A tightly bonded reduced graphene oxide coating on magnesium alloy with photothermal effect for tumor therapy
2022-12-30LidanLiuFengPengDongdongZhangMeiLiJianHuangXuanyongLiu
Lidan Liu,Feng Peng,Dongdong Zhang,Mei Li,Jian Huang,Xuanyong Liu,d,∗
a State Key Laboratory of High Performance Ceramics and Superfin Microstructure,Shanghai Institute of Ceramics,Chinese Academy of Sciences,Shanghai 200050,China
b Center of Materials Science and Optoelectronics Engineering,University of Chinese Academy of Sciences,Beijing 100049,China
c Department of Orthopedics,Guangdong Provincial People’s Hospital,Guangdong Academy of Medical Sciences,Guangzhou 510080,China
d School of Chemistry and Materials Science,Hangzhou Institute for Advanced Study,University of Chinese Academy of Sciences,1 Sub-lane Xiangshan,Hangzhou 310024,China
Abstract Photothermal therapy becomes a hotspot in the treatment of bone tumors.Magnesium and its alloys are regarded as potential bone implants for their favorable mechanical property and biodegradable in vivo.However,there is few research devoted to fabricating a photothermal coating on Mg alloy.In the present study,reduced graphene oxide coating with a strong photothermal effect was prepared on the surface of AZ31 via two steps.Firstly,graphene oxide coating was deposited on the surface via electrophoresis deposited (GO#),followed by a reduction process of the graphene oxide coating in ultrapure water (rGO#).GO# and rGO# coatings were characterized by SEM,Raman,XRD,FTIR,and XPS.The results revealed that,compared with GO# coating,the content of oxygen-containing (C–O/C–O-C,C=O,O–C=O) groups on rGO# coating was significantl decreased.rGO# coating was found tightly adhered to AZ31 substrate.According to the first-principle calculations,the well-bonded heterostructure between MgO and rGO is the main reason for the strong bonding force.Moreover,the prepared rGO# coating showed a superior photothermal effect,which brings a new strategy to the treatment of bone tumors with Mg-based implants.
Keywords: Magnesium alloy;Reduced graphene oxide;Photothermal effect.
1.Introduction
Primary bone tumors often occur in adolescents and increasing attentions are paid to the treatment of bone tumors in the past few years [1–3].In addition,tumors originated in the other parts of the body (such as lung,mammary,renal,and so on) would easily metastasis to bone tissueviablood circulation and lymph circulation,which further highlights the importance of bone tumor therapy [2,4,5].The traditional therapeutic means for bone tumors are surgical treatment,chemotherapy,and radiotherapy.Generally,tumors are removedviasurgical treatment,followed by chemotherapy or radiotherapy to kill residual tumor cells [6].However,the utilization of chemotherapy and radiotherapy would also do huge damage to normal cells and cause a series of side effects.Moreover,the large defects caused by the surgery need bone fillin materials to accelerate the bone regeneration process.Taking these considerations into mind,researchers devoted to developing bone fillin materials with antitumor ability to avoid chemotherapy and radiotherapy.
Tumor cells and normal cells show different tolerances to high temperatures.When temperature rises to 42–44 °C,the structure of tumor cells would experience irreversible transformation and lead to apoptosis or necrosis,while normal cells can resist temperature as high as 45 °C [7].Therefore,photothermal therapy (PTT) is a common method in the fiel of tumor nanomedicine,which can modulate temperature via absorbing near-infrared (NIR) lightvia[8].Researchers prepared various photothermal agents (such as CuFeSe2,CuS,MoS2,polydopamine,and so on) on bone implants (such as akermanite,β-TCP,bioactive glass,and so on) for tumor therapy and bone reconstruction [3,9–11].These studies mainly focus on bioactive ceramics,which is poor in thermal conductivity.Owing to the poor thermal conductivity,bioactive ceramics would suffer sharply increasing temperature when irradiated with NIR light (up to 120 °C on the surface of the material,far higher than the tolerable temperature of normal cells),which makes it hard to precisely control the temperature.Moreover,only the temperature of the irradiated side would rise,which is unfavorable for its anticancer effect via thermal therapy.Thus,it is of great importance to developing photothermal coatings on new substrate materials with superior thermalviaconductivity.
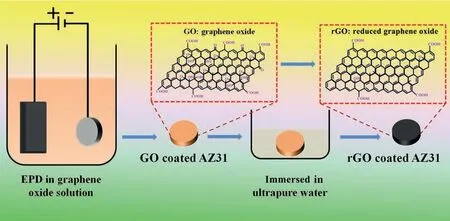
Fig.1.Preparation of rGO coated AZ31 alloy via EPD and self-reduction processes.
