Saponins from Sea Cucumber Residues Protect against Cyclophosphamide-Induced Immunosuppression in Mice via the Nucleotide Binding and Oligomerization Domain-Like Receptor Signaling Pathway
2022-11-30ZHANGLingDONGJingyuanCHENYongdeLIQianWUTaoLIURuiSUIWenjieZHANGMin
ZHANG Ling, DONG Jingyuan, CHEN Yongde, LI Qian, WU Tao,*, LIU Rui, SUI Wenjie, ZHANG Min,3
(1. State Key Laboratory of Food Nutrition and Safety, College of Food Science and Engineering, Tianjin University of Science &Technology, Tianjin 300457, China; 2. Bestlife Biological Technology Co., Ltd., Tangshan 118300, China;3. China-Russia Agricultural Processing Joint Laboratory, Tianjin Agricultural University, Tianjin 300392, China)
Abstract: Sea cucumber is a traditional Chinese tonic with various bioactivities. This study systematically investigated the immunomodulatory effect of saponins extracted from sea cucumber residues (SCRSS) at doses of 2.5, 5.0 and 10.0 mg/(kg mb·d) on immunosuppressive mice. The results showed that SCRSS at a dose of 5.0 mg/(kg mb·d) increased the body mass, spleen index, and thymus index by 15.3%, 23.8%, and 39.8%, respectively, compared to the model control group, and it also significantly increased the phagocytic index in immunosuppressive mice (P < 0.01). Moreover, All the doses of SCRSS remarkably increased serum cytokine levels, promoted the proliferation activity of splenic lymphocytes and peritoneal macrophages. Importantly, the middle dose (5.0 mg/(kg mb·d)) of SCRSS was more effective to upregulate the expression of nucleotide binding and oligomerization domain (NOD)1, NOD2, receptor interacting protein 2 (RIP2)and nuclear factor (NF)-κB in immunosuppressive mice. Thus, SCRSS may be used as a functional food supplement to improve immunity.
Keywords: sea cucumber residues; saponins; cyclophosphamide; immunomodulatory activity; nucleotide binding and oligomerization domain-like receptor
The immune system is a tight defense system that intelligently recognizes and eliminates foreign pathogens.It plays an irreplaceable role in maintaining normal physiological functions and health[1-2]. However, it is reported that the prevalence of immune-related chronic diseases is increasing due to the interaction of various factors.Many individuals have low immune functions. Currently,immunopotentiating agents (such as levamisole,BacillusCalmette-Guerin) are widely used to correct immune deficiency and enhance immunity[3-4]. However, these drugs generally cause a series of side effects, such as nausea,abdominal pain, and anaphylactic shock[1]. In recent years,active ingredients derived from marine resources have attracted attention due to their low side effect and toxicity.
Sea cucumber belongs to the phylum Echinodermata and is a marine invertebrate from the class Holothuroidea[5]. It is a traditional food ingredient as well as a valuable medicine[6].Sea cucumber has various bioactive substances, including amino acids, polysaccharides, saponins, collagen peptides and lipids[5]. These active substances play an essential role in regulating immune functions. For example, sulfated fucan AL1-1 from sea cucumberAcaudinaleucoproctacould enhance the immunomodulatory activity in RAW264.7 cells.Meanwhile,in vivoexperiments showed that AL1-1 reversed the immunosuppression by promoting the phagocytosis of macrophages and the secretion of cytokines and secretory immunoglobulin A (sIgA)[7]. Small molecule oligopeptides from sea cucumber enhanced the activity of RAW264.7 macrophages by upregulating the nuclear factor (NF)-κB and mitogen-activated protein kinase (MAPK) signaling pathways[8]. In addition, the immunomodulatory effect of sea cucumber oligopeptides may also be related to natural killer(NK) cells activation, cytokines secretion and the stimulation of Th cells[9].
