A novel nanoporous Mg-Li material for efficien hydrogen generation
2022-12-30JingruLiuQingxiYunWngxiHungXipingSong
Jingru Liu,Qingxi Yun,Wngxi Hung,Xiping Song,∗
a State Key Laboratory for Advanced Metals and Materials,University of Science and Technology Beijing,Beijing 100083,PR China
b Beijing Synchrotron Radiation Facility,Institute of High Energy Physics,Chinese Academy,Beijing 100049,PR China
Abstract Hydrogen generation material is a new kind of energy material that can supply hydrogen by reacting with water and is drawing more and more attention with the development of hydrogen economy.In this study,a novel nanoporous magnesium-lithium material prepared by a physical vapor deposition method exhibits an excellent hydrogen generation property.It generates hydrogen efficientl and quickly with saltwater,reaching a hydrogen generation amount of 962 mL g−1 and hydrogen generation rates of 60 mL g−1 min−1,109 mL g−1 min−1,256 mL g−1 min−1 and 367 mL g−1 min−1 at 0 °C,25 °C,35 °C and 50 °C,respectively.The nanoporous magnesium-lithium material is composed of a solid solution phase with a magnesium-lithium atomic ratio of 17:3.By synchrotron radiation analysis,the sizes of the nanopores are in the range of 100 nm ∼600 nm with an average size of 280 nm,and the porosity is calculated to be ∼42.4%.The improved hydrogen generation property is attributed to the nanoporous structure with a high specifi surface area,and the addition of lithium element which acts as active sites in hydrogen generation process.
Keywords: Nanoporous Mg-Li material;Physical vapor deposition;Hydrogen generation.
1.Introduction
Environmental pollution and traditional energy shortage make it urgent to develop a green energy system.Hydrogen is recognized as the most potential candidate to replace the fossil fuels with the advantages of zero emission,high energy density,and renewability [1,2].In general,the hydrogen generation methods include decomposition of fossil fuels[3,4],electrolysis/photolysis of water [5–7],and biohydrogen production [8,9].Decomposition of fossil fuels such as pyrolysis of petroleum is widely used in industry,but the gas products usually contain impurity gasses,and it needs complicated and costly purificatio process to produce high-purity hydrogen.Electrolysis of water needs a lot of energy consumption,and photolysis of water needs expensive catalysts and has high requirements on the environment.As for the biohydrogen production,maintaining a stable and continuous hydrogen generation is technically challenging.Besides these disadvantages,the hydrogen produced by the above methods needs to be stored and transported by high pressure devices,which adds extra costs on the hydrogen application and potential security risks to the gas manipulators and users [10].Therefore,to explore a new hydrogen supply method is essential for the hydrogen economy development.
Recent studies demonstrated that some hydrogen generation materials can directly produce hydrogen by hydrolysis reaction with water and have many advantages,such as onsite and rapid hydrogen supply,convenient storage and carrying,simple operation and high hydrogen purity.The hydrogen generation materials include magnesium [11–13],aluminum [14,15],and sodium borohydride [16,17].Aluminumbased material has a high hydrogen yield,but the alumina fil formed as a result of the oxidation of aluminum usually attaches on the metal surface and interrupts the hydrolysis reaction.The hydrogen generation amount of the aluminum-based material has been improved by adding other active agents such as LiH,NaBH4and alkaline salt [18–20],or doping other elements into the aluminum matrix,such as Fe,Co,Ni [21].However,the hydrogen generation rates of the aluminumbased materials are still very low.The sodium borohydride has a high hydrogen yield and a quick hydrogen generation rate,but its synthesis process is very complicated [22,23] and it hydrolyzes only when the noble metal works as a catalyst,such as Pt,Pd and Ru [24–26],which significantl increases the hydrogen preparation cost.In contrast,magnesium is abundant,cheap,and easy to be recycled,and it is active enough to react with water.The main problem for magnesium is the byproduct of the hydrolysis reaction,that is the magnesium hydroxide,will deposit on the surface of the material and form a passivation layer,which will slow or even stop the reaction [27].Recent studies reveal that the addition of lithium in magnesium is an efficien way to improve the hydrolysis activity and reduce the passivation[28].However,the commonly used method to add lithium into magnesium is the ball milling method,which is faced with the agglomeration problem and hard to produce homogenous Mg-Li material with a high specifi surface area.