Magnesium (Mg) alloys as potential biodegradable material provide both biocompatibility and suitable mechanical properties [12].Mg2+is an essential element and presents in large amounts in the human body.The presence of Mg in the bone system is beneficia to bone strength and growth.Mg alloys have a specifi density (1.74–2g/cm3) and Young’s modulus (41–45GPa) most close to those (1.8–2.1g/cm3,3–20GPa) of human body’s bone.Therefore,in orthopedic and bone repairing or replacement applications,Mg alloys are particularly superior to any other metallic or polymer implants in terms of physical and mechanical properties,as the dissimilarity in Young’s modulus between an implant and natural bone can result in stress shielding effects,leading to the concentration of stress at the interface between the bone and implant,reducing stimulation of new bone growth and decreasing implant stability.More importantly,as a metal,Mg possesses a good thermal conductivity property.Though many researchers devote to designing Mg-based orthopedic implantsviaalloying or surface modificatio in the past two decades [13-15],researches on Mg-based anti-bone tumor implants are limited to self-released H2and medicine loading.For example,Yang et al.took advantage of H2released in the biodegradation process of Mg to kill tumor cells [16,17],and Li et al.loaded zoledronic acid on Mg-Sr alloy to anti-Giant Cell Tumors of Bone (GCTB) [18].No literature reported photothermal coating on the surface of Mg-based implants for bone tumor therapy by now.
The objective of the present study is to fabricate a stable photothermal coating on Mg alloy.For this purpose,graphene oxide (GO) was electrophoresis deposited (EPD) on AZ31 alloy,and then the graphene oxide layer was reduced in ultrapure water to obtain a black reduced graphene oxide (rGO)coating (Fig.1).The reduced process of GO coating and the adhesion strength of rGO coating were evaluated;first principles calculations were carried out to identify the mechanism of interface reaction;the photothermal effect and relevantin vitroanti-tumor ability of rGO coating were also investigated.
2.Materials and methods
2.1.Sample preparation
Commercial AZ31 alloy was cut into 10mm in diameter and 2mm in thickness.The cut plates were ground with 1000#SiC abrasive paper and then ultrasonically cleaned with ethyl alcohol,and then dried in air.The electrolyte was composed of 12mg graphene oxide (Zhejiang Carbon Valley Materials Science and Technology Co.,Ltd.),50mg Zn(NO3)2•6H2O,50mg Zn(CH3COO)2•2H2O,and 250mL ethanol.The EPD process was applied using a DC power supply (62024P-600–8,Chroma)with a graphite plate as anode and the AZ31 plate as cathode.The process was conducted with a constant potential of 80V for 1,2,and 4 mins,and then the obtained sample was denoted as GO-1#,GO-2#,and GO-3#,respectively.Afterward,GO-1#,GO-2#,and GO-3# samples were immersed in ultrapure water and kept at 60 °C for 2h and the prepared samples were denoted as rGO-1#,rGO-2#,and rGO-3#,respectively.
2.2.Sample characterization
The surface views of GO-1#,GO-2#,GO-3#,rGO-1#,rGO-2# and rGO-3# samples were observed by scanning electron microscopy (SEM;Hitachi-S3400N,Hitachi,Japan).The Raman spectra of all the samples were detected using a Raman microscope system (LabRAM,Horiba Jobin Yvon,France).The phase compositions of the samples were evaluated by X-ray diffraction (XRD,D2PHASE,BRUKER,USA).X-ray photoelectron spectroscopy (XPS;PHI 5802,Physical Electronics Inc.,Eden Prairie,MN,USA) was applied to analyze the chemical states of the C element on the surface of specimens.
2.3.Coating adhesion force test
The coating adhesion force of GO-2# and rGO-2# samples were evaluated using tape test and ultrasonic clean test.For the tape test,adhesive tape was firs stuck on the surface of the samples,and then the tape was pulled back at an angle close to 180° The process was repeated 10 times.For the ultrasonic clean test,samples were immersed in ethyl alcohol,and then ultrasonic clean with an ultrasonic cleaning instrument (SK5210HP,KUDOS ULTRASONIC INSTRUMENT,Shanghai) at 200W of powder and 53kHz of frequency.The process was kept for 60min.For both of the tests,photos of the samples were taken by a digital camera,and microscopic views were observed by SEM.
