Prediction of concentration of toxic gases produced by detonation of commercial explosives by thermochemical equilibrium calculations
2022-12-23MuhamedSuceskaStimacTumaraVinkoSkrlecSinisaStankovic
Muhamed Suceska,B.Stimac Tumara,Vinko Skrlec,Sinisa Stankovic
University of Zagreb,Faculty of Mining,Geology and Petroleum Engineering,Pierottijeva 6,10 000,Zagreb,Croatia
Keywords:Mining ANFO Emulsion explosive Toxic fumes Thermochemical code EXPLO5
ABSTRACT An adverse effect resulting from explosive mine blasts is the production of toxic nitrogen oxides(NO and NO2)and carbon monoxide(CO).The empirical measurements of the concentration of toxic gases showed that it depends not only on the composition of an explosive and properties of its ingredients but also on several other parameters,such as volume of blasting chamber,explosive charge mass and design,confinement characteristics,surrounding atmosphere,etc.That explains why measured concentrations of toxic gases reported in literature significantly differ.I
1.Introduction
Blasting,heavily used in civil engineering and mining industry,has some negative impacts on the environment and workforce.In addition to direct physical adverse effects,such as vibrations,air blast,and flyrock,the blasting of commercial explosives produces some toxic gaseous detonation products within a detectable range of concentrations[1,2].When dealing with ammonium nitrate(AN)based commercial explosives(ANFO,emulsion,and their blends)the main detonation products are water(H2O),carbon dioxide(CO2),and nitrogen(N2).However,some amounts of toxic nitrogen dioxide(NO2),nitrogen monoxide(NO),and carbon monoxide(CO)are also formed,depending on the composition of the explosive and blasting conditions[3-10].
Gases like NO and NO2are toxic and can cause serious health risks to the personnel exposed,with NO2being about five times more toxic than NO[9,11,12]with different minimum lethal doses.The concentration of 805 ppm of NO2will cause lethal reflex choking after 15 s of exposure,while 8000 ppm of NO will result in sudden unconsciousness followed by death after 60 s exposure[7].Carbon monoxide(CO)is an asphyxiating gas[13]that cannot be detected by sight or smell[14].Even though CO is not dangerous in open pit excavation,its danger lies in its possibility to migrate through the ground and collect in enclosed spaces causing carbon monoxide poisoning[2,14].
The production of toxic gases is usually studied through experimental measurements of the concentration of gases produced by detonation of an explosive charge in a blasting chamber[5].The experiments reported in the literature differ vastly in terms of the quantity of explosive charge,the volume of the blasting chamber,presence and type of confinement used.Laboratory tests,such as Ornellas’detonation calorimetry[15]and Crawshaw-Jones test[16]involve smaller quantities of explosives(30 g and 450 g,respectively)and smaller chamber volumes(5.3 dm3and 50 dm3,respectively)which makes them unsuitable for highly non-ideal explosives,such are explosives based on ammonium nitrate(AN).
One of the earliest large-scale experiments conducted with ANFO was done by Chaiken et al.[16].The authors used a large closed system(cylindrical steel chambers with a volume of about 72 m3and about 0.5 kg of explosive charge encased in a glass tube).Similar work was done by Mainiero[5],Rowland and Mainiero[8],Rowland et al.[12],Sapko et al.[9],and Mainiero et al.[6],where a large chamber(section of a mine with an approximate volume of 274 m3)was used along with light and heavily confined explosive charge each weighing about 4.5 kg.Attempts to standardize the method of determination of toxic gases resulted in a European standard(‘EN 13631-16:2004’,[17])which recommends using chambers at least 15 m3in volume and a minimum explosive charge mass of 0.5 kg placed in a glass tube.The chamber is equipped with temperature and pressure probes and equipment for continuous measurement of CO,CO2,NO,and NO2concentrations[3,10,18,19].
Numerous factors affect the measured concentration of toxic gases:oxygen balance,presence of additives,nature,and stability of the explosive ingredients,presence and type of confinement,the way of priming,water resistance of the explosive,the reactivity of the detonation products with the surroundings,non-ideal detonation characteristics,etc.It was shown that the oxygen balance of an explosive plays the most important role in CO and NOXproduction;oxygen-deficient formulations produce more CO and less NOX[3,8,9,16,20].Biessikirski et al.[3]found that not only the percentage of AN but also the absorption index of its prills have an effect on the resulting gases;the larger the absorption index,the slower the detonation velocity and a lower concentration of toxic gases was observed.Rowland & Mainiero[8],Onederra et al.[7],and Sapko et al.[9]found that the degree of confinement affects NOXand NH3production but does not play an important role in CO production.In the case of ANFO,light confinement results in increased NOXconcentration,which the authors attributed to increased non-ideal behavior.The authors also found that additives in ANFO(such are excess fuel,water,aluminum powder,etc.)affect CO and NOXconcentrations.In addition,explosive charge diameter affects the production of toxic gases for both ANFO[8]and emulsion explosives[19].The volume of CO and NOXincreases with a decrease in charge diameter and strength of confinement.Maranda et al.[18],Rowland&Mainiero[12],Sapko et al.[9],and Harris et al.[11]state that oxygen from surrounding air may oxidize NO to NO2hence measured NO concentration decreases with time.The aforementioned suggests that the concentration measured under one experimental setup tells us a little about the concentration produced under different experimental conditions[9].
Based on available literature data,theoretical calculation of the concentration of toxic gases has not been widely reported.In the work of Chaiken et al.[16],the thermochemical code TIGER,based on the Chapman-Jouguet(C-J)detonation theory,was used for the prediction of detonation properties and product concentrations of ANFO explosives.The authors found that calculated concentrations were not in exact agreement with experimental data but could approximate the trends observed with compositional variations.De Souza and Katsabanis[4]used TIGER,BKW thermochemical codes,and C-J detonation theory to predict concentrations of toxic gases of a series of ANFO and emulsion explosives.The authors found that the calculated and experimental concentrations were in reasonable agreement,except for NO in the negative oxygen balance region and CO in the positive oxygen balance region.