In this work,a nanoporous magnesium-lithium (Mg-Li)material was successfully prepared by a simple physical vapor deposition method.The nanoporous structure can significantl increase the surface area and provide numerous channels for the hydrolysis reaction,and the addition of lithium can frequently break the hydroxide magnesium passivation layer and bring in a lot of active sites for the hydrolysis reaction.The nanoporous Mg-Li material has an excellent hydrogen generation property,and the preparation is simple,quick,and costeffective.The structure of the nanoporous Mg-Li material was characterized by the scanning electron microscope (SEM),transmission electron microscope (TEM),X-ray diffractometer (XRD) and the synchrotron radiation imaging technology.The formation mechanism of the nanoporous structure and the remarkable hydrogen generation property of this nanoporous Mg-Li material were discussed.
2.Experimental
2.1.The preparation
A self-designed physical vapor deposition device was used to prepare the nanoporous Mg-Li materials,as shown in Fig.1.The device mainly includes a furnace,a chamber,a pump and a circulating water cooling system.The furnace was used to heat the evaporation source,which was put into a quartz boat and placed at the bottom of the chamber.When heated,the Mg and Li atoms will evaporate from the evaporation source and deposit on a substrate,which is a 304 stainless steel net (1500 mesh) and placed 11∼18 cm above the evaporation source.Overhead the substrate,a circulating water system was used to lower the substrate temperature and a thermocouple was inserted to detect the substrate temperature.The pump connected to the chamber was used to create a vacuum environment (10−1Pa) and also as a low-pressure source to suck the Mg/Li vapor upward.In consideration of the high activity of pure lithium,we used a Mg-Li alloy as the evaporation source instead of the pure Mg and pure Li.The Mg-Li alloy was purchased from Aluminum Corporation of China and has a chemical composition of 85 wt.% Mg,14 wt.% Li and 1 wt.% Al (ASTM,LA141).It was cut to a cubic (6 × 6 × 6 mm),and polished with sandpaper in the order of 400#,1000#,2000# until the surface turned bright.The evaporating temperature and the depositing temperature were in the range of 570 °C ∼640 °C and 90 °C ∼200 °C,respectively.The evaporation and deposition lasted for one hour.After that,the furnace was turned off and the chamber was moved out from the furnace.The chamber was cooled in the air and the pump continued to work to maintain the vacuum level in the chamber.When the temperature in the chamber fell to the room temperature,the pump was turned off and the deposit together with the substrate was taken out from the chamber and vacuum sealed.

Fig.1.Schematic diagram for the preparation of the nanoporous Mg-Li material.
2.2.Characterization
Scanning electron microscope (SEM,Zeiss Auriga),X-ray diffractometer (XRD,TTR III) and the transmission electron microscope (TEM,FEI F30) were used to characterize the microstructures and phase compositions of the nanoporous Mg-Li material.The samples for SEM and XRD analysis were tested without any further treatments.The sample for the TEM analysis was firs dispersed by ultrasound in acetone solution and was dropped on the ultra-thin carbon film After the acetone evaporated completely,the carbon fil carrying the samples was used to observe.The Synchrotron radiation(Beijing Synchrotron Radiation Facility,4W1A-X-ray imaging station,with the spatial resolution of 50 nm) was used to characterize the three-dimensional structure of the nanoporous Mg-Li material.The sample for the synchrotron radiation imaging experiment was cut into a size of 10 × 10 × 15 μm3.