2.4.First-principles calculation
All calculations were performed using theVienna Ab initio Simulation Package(VASP) with projector augmented wave method (PAW) pseudopotentials [19,20].The Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation (GGA)was chosen to treat exchange-correlation effects [21].A cutoff of 520eV was imposed on the kinetic energy.The convergence threshold was set to 10−7eV for total energy and 0.01eV/°A for the force on atoms.Sampling of the Brillouin zone was performed using gamma point only.Supercells were built to model the interface reaction by combining GO or rGO (i.e.pure graphene) monolayer with bare surface of slabs while considering the lattice mismatch less than 2%.Most common surfaces,including MgO(111),Mg(001),and Mg(OH)2(001),were adopted to contact with the monolayer[22].The binding energy,Eb,was calculated byEb=Etot–Es–Eg,whereEtotis the total energy of the slab covered with GO or rGO,Esis the total energy of the bare surface of the slabs,andEgis the total energy of the unsupported GO or rGO.Similarly,the charge density difference which is closely related to bonding was obtained byρb=ρtot–ρs–ρg,whereρis the corresponding charge density as previously define forEb.
2.5.Photothermal effects of rGO coated AZ31 alloy
The photothermal effect of AZ31,GO-2#,and rGO-2#samples was detected in a 24-well culture plate in dry (air)or wet (the well was added with 700 μL ultrapure water) environment.An 808nm laser beam with a diameter of 12mm was used in the test.For dry environment observation,the laser beam with a power of 0.5W was focused on the samples for 5min,followed by natural cooling.For wet environment observation,the laser beam with a power of 1W was focused on the samples for 10min,and then naturally cooling.The real-time temperature and thermal images of the samples were monitored and record by an infrared thermal imaging system (FLIR A325sc,USA).
2.6.In vitro anticancer effects of rGO coated AZ31 alloy
MG63 cells (Human osteosarcoma cells) were cultured inα-MEM culture medium containing 10% fetal bovine serums (FBS,Hyclone,USA) and 1% antimicrobial of penicillin/streptomycin mixture (P/S,Hyclone,USA).Samples were sterilizedviaultraviolet irradiation.The cells were seeded in 24-well plates with a density of 2×104cells/well,and incubated at 37°C in a 5% CO2incubator.After cultured for 24h,AZ31 and rGO-2# samples were gently placed onto the plates.Subsequently,cells (with or without samples) were irradiated under 808nm laser beam (10min,0.6W).Furthermore,cells cultured without irradiation were also applied and cells cultured on plate without sample and irradiation were worked as control.After cultured for another 24h,cell viability was detectedviaalamarBlue according to our previous study [23].
The influenc of irradiation parameters on cell viability was also evaluated in this work.MG63 cells were cultured as described above.For the effect of irradiation powder,cells were irradiated with different power densities (0.3,0.6,and 1W) for 10min.For the effect of irradiation duration,cells were irradiated for different time (5,10,and 20min) at 0.6W.For the effect of irradiation times,cells were irradiated under 0.6W for 10min,and the process was repeated for two times.The cell viability was measuredviaalamarBlue.
3.Results and discussion
The surface morphologies of GO# and rGO# coated samples are shown in Fig.2.After deposited GO layer on AZ31 alloy,the original gully-like structures (Fig.S1) were uniformly covered by fla coatings(Figs.2a-2c).Typical wrinkles(red arrows) can be observed on the coatings,which are often presented on the surface of GO layer [24].It can be seen that the deposited time has little influenc on its surface morphology.GO coated samples were then immersed in ultrapure water,and their surface views are displayed in Figs.2d-2f.In comparison to GO# samples,the surfaces of rGO# samples were flatte,and fewer wrinkles were observed.Notably,the gully-like structure appeared again on rGO# samples.The cross-section of rGO-2# sample was observed,and it can be seen that the thickness of the coating was 863±119nm (Fig.S2).The optical images of AZ31,GO-2#,and rGO-2# samples are shown in Fig.S3.Uncoated AZ31 sample was silvery white,and became yellow after deposited with GO layer.However,rGO-2#sample exhibited a black surface,indicating the sample possesses favorable light absorption characterization [25].
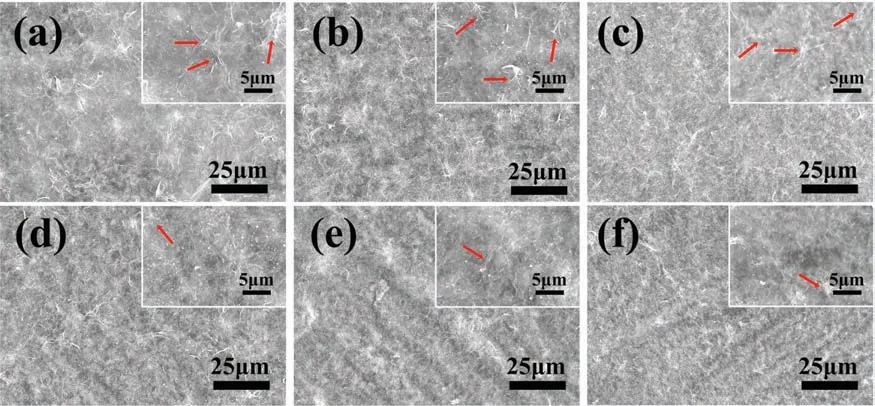
Fig.2.Surface views of GO-1# (a),GO-2# (b),GO-3# (c),rGO-1# (d),rGO-2# (e),rGO-3# (f).