This work aims to test the applicability of the C-J detonation model supplemented with a more accurate equation of state of the gas products and test the applicability of the deflagration explosion model to commercial explosives.
2.Numerical modeling of effects on concentration on production of toxic gases
2.1.Description of the calculation model
A comprehensive study of the production of toxic gases under mining conditions should consider all the processes involved;detonation,formation of detonation products,expansion,and cooling of the products,mixing with surrounding atmosphere followed by kinetically controlled post-detonation reactions.The thermochemical calculations can provide information on the initial composition and concentration of detonation products but cannot address how concentrations change in kinetically controlled postdetonation reactions with atmospheric oxygen.
In this study calculations are performed using EXPLO5 thermochemical code[21],applying two calculation models:a)the CJ detonation model and b)the deflagration model.The accuracy of the calculation of detonation parameters,including the concentration of detonation and combustion products,has so far been tested on many explosives and the results have been presented in several papers[22-26].Generally,detonation velocity can be predicted with the root mean square(RMS)error below 3% and detonation pressure with RMS error below 6%[25],while the calculated concentration of detonation products reproduces very well those obtained by detonation calorimetry[22].
The C-J detonation theory assumes an explosive instantaneously transforms reactants into detonation products which are initially under high temperature(T)and pressure(p).Under such conditions,the state of equilibrium establishes instantaneously,and the concentration of individual products is determined by the state of equilibrium at a given p,T,V state.In other words,concentration is thermodynamically controlled and can be calculated by solving thermodynamic equations between detonation products[21,27].After formation,the products expand isentropically and since they are still hot,they can react with each other.As the result,the concentration of products changes with time.Below a certain temperature,the so-called freeze-out temperature(typically 1800-2250 K)[27,28],the rate of reactions becomes too slow,so a state of equilibrium cannot be established quickly.Thus,below the freeze-out temperature,the product concentration is assumed to remain unchanged.
Kinetic analysis of some elementary reactions between combustion products in an internal combustion engine,described in Chaiken et al.[16],showed that within 5 ms of piston expansion,the concentration of NO at 2665 K remained at its initial value,while the concentration of CO reduced to about 50% of its initial value.This indicates that rates of equilibrium reactions of the reduction of NO to N2and oxidation of CO to CO2cannot keep pace with the cooling effects during the expansion.Based on this,it is assumed that the concentrations of CO and NOXremain relatively unchanged during the isentropic expansion of detonation products to atmospheric conditions.Thus,the concentrations of CO and NOxcan be predicted by calculating their concentrations at the C-J state using a suitable thermochemical code.Our ideal detonation model is based on this assumption.
It was suggested by Barnhart(described in Onederra et al.[7])that increased production of NOxin fuel-lean ANFO formulations can be better described by the deflagration model.Generally,this model seems applicable in all cases where reaction time is longer(i.e.,slower detonation velocities).In the deflagration model,the calculation was performed using the constant volume combustion module incorporated in the EXPLO5 code.The model requires blasting chamber volume and mass of explosive to be specified by the user.In addition,the model allows the atmosphere which fills the chamber(or some fraction of it)to take place in the combustion reaction.
2.1.1.Effect of the equation of state on the production of toxic gases
The equation of state(EOS)of gaseous detonation products plays a key role in determining the accuracy of the calculated detonation parameters and concentrations of detonation products[27,29,30].Therefore,we started this study with the analysis of the effects of EOS on calculated detonation parameters of ANFO explosives.In the analysis,we studied two EOS incorporated in EXPLO5;Becker-Kistiakowsky-Wilson(BKW)[23,31]and EXP-6 equations of state[30].BKW EOS is a semi-empirical,while EXP-6 is a theoretically grounded EOS,based on the Buckingham α-exponential-6 equation and an analytical representation of the excess thermodynamic functions for a classical fluid mixture.The interaction potential parameters in EXP-6 EOS for CO,NO,NO2,and N2O are taken from Refs.[32,33],which we found most accurately describe the detonation behavior of a series of ideal explosives[30].Values of covolumes in BKW EOS of these gases are taken from Refs.[21,27,29]and slightly modified to reproduce concentrations calculated by EXP-6 EOS(Table 1).For other detonation products,parameters from the built-in database in EXPLO5 database are used[21,30].
EXP-6 EOS predicts lower detonation velocity and pressure of ANFO than BKW EOS(by about 6.5% and 17%,respectively).Both EOSes give almost the same concentrations of detonation products(Table 2).In further calculations,we used BKW EOS since it predicts detonation velocity and pressure closer to experimental values reported in the literature[34].The covolumes of NO,NO2,and N2O were modified to reproduce the concentration of these products calculated using theoretically based EXP-6 EOS(Table 2).In this way,we obtained the covolume of NO as 340Å,which is slightly smaller compared to 394Åreported in Refs.[27,29].A lower covolume of NO results in a larger calculated concentration of NO;for example,k=340Ågives 0.035 mol/kg while k=394Ågives 0.021 mol/kg(40% difference).Similarly,we obtained a smaller covolume(k=520Å)for NO2,compared to literature(626Å[27]and 594Å[29]).It follows from Table 2 that the main products of ANFO 94/6(H2O,N2,and CO2)make up 98.3% of the total moles(volume)of gas products,while all other products,including toxic gases,make up only 1.7%.
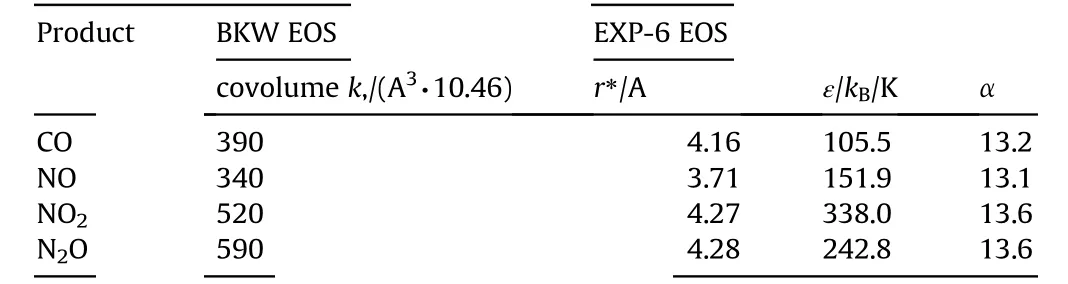
Table 1 Parameters of toxic gases used in BKW and EXP-6 EOS.
