnesium alloy with photothermal effect for tumor therapy
- Nucleation of recrystallization in magnesium alloy grains of varied orientation and the impacts on texture evolution
- A novel nanoporous Mg-Li material for efficien hydrogen generation