The XRD patterns of AZ31,GO#,and rGO# samples are displayed in Fig.3a.The characteristic peaks of all the samples were same and only Mg characteristic peaks were detected [26].Generally,the diffraction peak of GO would appear around 12° [27],but in this work,no feature peaks of GO were observed.This might ascribe to that the prepared film are too thin and their poor crystallization [28].To prove the existence of GO and rGO,Raman shift was used to measure molecular structures of coated samples and the results are displayed in Figs.3b and 3c.The Raman spectra of all three GO# samples were similar (Fig.3b).Intensive peaks at∼1350 cm−1and ∼1580 cm−1were detected,which represent the D band and G band of GO,respectively [29].The D band is caused by the symmetric expansion and contraction of sp2carbon atoms in the aromatic ring and requires a defect for activation,while the G band is brought by the vibrations of E2gphonon at the center of the Brillouin Zone[30,31].For comparison,the spectrum of AZ31 alloy shows a straight line (Fig.S4).The Raman spectra of rGO# samples were also similar to each other (Fig.3c) and both D band and G band of rGO were detected.It is worth noting that,different from GO# samples,the intensities of D band of rGO# samples were significantl stronger than that of G band.The ratios of intensity of D band to G band (Id/Ig) of various coatings are shown in Table 1 and the Id/Igvalues of GO# samples and rGO# samples were around 1 and 1.1,respectively.Generally,the higher value of the ratio means more defects in the graphene-derivative [32-34].The defects of rGO# samples might be introduced in the reduction process of oxygen-containing groups of GO.The above resultwas contrary to the study by Yuan et al.,which used GO to reinforce the matrix of Mg alloy and found that the value of Id/Igof GO was smaller after reduced by Mg substrate[35].The reasons for this difference might be that the reaction of this work was happed in ultrapure water at a relatively low temperature (60 °C),which was easy to introduce defect,while the reaction of GO and Mg in the literature was in Ar or vacuum and at a much higher temperature (600 °C),which is favorable for the defects to be healed.
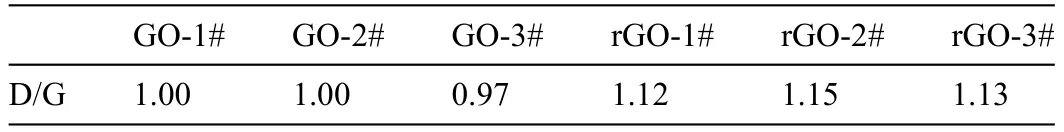
Table 1 The ratios of intensity of D band to G band (Id/Ig) of various f lms.
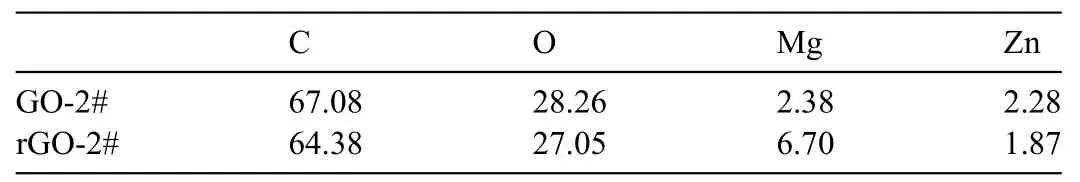
Table 2 Element compositions of GO-2# and rGO-2# coatings (at%).