2.3.Property test
Hydrogen generation properties of the nanoporous Mg-Li materials were determined by a saltwater immersion test and the LA141 alloy was used as the control group.The nanoporous Mg-Li with the substrate was cut to 6 × 6 mm2,then it was weighed and recorded before the test.The LA141 alloy was cut to 6 × 6 × 1 mm3and was polished with sandpaper of 400#,1000#,2000# to remove the oxide layer and then it was weighed and recorded.The solution is 5 wt.%NaCl and the test was conducted at temperatures of 0 °C,25 °C,35 °C and 50 °C,respectively.After the test,the substrate was cleaned and weighed again,so that the weight of the nanoporous Mg-Li material can be calculated.The hydrogen volumes were determined by displacement of water at room temperature and then they were converted into the volumes at standard conditions.The hydrogen generation amounts which is the generated hydrogen volume (at standard conditions)by one gram sample and the spend time were used to draw the hydrogen generation property curves.
To further investigate the hydrolysis activity of nanoporous Mg-Li alloys and the LA141 alloy,the electrode potential E and corrosion current Icorrwere measured using a CHI 660D electrochemical workstation through Tafel curves.During the test,the sample and Pt were used as the working electrode and the counter electrode,respectively,and Hg/HgCl was used as the reference electrode.The electrolyte was 2.5 wt.% NaCl solution,and the scan rate was 5 mV/s.
3.Results
3.1.Characterization
The nanoporous magnesium-lithium material was prepared using the self-designed physical vapor deposition device mentioned above (Fig.1).The effects of evaporating temperature,the substrate position as well as the substrate temperature on the microstructure of the deposit were investigated(see Fig.S1).It is found that when the evaporating temperature is 600 °C,the substrate is 13 cm above the evaporating source and the depositing temperature is around 110 °C,a nanoporous magnesium-lithium material is successfully prepared.
The microstructures of the prepared nanoporous Mg-Li material with different magnificatio are shown in Fig.2(a),(b) and (c).It can be seen from Fig.2(a) that the prepared materials consist of irregularly packed grains,which are separated by very thin gaps.The grains are surrounded by several straight grain boundaries and the sizes of these grains are about several tens of microns.A zoom-in view,as displayed in Fig.2(b),shows that there are numerous nanometersized pores on the surface of grains,and they distribute homogeneously on each facet.When zooming in further,as demonstrated in Fig.2(c),one can clearly see numerous nano scaled pores and sub-micron ligaments which have regular shapes and roughly equal sizes.The XRD result,as shown in Fig.2(d),reveals that the as-received material is aβ-Li (Mg)phase,while the as-prepared nanoporous Mg-Li material is aα-Mg (Li) phase.The structure of the ligament was further characterized by TEM,and the sample for TEM observation was prepared by the ultrasonic vibration method.Fig.2(e),(g) and (h) are bright fiel images of the ligaments,and it can be seen that the ligaments have different sizes and obvious changes of growth direction can be seen among some of them.The electron diffraction patterns (inset image) of the circled area in Fig.2(g) demonstrate that the phase of the ligament is Li3Mg17.Fig.2(f) and (i) are the high-resolution images of the ligaments,which can be used to determine the growth orientation and further confir the phase.The labeled interplanar spacings are 0.2770 nm and 0.1590 nm,and the angle between them are 90°,which are consistent with the(100) plane and the (110) plane of Li3Mg17.It can thus be inferred from Fig.2(e) and Fig.2(f) that the growth orientation of the ligament is along [110].The same growth orientation is determined from Fig.2(h) and Fig.2(i) which show a ligament having a much larger width.