In recent years, the sea cucumber body wall has been widely used to produce sea cucumber peptides due to their high edible and medicinal value. The residues are often discarded after enzymatic hydrolysis and extraction of peptides. However, the remaining residues are rich in various active components, such as polysaccharides and saponins.Saponins are the primary secondary metabolites and chemical defense substances secreted by the body wall of sea cucumber and Cuvierian organs[5]. They are triterpene glycosides that are composed of aglycones and carbohydrate chains.At present, the triterpenoid moieties found in the research are holostane-type with an 18(20)-lactone[10]. Studies have confirmed that sea cucumber saponins have significant health benefits, including hemolysis, anti-fungal, anti-tumor, antiobesity and improvement of non-alcoholic fatty liver[5,11-12].For instance, sea cucumber saponins could improve obesity by inhibiting lipid synthesis as well as activating the lipolysis in white adipose tissue[12]. Saponin-rich fractions of sea cucumberApostichopus japonicusexhibited anti-tumor activity by inhibiting the proliferation activity of HL-60 and B16F10 cells in a dose-dependent manner[11].
The current research on sea cucumber residues mainly focuses on the separation and purification of active ingredients and the development of sea cucumber peptiderelated products. In contrast, the immunomodulatory activity of saponins extracted from sea cucumber residues was still unknown. In the present study, we are aimed to investigate the immunomodulatory effect of saponins extracted from sea cucumber residues on cyclophosphamide (CTX)-induced mice.
1 Materials and Methods
1.1 Animals, materials and reagents
Male C57BL/6J mice (4-week-old, body mass(18 ± 2) g) were purchased from SPF (Beijing) Biotechnology Co., Ltd. with the production license number of SCXK(Jing) 2019-0010 and kept in Barrier System of Animal Laboratory in Tianjin University of Science and Technology with the permit number of SYXK(Jin)2018-0001. Sea cucumber residues were prepared and provided by Bestlife Biological Technology Co., Ltd.
CTX, concanavalin A (Con A), lipopolysaccharide(LPS), dimethyl sulfoxide (DMSO) and red blood cell lysate Beijing Solarbio Science & Technology Co., Ltd.;fetal bovine serum and Roswell Park Memorial Institute(RPMI)-1640 medium USA Hyclone Co.; enzyme linked immunosorbent assay (ELISA) kits of sIgA, interleukin(IL)-1β, IL-6, IL-18 and tumor necrosis factor (TNF)-α Wuhan Servicebio Technology Co., Ltd.; bicinchoninic acid(BCA) protein assay kits Nanjing Jiancheng Bioengineering Institute; All other chemical reagents used in the experiments were analytical grade;β-actin, nucleotide binding and oligomerization domain (NOD)1, NOD2, activator protein 1(AP-1), inhibitor kappa B kinase α/β (IKKα/β), NF-κB, p38,receptor interacting protein 2 (RIP2), transforming growth factor kinase 1 (TAK1) and secondary antibody Beijing Bioss Technology Co., Ltd.
1.2 Instruments and equipment
DH-101 oven Tianjin Zhonghuan Experimental Electric Furnace Co., Ltd.; MODULYOD-203 freeze dryer and ST40R refrigerated centrifuge USA Thermo Fisher Technology Co., Ltd.; LC-20A high performance liquid chromatography Japan Shimadzu Corporation;RE-52AA rotary evaporator Shanghai Yarong Biochemical Instrument Factory; Rt2100c enzyme label detector Shenzhen Lei Du Life Science Co., Ltd.; MX-F vortex mixer,TSY-B decoloring shaker and KZ-II homogenizer Wuhan Servicebio Technology Co., Ltd.
1.3 Methods
1.3.1 Experimental design
All experimental procedures were approved by the Committee on the Ethics of Animal Experiments of Tianjin University of Science and Technology (TUST20200908) and conformed to the Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0-309-15396-4.Sixty male C57BL/6J mice were housed under standard environmental conditions (temperature is (25 ± 2) ℃, relative humidity is (55 ± 10)%, and 12 h/12 h light/dark cycle).