To further identify the content of oxygen-containing groups of GO and rGO coated samples,XPS analysis was applied.Figs.4a and 4b show the full XPS spectra of GO-2# and rGO-2# samples.Element Zn,Mg,O,and C were detected for both the samples.Corresponding semi-quantitative analysis results are shown in Table 2.For GO-2# sample,Zn was originated from the electrolyte,while Mg was from the corrosion of AZ31 alloy substrate.C and O were ascribed to the deposited GO layer.Compared with GO-2# sample,the Mg content of rGO-2# sample was significantl higher,indicating the possible reaction between the AZ31 substrate and GO layer when immersed in ultrapure water.The high resolution of C 1s spectra of GO-2# and rGO-2# samples are shown in Figs.4c and 4d,respectively.The C1s spectra can be fitte into four peaks: peak at 284.8eV represents carbon skeletons(C–C/C=C),at 286.6eV represents C–O/C–O-C,at 287.4eV represents C=O,and at 288.6V represents O–C=O [36-38].The area ratios of four peaks of the two measured samples are shown in Table 3.The content of oxygen-containing groups,including C–O/C–O-C,C=O,O–C=O,of GO-2# sample were 16.07%,8.96%,and 6.44%,respectively.However,after immersed in ultrapure,the content of oxygen-containing groups sharply decreased to 6.31%,0%,and 4.6%,respectively.The above results suggested that GO layer on AZ31 alloy would experience a reduction process when contacting with liquid.
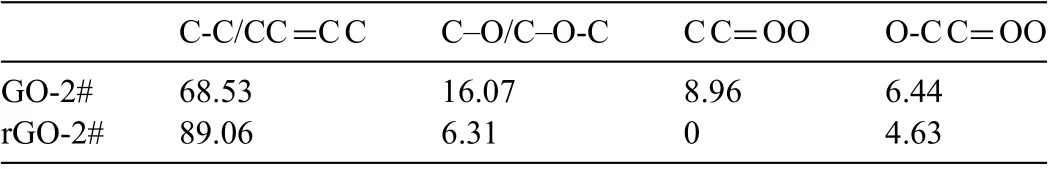
Table 3 Area ratios of fitte peaks from C 1s spectra of GO-2# and rGO-2# samples(%).
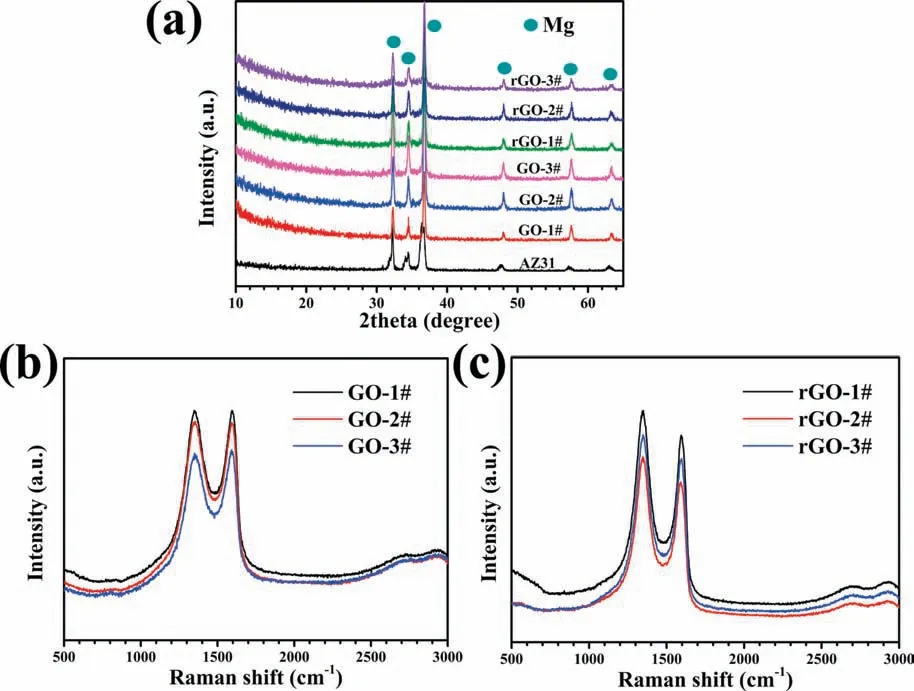
Fig.3.XRD patterns of all the samples (a),Raman shift of GO coated samples (b) and rGO coated samples (c).
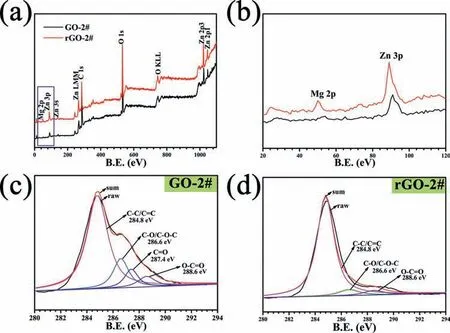
Fig.4.XPS full spectra of GO-2# and rGO-2# samples (a).The magnificatio of blue rectangle in (a) is shown in (b).High resolution of C 1s spectra of GO-2# (c) and rGO-2# (d) samples.