3.2.3D reconstruction of the nanoporous Mg-Li material
The synchrotron radiation imaging technology was used to further characterize the internal structure of the nanoporous Mg-Li material,and the result data were processed using a reconstruction software Avizo.Fig.3(a) displays the distribution of the nanopores and gaps in three dimensions,and a cross-section image drawn from the three-dimensional image is shown in Fig.3(b).It can be seen that the nanopores distribute homogeneously throughout the material,and the statistical analysis in Fig.3(c) shows that the sizes of these nanopores are in the range of 100 nm ∼600 nm and have an average size of ∼280 nm.In addition to the nanopores,there are also gaps between the grains,as displayed in Fig.3(d).The total volume fraction of the nanopores and gaps is about 0.42,as shown in Fig.S2,and the specifi surface area of the sample is calculated to be 2.12 m2/g (The detailed calculation process is shown in supplementary files Fig.S3).The threedimensional images of Mg atoms and Li atoms are exhibited in Fig.3(e)and(g),respectively,and the cross-section images drawn from the three-dimensional images,as demonstrated in Fig.3(f) and (h),show that the Mg atoms and the Li atoms also distribute homogenously in the solid component.
3.3.Hydrogen generation property
The hydrogen generation property of the as-received Mg-Li alloy plate and the as-prepared nanoporous Mg-Li materials at different temperatures are shown in Fig.4(a).The Mg-Li alloy plate does not hydrolyze in 5 wt.% NaCl solution at the temperature range of 0 °C ∼50 °C.By contrast,the prepared nanoporous Mg-Li materials show an excellent hydrogen generation property.The hydrogen generation amount of the nanoporous Mg-Li material reaches 962 mL g−1(at standard conditions),over 99% theoretical output,at all the test temperatures.According to the hydrolysis reaction Eq.(1) and the hydrogen volume calculation Eq.(2),the theoretical hydrogen generation amount of Mg and Li is calculated to be 933 mL/g and 1600 mL/g (at standard conditions)respectively.
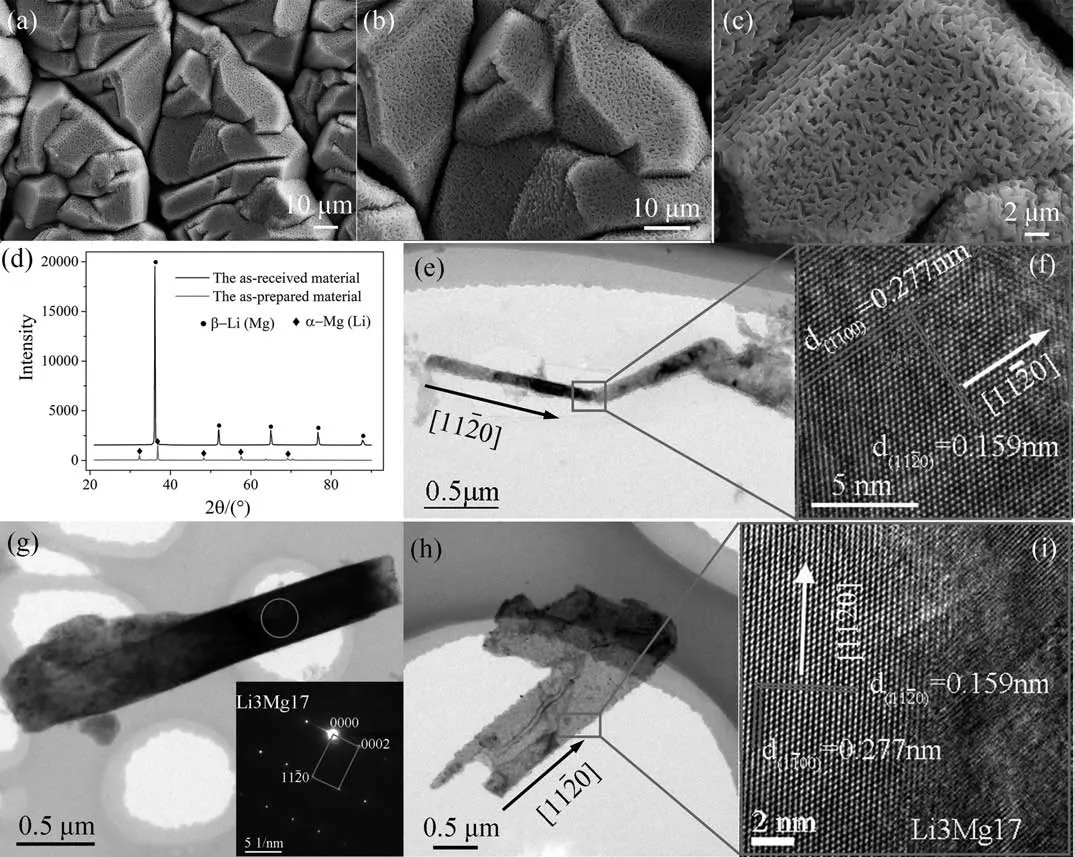
Fig.2.(a),(b) and (c) are the morphology of the nanoporous Mg-Li material at different magnifications (d) is the XRD patterns of the as-received material and the prepared nanoporous material,(e),(g) and (h) are the TEM images of ligaments in the nanoporous materials,the insert of (g) is diffraction patterns of the red cycle area in (g),(f) and (i) the corresponding lattice images of the area in (e) and (h).