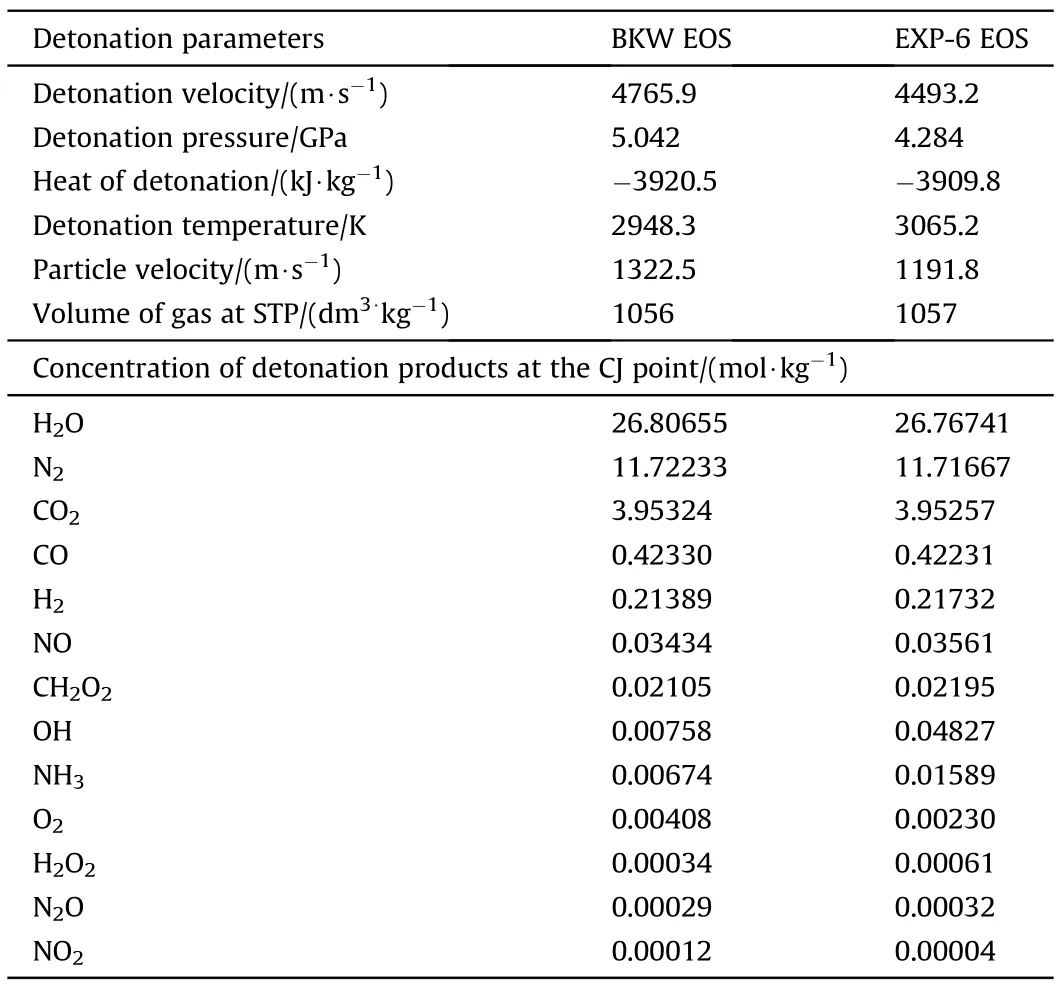
Table 2 Calculated detonation parameters and concentration of detonation products for ANFO(94/6,0.8 g/cm3).
From the expansion isentrope of detonation products(Fig.1)with the freeze-out temperature set to 2250 K,it is clear that the concentrations of CO,NO,CH2O2,and NO2decrease in the early stages of the expansion,while CO2and N2concentrations slightly increase.This indicates that CO oxidizes to CO2,while NO reduces to N2and O2.This agrees with the analysis of Chaiken et al.[16]for high-temperature low-pressure expansion of combustion products.The freeze-out temperature of 2250 K was determined in previous work[28]as the temperature which best reproduces the experimental concentrations of detonation products for a series of explosives[15].
Unlike Chaiken et al.[16],who stated that CO concentration is frozen at 50% of its initial concentration at 2665 K,we found that the decrease in CO concentration at a freeze-out temperature of 2250 K changes with ANFO oxygen balance(OB).For OB<0,decrease of CO concentration is 15-25%,for OB≈0%about 5%,and about 90% at OB=12%.For highly negative OB explosives,CO concentration can even increase during the isentropic expansion due to the oxidation of solid carbon.Thus,the assumption that COconcentrations at the freeze-out temperature roughly correspond to that at the C-J point seems valid for ANFO with OB≈0%.The assumption is also valid for emulsion explosives whose detonation temperature of about 2200 K is below freeze-out temperature.
The concentration of NO at the freeze-out temperature is about 5%of its initial value at the C-J point.Chaiken et al.[16]stated that NO concentration is frozen at 2665 K(at p=27.1 bar).Similarly,Mainiero et al.[6],also claimed that NO concentration does not change during the expansion,unless there is an interaction with oxygen from the surrounding atmosphere(on contact with the air,NO slowly oxidizes to NO2).Our calculation results given in Table 2 show that NO concentration at the C-J point roughly corresponds to that measured,which indicates that NO concentration for ANFO is frozen at the C-J point(at 2950 K).