GO contains a large number of oxygen-containing groups and these groups can be reduced by alkali metals,including Mg,Al and Zn et al.[39-41].Many studies used GO to reinforce the Mg substrate and GO would be reduced during the process of processing [33,35,42].However,it is the firs time that rGO was prepared on the surface of Mg alloyviaEPD and self-reduction process.During the process of EPD,the current was dramatically decreased from 65mA to 25mA in the initial 1min,and then reposefully decreased to 15mA at 2min and 5mA at 4min (Fig.S5).This phenomenon demonstrated that the GO layer was mostly deposited on AZ31 alloy in the firs 1min.Therefore,few differences in surface morphology,phase composition,and Raman shift were observed between the three GO coated samples.As the characteristics of GO coated samples were similar,the features of the three rGO coated samples were also almost the same.Hence,the adhesion force and photothermal tests were only applied to GO-2# and rGO-2# samples.
As a coating,especially used in bone implants,it requires strong adhesion with the substrate to avoid peeling.Figs.5a and 5c show the results of the tape test.The diagrammatic sketch of the experiment process can be seen in Fig.S6.After 1 time of sticking and tearing,the original yellow coating on the surface of GO-2# sample almost disappeared,and the AZ31 substrate was exposed.However,even experienced 10 times of sticking and peeling,the surface of rGO-2# sample was covered by black coating just as its original state and almost no substrate can be observed(Fig.5a).It can be obviously seen in Fig.5c that only a few GO plates (indicated by red arrows) were left on the surface of GO-2# sample,while integrity rGO coating was observed on the surface of rGO-2# sample.Ultrasonic test was also used to evaluate the adhesion force of the coating with the substrate,and the results are shown in Figs.5b and 5d.After ultrasonic for 10min,the GO coating was peeled off from the substrate.Nevertheless,most of the substrate of rGO-2# sample was still covered by black rGO coating even after ultrasonic for 60min.The SEM results in Fig 5d further confirme the phenomenon.Both the results of the tape test and the ultrasonic clean test revealed that rGO coating exhibited strong adhesion force to the substrate.
To better understand the interaction between the interfaces of A (Mg,MgO,Mg(OH)2) and B (GO,rGO),first-principle calculations were carried out usingVienna Ab initio Simulation Package(VASP).As the GO and Mg were immersed into the water,different scenarios can be proposed as following:(1) Mg reacts directly with GO or (2) Mg reacts with water to produce H2and Mg(OH)2that further binds with GO.As shown in Figs.S7a and S7b,the GO rarely interacts with H2or Mg(OH)2.In contrast in Fig.S7c,the bare (001) surface of Mg seizes the O atom of GO and forms new Mg-O bonds and the GO reduces back to rGO layer.Mg also shows similar strong interaction with C–OH of GO and results in the formation of new Mg-OH bonds (Fig.S7d).Thus,as the firs step,the GO will be mostly reduced and new Mg-O and Mg-OH bonds will occur,leading to the formation of compounds of MgO and Mg(OH)2at the interface.Further,the calculations were applied to compare the interaction between rGO layer and newly formed MgO or Mg(OH)2compounds.Interestingly,only weak cohesion is found for rGO/Mg(OH)2interface (Fig.S7e),compared with the strong anchoringviathe distorted rGO layer with newly formed covalent C–O bonds bridging the interface (Fig.S7f).The bond length of C–O ranges from 1.478 to 1.515 °A,which is comparable with other calculations [43].Based on the geometry optimization,it is likely that the fina stable interface is only MgO/rGO heterostructure after two steps reaction of GO reduction and chemisorption on MgO surface.The binding energy between MgO and graphene is -0.18eV per C atom,which is similar to other strong-bonded systems [44].As shown in Fig.6a,the charge density difference between rGO and O atoms shows clear p-p orbital coupling.The exposed O atoms at the polar MgO surface provide an anchor point to the graphene raft,suggesting that the rGO layer acts as a donator of charge to the MgO surface.It should be noted,our analysis of charge localization based on the electron localization function (ELF)method (Fig.S8) indicates the electronic structure is not localized around the chemisorption region but uniformly delocalized [45].The density of state (DOS) of MgO/rGO heterostructure in Fig.6b indicates that the electron redistribution between the C and O atom by forming the covalent bonds.The C atoms at corrugated sites show strong orbital hybridization with the oxygen atoms.The comparison of partial DOS for the atoms at different sites indicates that the C atom on the O site has a sp3character while forming the chemical bond with O atom.This hybridization induces aπ–π∗state degeneracy and the formation of new energy states between theπstates and the 2p orbital of O.Our Bader charge analysis also verifie this findin (Fig.6c),and the C atoms bonded to O atoms have less charge thus acting as a donator of charge to the MgO surface.As shown in Fig.6d,a drop of electrostatic potential by 10.76eV across the interface of MgO/rGO heterostructure is expected to result in a large built-in electric field The built-in electric fiel across the interface is an important property to separate the photogenerated electron–holes[46].In addition,the calculated work function of 4.55eV for MgO/rGO is higher than 4.30eV of free graphene,representing a p-type doping behavior.These characters are believed to attribute the photothermal performance for our application in tumor therapy.