The nanoporous Mg-Li material is the Li3Mg17phase according to the X-ray diffraction result,and its theorical output is calculated to be 965 mL/g (at standard conditions) based on the Eq.(3).

The nanoporous Mg-Li material can generate 962 mL g−1H2(at standard conditions) in 15 min at 0 °C and in 3 min at 50 °C,which is an efficien and fast hydrogen supply.The specifi hydrogen generation rate is calculated to be 60 mL g−1min−1at 0 °C,109 mL g−1min−1at 25 °C,256 mL g−1min−1at 35 °C and 367 mL g−1min−1at 50 °C.
3.4.Electrochemical property
The Tafel curves,as shown in Fig.4(b),further characterize the activity of these two materials,the as-received Mg-Li alloy plate and the nanoporous Mg-Li material.The electrode potentials of the as-received Mg-Li material and the nanoporous Mg-Li material are determined to be −0.576 V and −1.578 V (vs.SCE),respectively.The more negative electrode potential indicates a greater tendency of corrosion[29].This result demonstrates that the nanoporous Mg-Li material has a higher hydrolysis activity.The corrosion current can be determined by Tafel plot by an extrapolation method,and it is 4.89 mA for nanoporous Mg-Li material,0.25 mA for Mg-Li plate,and the current density is calculated to be 0.95 μA cm−2for nanoporous Mg-Li material and 0.25 mA cm−2for Mg-Li plate.During the test,the hydrogen evolution on the working electrode of the nanoporous Mg-Li material happens,so the corrosion current cannot be used to represent the hydrogen generation rate.Therefore,the corrosion current and the current density just can be used as a reference.

Fig.3.Three dimensional images of nanoporous Mg-Li materials.(a) the three-dimensional distribution of nanopores and gaps,(b) the cross-section image drawn from (a),(c) frequency of nanopore size,(d) the three-dimensional image of the gaps,(e) the three-dimensional distribution of the Mg atoms,(f) the cross-section image drawn from (e),(g) the three-dimensional distribution of Li atoms,(h) the cross-section image drawn from (g),the line represent 1 μm.