2.2.Analysis of literature reported measured concentrations of toxic gases
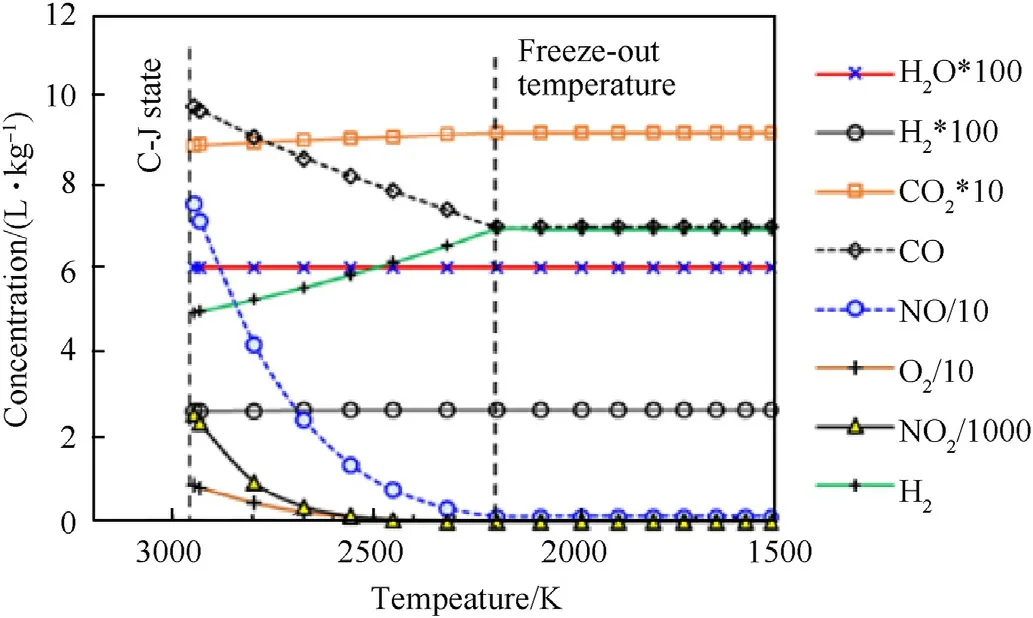
Fig.1.Calculated concentrations of detonation products along expansion isentrope of ANFO 94/6.
The measured concentrations of toxic gases for ANFO and emulsion explosives are collected from different literature sources(Table 3 and Table 4).Since these results were obtained under different experimental conditions,some key informations on experimental conditions are also indicated.The purpose of the comparison is to highlight the differences in the results from different authors and to get a mean value of NOxand CO concentrations which could serve as validation of calculation results.
Even though the comparison of measured concentrations of toxic gases is difficult due to different experimental conditions,several facts can be elucidated from the comparison.First,there is a large difference in measured NOxand CO concentrations reported by different authors,especially for NO and NO2concentrations.The prilled ANFO measured concentrations are fairly uniform-CO concentrations ranging from 13.2 to 19.7 L/kg and from 4.7 to 13.1 L/kg for NOx.However,for pulverized ANFO,NOxconcentration is more than an order of magnitude lower than for prilled(0.1-1.35 L/kg),which indicates an effect of AN characteristics on the production of toxic fumes.Concentrations of CO for emulsion explosives are slightly more scattered(from 5.3 to 21.4 L/kg,with a mean value of 11.2 L/kg)but still relatively consistent,while NOxconcentrations are significantly different(0.04-2.4 L/kg,with a mean value of 1.04 L/kg).A comparison of the reported minimum and maximum concentrations shows that CO concentrations differ by a factor of approximately 1.5 times for prilled ANFO and a factor of 4 times for emulsion explosives,while NOxconcentrations differ by a factor of 3 times for prilled ANFO and 60 times for emulsion explosives.
Secondly,it should be added that in the cases where the products are too diluted(large chamber volume,small sample mass)the accuracy of measurement may be affected.This is especially true for NO and NO2,which are present in small amounts,about 0.08% of the total volume of gases.
Large scattering of experimental results is usually attributed to differences in experimental conditions,however,our analysis shows that even in the cases where very similar experimental conditions are used,the difference between reported measured concentrations for CO can go up to 1.7 times,and up to 7 times for NO concentrations,while differences in NO2concentration may exceed 100 times.In our opinion,one of the most important reason behind these lathe discrepancies may be the reproducibility of post-detonation reactions,in particular mixing and oxidation of CO and NO with atmospheric oxygen.
2.3.Comparison of measured and calculated concentrations of toxic gases
The concentration of toxic gases generated by the detonation of ANFO and emulsion explosives is calculated based on models described previously:
·Ideal detonation model(IDM):based on the C-J detonation theory and assumption that measured NOxand CO concentrations correspond to those in the C-J state
·Deflagration model(DM1):assumes explosive deflagrates under constant volume conditions without interaction with the atmosphere,and calculated concentrations at the combustion temperature corresponds to measured concentrations
·Deflagration model(DM2):a modification of DM1 which assumes detonation products react with a fraction of the surrounding atmosphere at the combustion temperature.
Although IDM seems to be the logical choice when calculating the concentration of toxic gases(since an explosive charge is initiated to detonate),deflagration models(DM1 and DM2)have a background in Barnhart's(in Onederra et al.[7])and Ornellas'research[15].According to Barnhart,the deflagration model is better suited to describe the increased production of NOxin cases of lower detonation velocities(when the explosive behaves more non-ideally and reactions last a longer time).According to Ornellas,the products from the detonation of unconfined charges and from deflagration in the blasting chamber are comparable since in both cases they equilibrate under conditions of high temperature and lower pressure,i.e.,the concentration of the product does not correspond to those on the expansion isentrope.Therefore,unconfined charges produce more CO,H2,and NO than those confined.In the case of heavily confined charges,a large fraction of released energy converts to the kinetic and internal energy of confinement,which largely reduces reverberation of shock from the chamber walls.Consequently,detonation products from heavily confined charges correspond to those found on the expansion isentrope,in the region between C-J temperature and freeze-out temperature.