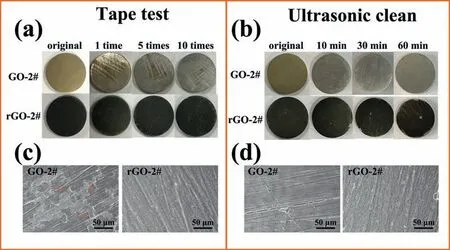
Fig.5.Optical images of GO-2# and rGO-2# samples after experiencing different number of times of tape test (a),and corresponding SEM views after 10 times of tape test (c).Optical images of GO-2# and rGO-2# samples after experiencing different duration time of ultrasonic clean (b),and corresponding SEM views after 60min of ultrasonic clean (d).
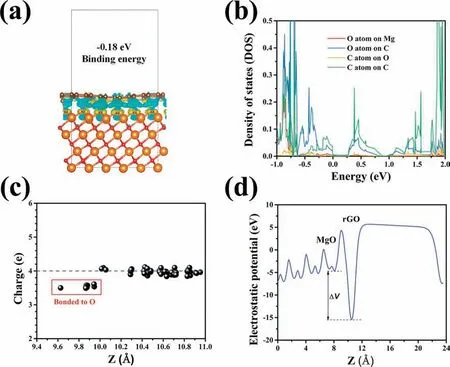
Fig.6.Charge density difference of interface between MgO and graphene,wherein yellow and blue colors correspond to positive and negative charge densities(a).C,Mg,O atoms are represented as brown,orange and red spheres,respectively.Partial density of state (DOS) of O and C atoms at the MgO/graphene interface (b);Bader charge distribution of C atoms covering MgO surface (c);the potential drop across the interface of the MgO/graphene heterostructure (d).
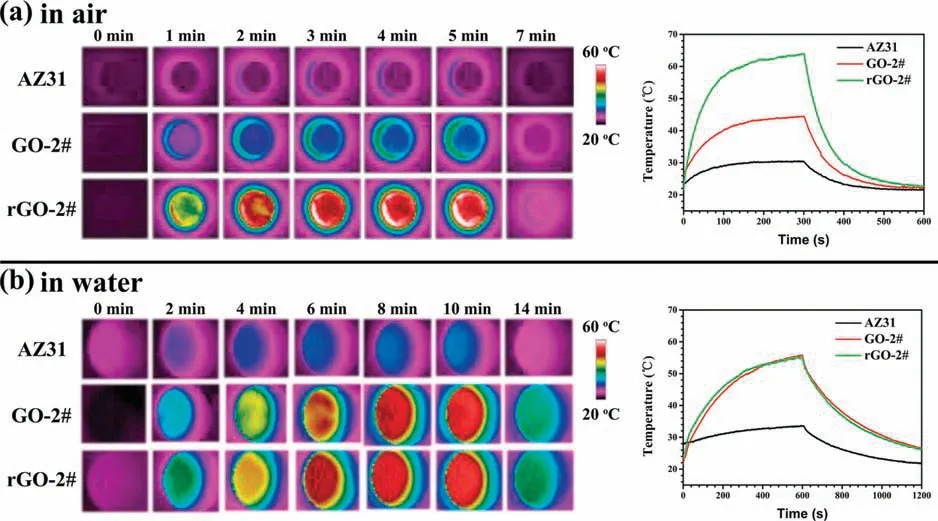
Fig.7.Thermal images and real-time changes of temperature of AZ31,GO-2#,and rGO-2# samples in a dry environment (a) and wet environment (b).