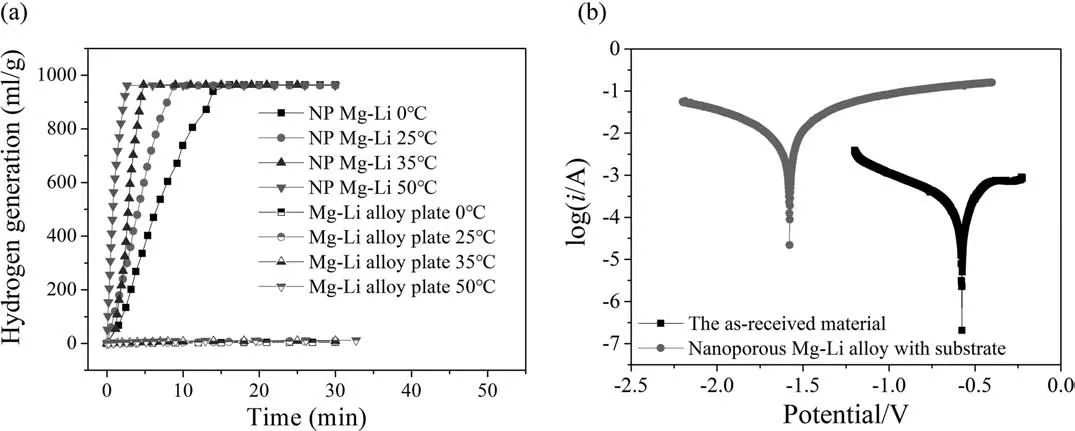
Fig.4.(a) hydrogen generation property of the nanoporous Mg-Li material (NP Mg-Li) and the as-received Mg-Li alloy plate at different temperatures,(b)Tafel curves of the nanoporous Mg-Li material and the as-received Mg-Li alloy plate.
4.Discussion

Fig.5.Schematic of the growth process of the nanoporous Mg-Li material.
4.1.Growth mechanism of the nanoporous Mg-Li material
Based on the morphology of the nanoporous Mg-Li material and the crystallographic orientation of its ligaments,we propose a growth mechanism of the nanoporous Mg-Li material,as shown in Fig.5.The metal atoms,namely Mg and Li atoms,evaporate from the bottom of the chamber and drift upwards.During the drift,the metal atoms collide with each other and form some clusters containing Mg and Li atoms.When these clusters deposit on the substrate,they act as crystal nuclei and form some particles.This process is known as nucleation,as shown in Fig.5 step 1.As the deposition goes on,atoms or clusters continuously arrive at the substrate and they diffuse on the surface under the attracting force of other atoms and eventually settle down at a position with the lowest energy.In the metal crystal structure,the bond length (bl)determines the attracting force and the position energy,that is,the shorter the bond length,the bigger attracting force and the lower position energy [30].The Li3Mg17phase has a hexagonal crystal structure,in which the [110] orientation is the closest packed direction and has the shortest bond length.Atoms in [110] orientation hence have the largest force to attract the arriving atoms or clusters,and similarly the lattice sites along this direction have the lowest position energy which makes it easier for these arriving atoms or clusters to settle down there.As a result,the particles prefer growing along the [110] orientation and form the straight ligament,as shown in Fig.5 step 2.As the deposition continues,the directional growth branches out along the [210] or [210] orientation,the equivalent orientations of the closest packed[110],as shown in Fig.5 step 3.When the branch encounters other branches,a frame which consists of many ligaments and nanopores will form,as demonstrated in Fig.5 step 4.The low deposition temperature also plays an important role in the formation of the nanopores.The temperature near the substrate cooled by the circulating water is only about 110°C,and under such low temperature the saturated vapor pressures are only about 6 × 10−11Pa for Mg and 1 × 10−12Pa for Li,which are too low to fil these nanopores.As many crystal nucleus grow into big grains and contact with each together,a polycrystalline nanoporous Mg-Li material will finall form,as shown in Fig.5 step 5.

Fig.6.The hydrogen generation mechanisms for three different Mg-Li alloys: (a) the as-received Mg-Li alloy plate,(b) the nanoporous Mg-Li material,(c)the nanoporous Mg-Li material with substrate.