The calculations for ANFO were performed for two formulations:a generic formulation containing 94% AN and 6% fuel oil(ANFO1)and a formulation with 5.7% of fuel oil and an oxygen balance closer to zero(ANFO2).For emulsion explosives,three typical formulations are used:one AN-based emulsion and two AN and SN-based emulsions.The results of the calculation are given in Table 5 and Table 6.DM1 and DM2 calculations are done assuming a chamber volume of 15 m3and mass of explosive of 1 kg,i.e.,loading density is 1/15 kg/m3.The amount of atmospheric air that participate in reactions is set to be 10% of the total mass.
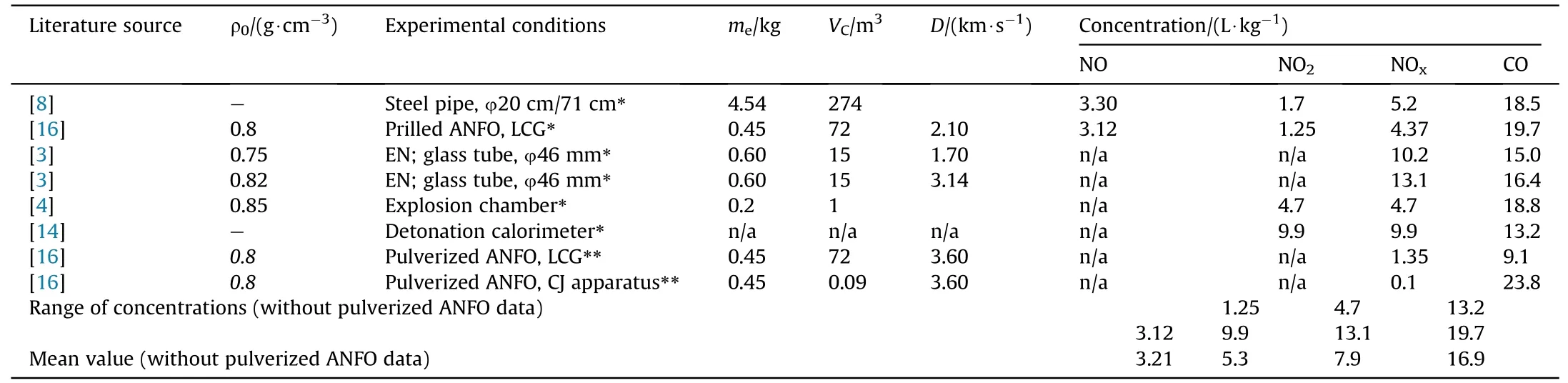
Table 3 Literature reported measured NOx and CO concentrations for ANFO explosive.
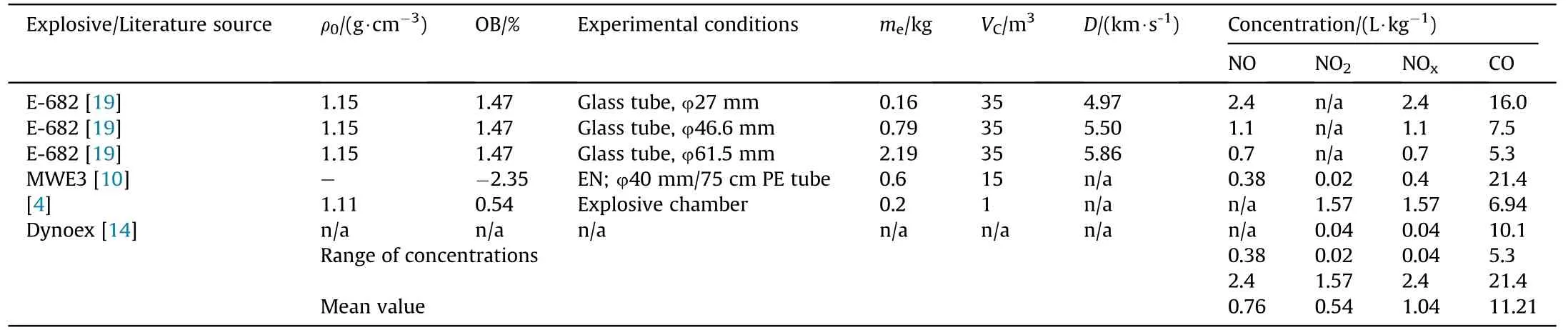
Table 4 Literature reported measured NOx and CO concentrations for emulsion explosive.
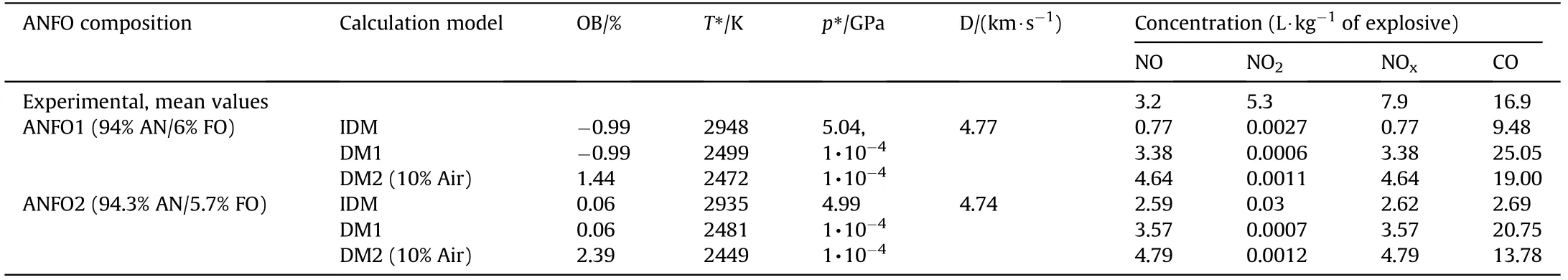
Table 5 Calculated NOx and CO concentrations for ANFO explosives with two different amounts of fuel oil.