The photothermal performance of AZ31,GO-2#,and rGO-2# samples are depicted in Fig.7.In a dry environment,after irradiation for 5min,the surface temperature of rGO-2# sample reached 66°C,while the temperature of AZ31 and GO-2# samples only increased to 30°C and 42°C,respectively(Fig.7a).In a wet environment,the temperature of AZ31 alloy after irradiation for 10min was about 30°C (Fig.7b).It is worth noting that the heating curves of GO-2# and rGO-2# samples were almost the same,and increased to 56°C in 10min.As discussed earlier,GO coating would be reduced when contacted with liquid,so the photothermal effect of GO-2# sample was consistent with that of rGO-2# sample.The phenomenon can also be verifie by the fact that the color of GO-2# sample changed to black after irradiation in water(Fig.S9).The temperature can easily be adjusted by changing irradiation power.When irradiation power was 0.5 and 0.8W,after irradiation for 10min in wet condition,the temperature on rGO-2#surface would reach 44.3 and 51.5°C,respectively(Fig.S10).In addition,rGO-2# samples were implanted into the subcutaneous of a BALB/C rat,and the irradiation results suggested that the surface temperature of rGO-2# samples increased to 53.8°C after being irradiated at 1W for 5min (Fig.S11).Both thein vitroandin vivoresults revealed a favorable photothermal effect of rGO-2# sample.
MG63 cells were used as models to investigatein vitroanticancer effects of various samples.The influenc of laser,power density,irradiation duration,and irradiation times were studied,and the results are shown in Fig.8.Only 40% of cells were alive when the rGO-2# samples were under irradiation (Fig.8a).For comparison,cells cultured with AZ31(with or without irradiation) and rGO-2# (without irradiation) showed no significan difference with that cultured on the control group (Blank).Moreover,cell viability was decreased with the increase of power density and totally died when power density reached 1W/cm2and kept for 10min(Fig.8b).Cell viability was also decreased with the prolongation of irradiation duration and increase of irradiation times(Fig.8c and d).Cells are sensitive to ambient temperature.It has been reported that cancer cells would experience irreversible changes in biochemical metabolism and cell structure at 42–44°C and eventually resulting in apoptosis or necrosis,while normal cells can tolerate a high temperature of 45°C [7].When the surrounding temperature is above 48°C,it would lead to drastic cell death [47].In the present study,the temperature of rGO-2# sample can reach 53.8°Cin vivo,which is high enough to induce cancer cell death.Therefore,rGO modifie AZ31 alloy with a desirable photothermal effect may be a promising biomaterial used in bone cancer therapy.
4.Conclusions
In summary,rGO coatings were successfully fabricated on AZ31 alloyviatwo steps.Firstly,GO coatings were deposited on AZ31 alloy by EPD method.Secondly,the GO coatings were reduced by immersing in ultrapure water.XPS results revealed that C=O,C–O/C–O-C,and–COOH groups in GO coatings were reduced.The newly formed rGO layer showed strong adhesion with AZ31 substrate.First-principles calculations indicate that the fina stable interface is the heterostructure MgO and reduced graphene oxide,where C and O atoms form the chemical bond site with sp3character verifie by the electronic structure of the interface.Moreover,the rGO coating endowed the sample with favorablein vitroandin vivophotothermal effects.With a desirable photothermal effect,rGO coated Mg sample showed significanin vitroinhibition of tumor cell viability.Therefore,such a photothermal coating modifie biodegradable Mg alloy provides a new strategy for the therapy of tumor-induced bone defects.
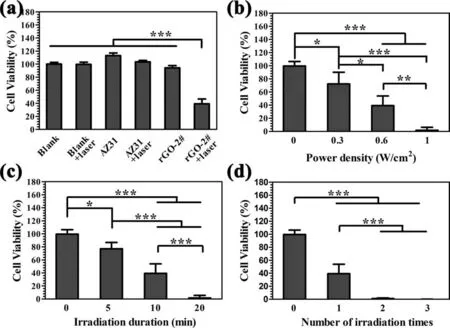
Fig.8.Cell viability of bone tumor cells MG-63 after being cultured with AZ31 and rGO-2# samples under or not under irradiation (a).Cell viability of MG-63 after being cultured with AZ31 and rGO-2# samples under different powder densities of irradiation (b),different duration time of irradiation (c),and different number of irradiation times (d).
Declaration of Competing Interest
None declared.
Acknowledgments
This work is financiall supported by the National Natural Science Foundation of China (31771044),China Postdoctoral Science Foundation(2019M662830),Shanghai Committee of Science and Technology,China (19JC1415500,18ZR1445000),International Partnership Program of Chinese Academy of Sciences(GJHZ1850)and Natural Science Foundation of Guangdong Province,China (2020A1515011447).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2021.03.003.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Grain refinemen of Mg-alloys by native MgO particles: An overview
- Tailoring MgH2 for hydrogen storage through nanoengineering and catalysis
- In silico studies of magnesium-based implants: A review of the current stage and challenges
- Investigating local corrosion processes of magnesium alloys with scanning probe electrochemical techniques: A review
- Nucleation of recrystallization in magnesium alloy grains of varied orientation and the impacts on texture evolution
- A novel nanoporous Mg-Li material for efficien hydrogen generation