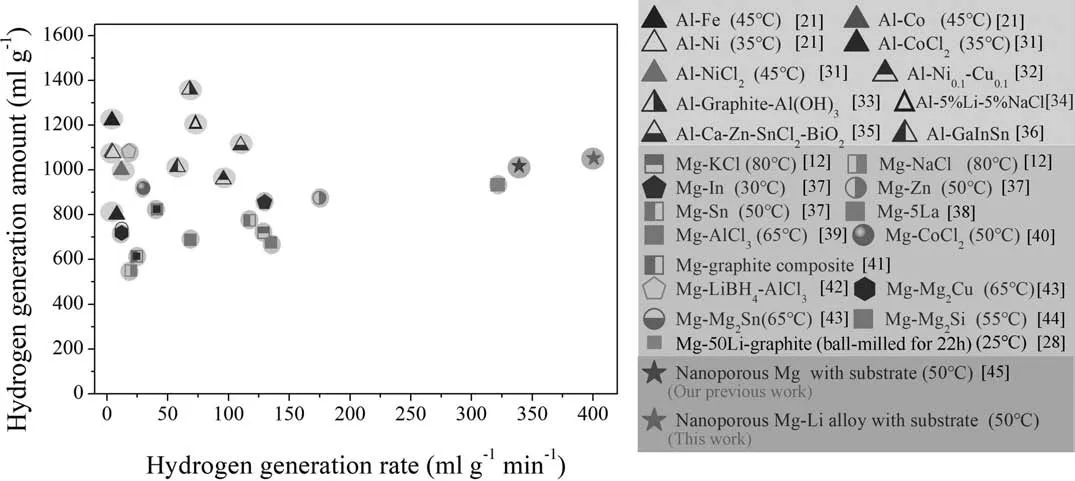
Fig.7.The comparison of hydrogen generation properties of several metal-based materials [12,21,28,31–45].
4.2.The hydrogen generation mechanism of the nanoporous Mg-Li material
The excellent hydrogen generation property of the nanoporous Mg-Li material in one hand comes from the nanoporous structure.As shown in Fig.6(a),the byproducts of the hydrolysis reaction of Mg-Li plate are Mg(OH)2and LiOH,and the Mg(OH)2will deposit on the plate surface.A passivation layer will be eventually formed as the deposition increases,which will make the hydrolysis reaction activity (ACT) of the Mg-Li plate significantl decrease and even stop the hydrolysis reaction.For the nanoporous Mg-Li materials,as displayed in Fig.6(b),numerous nanopores frequently break the deposition of Mg(OH)2,and only a partial passivation layer forms on the surface.Meanwhile,the ligaments in the nanoporous Mg-Li material are very fin and have a high surface energy,which make it easy to dissolve and collapse during the hydrolysis reaction.As a result,the byproduct is difficul to deposit,and the passivation layer can not easily form.In addition,the nanopores and gaps provide sufficien channels for the hydrolysis reaction and the hydrolysis reaction activity stay a high level after a little decrease due to the partial Mg(OH)2deposit.
On the other hand,the substrate (stainless steel nets) also helps to accelerate the hydrolysis reaction by forming a galvanic cell with Mg-Li materials,as demonstrated in Fig.6(c).The potential difference between the substrate(represented by Fe atoms) and Mg/Li materials causes the electron transportation from the Mg/Li to the substrate,which accelerates the reduction reaction of hydrogen ion (H+).That is,the chemical reaction between the nanoporous Mg-Li material and water become a faster electrochemical reaction.The passivation layer also forms partially,and the galvanic effect accelerates the hydrolysis significantl ,which leads the structure to collapse more quickly.The hydrolysis activity increases a lot due to the galvanic cell effect though a little decrease due to the partial Mg(OH)2layer formation.Thus,the hydrolysis reaction of the nanoporous Mg-Li material is further enhanced.