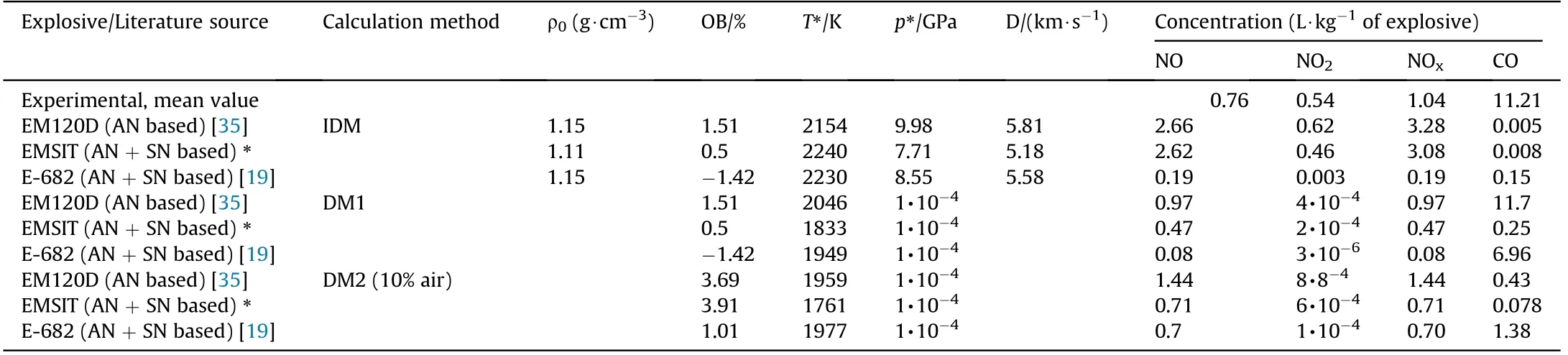
Table 6 Calculated NOx and CO concentrations for four emulsion explosives having different compositions.
Several observations can be drawn from the comparison ofmeasured and calculated concentrations.The ideal detonation model(IDM)predicts twice less CO comparing to the measured mean value for ANFO1(OB=-0.99%)and much less NOx.On the contrary,for ANFO2(OB=0.06%)the concentration of NO is quite accurately predicted,but CO is severely underestimated.Such results indicate high sensitivity of the calculations to variation in composition-a 0.3% change of fuel oil content causes changes of CO and NOxconcentration by a factor of 3.5.This issue will be discussed in greater detail in the next section.The deflagration model(DM1)predicts CO concentration(25.5 L/kg for ANFO1 and 20.75 L/kg for ANFO2)and NO concentration(3.38 and 3.57 L/kg,respectively)quite accurately.DM2 results in slightly more NO and less CO than DM1,which is a consequence of the reaction between a fraction of oxygen from air with combustion products.Also,it follows from the calculation that deflagration models are not as sensitive to compositional variations as the IDM.
The findings are similar when it comes to emulsion explosives.IDM and DM2 predict NO concentration reasonably well but underestimate the CO concentration.On the other hand,DM1 predicts CO more accurately(except for EMSIT).Generally,emulsion explosives produce less NO and CO than ANFO.From a thermochemical point of view,there are two possible reasons for this.Firstly,emulsion explosives have approximately 25% less nitrogen per kg of explosive than ANFO.Secondly,the detonation(and combustion)temperature of emulsion explosives is about 800 K lower than for ANFO(our thermochemical calculation shows that lower temperature favors the formation of CO2and N2).Unlike NO and CO concentrations,NO2concentrations cannot be predicted correctly by the models presented.DM1 and DM2 predict a negligible amount of NO2,while IDM is more accurate but still highly underestimates NO2concentration.This may be explained by the fact that NO2is produced in slow post-detonation oxidation of NO(2NO+O2→2NO2)by atmospheric oxygen.
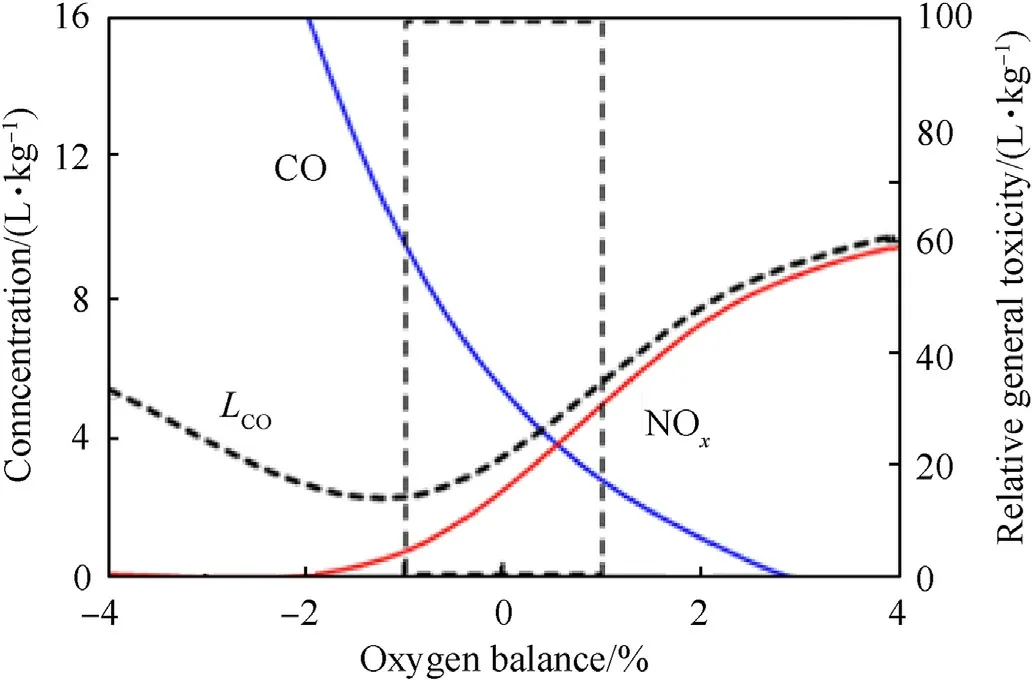
Fig.2.Effect of oxygen balance on calculated NOx and CO concentration for ANFO explosive(calculated by IDM).
The general conclusion is that DM predicts the concentration of toxic gases more accurately for both studied explosives than IDM.The difference between calculated and mean experimental values of NO and CO is still high;about 2 times for CO and 10 times for NO.However,bearing in mind that measured CO concentrations differ 1.5 times and NOxconcentration almost 3 times(Tables 2 and 3),the calculated results may be considered quite acceptable.The fact that DM1 predicts more accurately the concentration of toxic gases than IDM may be somewhat surprising,particularly for emulsion explosives.However,since most tests for the determination of the concentration of toxic gases use light confinement,deflagration models better reproduce measured concentrations.This agrees with Ornellas’previously mentioned research.
2.4.Effect of oxygen balance on the production of toxic gases
It is clear from the previous section that oxygen balance,i.e.,fuel oil content in ANFO explosives,plays an important role in the production of toxic gases.Calculations by IDM showed that a variation in fuel oil content in ANFO from 6% to 5.4%,results in a change in OB of nearly 2%.This in turn results in a change of CO concentration from 9.48 L/kg to 2.69 L/kg and NOxfrom 0.77 to 2.62 L/kg(Fig.2).As OB increases,NOxconcentration increases while CO concentration decreases.According to Zawadzka-Małota[10],minimum relative general toxicity(LCO=(CO+6.5NOx))in obtained at slightly negative OB.
Fig.3 shows the calculated CO and NOxconcentrations for ANFO explosive at different fuel oil fuel oil contents,compared against the experimental data reported in the literature.All three calculation models give good agreement with the experimental CO data as well as the trends observed with the change of fuel oil content.Close to zero oxygen balance(at FO≈5.7%),CO concentration sharply increases with the increase of FO.In the region of fuel-lean formulations,CO concentration is low compared to measured values.Similarly,IDM predicts a sharp change of NOxconcentration at FO≈5.7%,while,in the fuel-rich region,NOxconcentrations tend to zero,which does not agree with experimental data.Both DM1 and DM2 do not predict a sharp change of NOxand CO near-zero oxygen balance.Generally,these two models more accurately predict CO and NOxconcentrations in a broader region of FO(from 2% to 9%)than IDM.
As mentioned above,the DM2 calculations assume that a fraction of air that fills the explosion chamber takes part in a reaction at the combustion temperature.We put this fraction to be 10% of air(regarding the mass of the mixture of explosive and air)because it gives the best reproduction of experimental NO and CO concentrations for ANFO explosives.However,from Fig.4 it follows that for emulsion explosives,this amount is lower.
The calculation shows that CO concentration decreases with an increase of air(due to its oxidation to CO2)for both ANFO and emulsion.The concentration of NO increases to a maximum value of about 40%of air for ANFO and 30%for emulsion explosives(i.e.,at OB≈8-10%).The concentration of NO2remains very low but increases with the amount of oxygen.Higher amount of air involved in reactions will give higher NOxconcentrations than measured,but underestimate CO concentrations.The amount of air that best reproduces experimental mean values of CO and NOxconcentrations is 10-15%for ANFO,and close to zero for emulsion explosives.The reason for this may be higher deflagration temperatures for ANFO(by about 500 K)and consequently faster reactions,as well as longer duration of reactions.
2.5.Effect of additives and wrappers on the production of toxic gases
The approach we have described enables an approximate prediction of the composition and concentrations of toxic(and other)detonation gases.It may be particularly useful in the analysis of the effect of explosive composition on the production of toxic gases(Fig.3).Fig.5 gives an example of the calculated effect of additives(water content)on NOxand CO production.
Calculations show that CO concentration increases slightly with an increase of water content,which is consistent with the experimental results of Rowland and Mainiero[8].However,contrary to experimental results(which showed an increase of NO concentration with water content),our calculation predicts a small decrease of NO concentration.Since oxygen balance does not change significantly with the addition of water(changing the water concentration from 0% to 10%,oxygen balance changes only 0.1%),the main cause of the decrease of CO and NO concentrations may be attributed to a decrease in the detonation temperature with the addition of water(about 400 K for studied range).Lower temperatures favor the formation of CO2and N2,while higher temperatures result in more CO and NO.
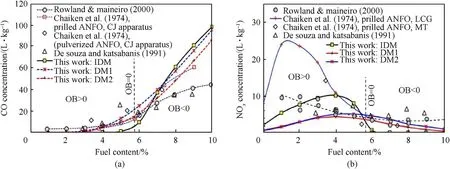
Fig.3.Comparison of calculated and measured(a)CO and(b)NOx concentrations for ANFO with different fuel oil content.
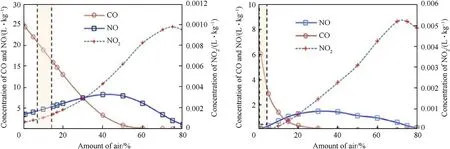
Fig.4.Change of NOx and CO concentration with the amount of air that takes place in reaction with a)ANFO 94/6 and b)E-682(calculated by DM2).
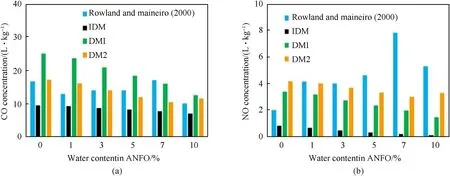
Fig.5.Effect of water content in ANFO 94/6 on(a)CO and(b)NO concentrations.