4.3.Comparison of the hydrogen generation properties of metal-based materials
The hydrogen generation properties of our nanoporous Mg-Li material and several reported metal-based materials,including the aluminum materials and the magnesium materials,are summarized in Fig.7.In the references,most of the hydrogen volumes were determined at room temperature,so the hydrogen generation volumes of the nanoporous Mg-Li material at room temperature are used to compare.In this figure the hydrogen generation amount refers to the max hydrogen generation amount and the hydrogen generation rate refers to the ratio of the max hydrogen generation amount to the spent time.It can be seen that the hydrogen generation amounts of Al-based materials are in the range of 900 mL g−1to 1400 mL g−1and are generally higher than those of Mg-based materials,which ranges from 500 mL g−1to 1100 mL g−1.However,the hydrogen generation rates of Al-based materials can only achieve 120 mL g−1min−1,which are much lower than that of 175 mL g−1min−1by Mg-based materials.In contrast,the hydrogen generation amount of the nanoporous Mg(in our previous work)reaches 1018 mL g−1,approaching the upper bound of the Mg-based materials,and the hydrogen generation rate of the material reaches 339 mL g−1min−1at 50 °C,nearly twice the value of the Mg-based materials,and three times the value of the Al-based materials.The addition of Li element in present work makes the nanoporous Mg material become lighter,and it provides the sample more active sites for hydrolysis reactions.As a consequence,the nanoporous Mg-Li materials obtain an even larger hydrogen generation amount of 1050 mL g−1and a higher hydrogen generation rate of 400.6 mL g−1min−1at 50 °C,distinctly better than those reported metal-based hydrogen generation materials.The Mg-50%Li materials has a comparable property with the nanoporous Mg-Li material,but its lithium content is as high as 50%,which will greatly increases the cost and risks of the preparation.
5.Conclusions
By using a self-designed physical vapor deposition device,we successfully prepared a nanoporous Mg-Li material.The hydrogen generation property of the nanoporous Mg-Li material was determined by a saltwater immersion test.The microstructures of the nanoporous Mg-Li materials were characterized by SEM,TEM,and the synchrotron radiation imaging technique,the phase compositions were determined by XRD,and the electrochemical property was tested by the electrochemical working station.The conclusions are as follows:
(1) The nanoporous Mg-Li material is composed of a pure Li3Mg17 solid solution phase which has a hcp structure.The nanopores with sizes in the range of 100 nm to 600 nm distribute homogeneously in the whole material,and the porosity is calculated to be ∼42.4%.These nanopores are formed as a result of the oriented growth of the ligaments along the most close-packed direction of the hcp structure.i.e.,the [110] directions.
(2) The amount of the hydrogen generated by the nanoporous Mg-Li materials reaches over 99% theoretical hydrogen generation amount in the temperature range from 0 °C to 50 °C.The hydrogen generation rate of the material significantl increases from 60 mL g−1minat 0 °C to 367 mL g−1min−1at 50 °C,which is more than twice the value of most Mg-based materials and Al-based materials,and is also higher than the value of nanoporous pure Mg.
(3) The remarkable hydrogen generation property of the nanoporous Mg-Li material mainly comes from the nanoporous structure and the Li element addition.The nanoporous structure prevents the byproduct of hydrolysis rection,Mg(OH)2,from forming the passivation layer on the surface of the material.The Li element not only improves the hydrogen generation amount by lowering the density of the nanoporous Mg material,but it also increases the hydrogen generation rate by providing the sample a large number of active sites for hydrolysis reaction.In addition,the galvanic cell formed between the substrate and magnesium/lithium nanoporous material also accelerates the hydrogen generation process.
Acknowledgment
This work was supported by National Natural Science Foundation of China [grant No.51271021] and Natural Science Foundation of Beijing Municipality[grant No.2162025].
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2021.09.022.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Effect of crystallization on purity of volatile metallic magnesium prepared from a one-step multi-region condensation process under vacuum condition
- Tribological behaviour of AZ31 magnesium alloy reinforced by bimodal size B4C after precipitation hardening
- Thermodynamic assessment of Mg−Ni−Y system focusing on long-period stacking ordered phases in the Mg-rich corner
- Primary Mg2Si phase and Mg2Si/α-Mg interface modifie by Sn and Sb elements in a Mg-5Sn-2Si-1.5Al-1Zn-0.8Sb alloy
- Quasi-in vivo corrosion behavior of AZ31B Mg alloy with hybrid MWCNTs-PEO/PCL based coatings
- The microstructure evolution and deformation mechanism in a casting AM80 magnesium alloy under ultra-high strain rate loading