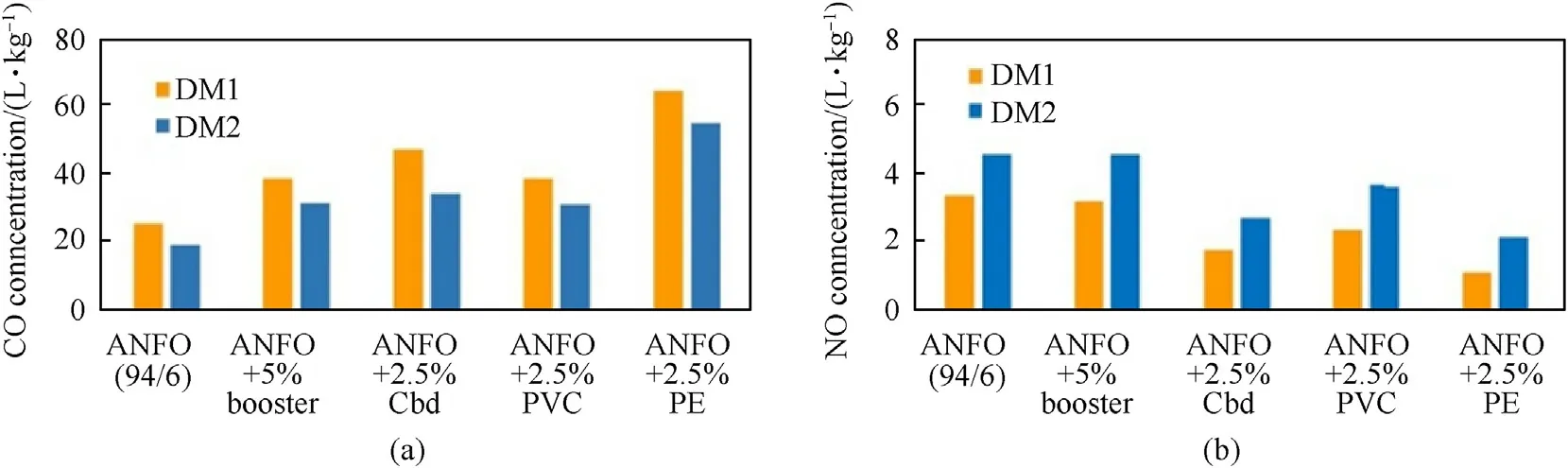
Fig.6.Calculated effect of booster and wrapper material on(a)CO and(b)NO concentrations(Legend:PVC-polyvinyl-chloride,PE-polyethylene,cardboard-70%lignin and 30%cellulose;Booster-pentolite booster).
The calculated effect of wrapper material on the concentration of CO and NO is illustrated in Fig.6.The calculation was performed using DM1 and DM2 and assuming the wrapper reacts with the explosive at the combustion temperature.A small amount of wrapper material(2.5% relative to the weight of explosive charge)can double CO concentration.All wrappers studied result in a decrease of OB of explosive,particularly PE.This causes an increase in CO concentration(almost 3 times)and a decrease in NO(about 2 times).This analysis shows a large sensitivity of CO and NOxconcentration to the presence and type of wrapper and confinement used.
3.Conclusions
We explored the possibility of theoretical prediction of the concentration of toxic gases by thermochemical equilibrium calculations by applying two models:ideal detonation model and deflagration model.Validation of the calculations is done by comparing calculated and reported measured concentrations of NOxand CO in the literature.
It was demonstrated that the calculation results can approximate measured concentrations quite well and reproduce the experimentally observed effect of fuel oil(i.e.,oxygen balance)on the production of toxic gases.The ideal detonation model(IDM),which assumes that the calculated concentration of the products at the C-J state corresponds to those measured experimentally,yields NOxconcentrations that agree reasonably well with the measured data,but significantly underestimates measured CO and NO2concentrations.The IDM was found to be very sensitive to explosive composition,so a small variation in the composition may result in a significant change in CO and NOxconcentrations.
Deflagration models,which assume that the explosive deflagrates under constant volume conditions without interaction with the oxygen from the air(DM1)or that only a fraction of the oxygen takes part in reactions(DM2),predict CO and NOxconcentrations for both ANFO and emulsion explosives more accurately than IDM model.This agrees with Barnhart's assumption that deflagration seems to be a more plausible mechanism in the case of lower detonation velocity,i.e.,longer duration of reactions.This is particularly true for ANFO(a highly non-ideal explosive)in which a significant fraction of unreacted explosive deflagrates during the expansion of the detonation products.In addition,findings of this work agree with Ornellas'detonation calorimetry results which showed that the concentration of detonation products from bare explosive charges(or charges with light confinement)is comparable to the concentration of deflagration products.On the other hand,the concentration of detonation products from heavily confined explosive charges should correspond to those at the freeze-out temperature on the expansion isentrope.
One of the biggest challenges when comparing calculated and measured concentrations lies in the fact that the calculation predicts the concentrations at the C-J point or at the freeze-out temperature on the expansion isentrope(IDM model)and at the combustion temperature(DM model).Meanwhile,the measured concentrations include post-detonation reactions such as oxidation of CO to CO2and NO to NO2by atmospheric oxygen.Under such conditions,the degree of oxidation is non-reproducible,and this is why some authors[16]even suggested that the best that can be done in any form of toxic fume test would be to accurately measure the maximum concentration of CO,before oxidation by the atmospheric air.This is the state at which the current thermochemical calculations can elucidate.
Nonetheless,with all the above-mentioned limitations,thermochemical calculations remain useful in approximating the measured concentrations of toxic gases,and they are especially relevant in compositional analysis-for example for prediction of effects of additives,wrappers,etc.on the production of toxic gases.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was supported by the Croatian Science Foundation(HRZZ),Croatia,under the projects IP-2019-04-1618“NEIDEMO”.
杂志排行
Defence Technology的其它文章
- Influence of local stiffeners and cutout shapes on the vibration and stability characteristics of quasi-isotropic laminates under hygrothermo-mechanical loadings
- General design principle of artillery for firing accuracy
- Data-driven prediction of plate velocities and plate deformation of explosive reactive armor
- Modeling of unsupervised knowledge graph of events based on mutual information among neighbor domains and sparse representation
- Analysis of the effect of bore centerline on projectile exit conditions in small arms
- Adaptive sliding mode control of modular self-reconfigurable spacecraft with time-delay estimation