After adaptive feeding for one week, the mice were weighed and randomly divided into five groups (n= 12)according to body mass. The groups were normal control group (NC group), model control group (MC group), SCRSS-treated groups (2.5, 5.0 and 10.0 mg/(kgmb·d)). The mice in the NC group and the MC group were orally administrated with 0.2 mL of physiological saline. The SCRSS-treated group was orally administrated with an equal volume of SCRSS at 2.5, 5.0 and 10.0 mg/(kgmb·d) for 14 days,respectively. Except for the NC group, the other groups were intraperitoneally injected with 80 mg/kgmbCTX on the 8thto 10thday to establish the immunosuppressive mice model. Twenty-four hours after the last administration, the mice were weighed. Each mouse was injected with 10%Indian ink at a dose of 0.1 mL/10 g through the tail vein,and 20 µL of blood samples were collected from the retroorbital vein plexus 2 min (t1) and 10 min (t2). Blood samples were collected from eyeballs of mice and centrifuged at 1 000 ×gfor 20 min at 4 ℃. The serum was immediately stored at –80 ℃. Subsequently, the mice were sacrificed by dislocation, and spleen and thymus of each animal were immediately removed, weighed and stored at –80 ℃[13]. In addition, intestinal samples were collected from the distal 5 cm posterior to the cecum of sacrificed mice.
1.3.2 Calculation of spleen and thymus indices
As previously reported, spleen and thymus indices were calculated according to formula (1)[14].
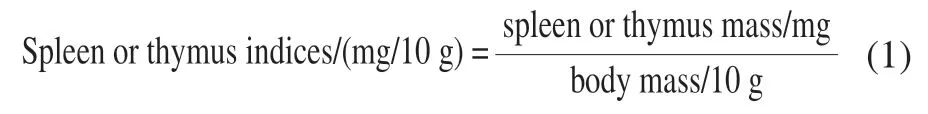
1.3.3 Determination of the phagocytic capacity of macrophages
The 20 µL blood sample obtained in section 1.3.1,which was collected from the retro-orbital vein plexus 2 min (t1) and 10 min (t2), respectively, was mixed with 2 mL of 0.1% Na2CO3solution. The absorbance at 600 nm was determined[14]. Subsequently, the carbon clearance index (K)and phagocytic index were calculated as formula (2) and (3),respectively.
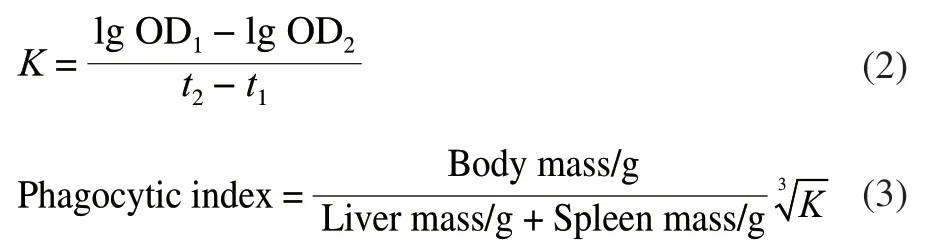
Where OD1was the absorbance value att1, and OD2was the absorbance value att2.
1.3.4 Histopathological analysis of spleen
Spleen tissues with 5 µm thick were cut and stained with hematoxylin and eosin (HE) as previously described[15].Briefly, the samples were fixed in 10% formaldehyde solution for 12 h, then rinsed with phosphate buffered saline (PBS)(0.1 mol/L, pH 7.4, the same as follows) and stained with HE.Finally, the sections were observed under a light microscope.
1.3.5 Peritoneal macrophages proliferation assay
The sacrificed mice were immersed in 75% (V/V)alcohol. After 30 s, 10 mL of sterile PBS was injected into the abdominal cavity of mice, and the abdomen was massaged for 1–2 min. Subsequently, ascites fluid was drawn into a centrifuge tube with a sterile syringe, centrifuged at 1 000 ×gfor 5 min at 4 ℃. The precipitation was collected and mixed with 2 mL PBS. The cell was suspended in RPMI-1640 complete medium, seeded in 96-well plates at a density of 2 × 106cell/mL and incubated for 48 h (37 ℃, 5%CO2). Afterward, 20 µL of methylthiazol tetrazolium (MTT)solution (5 mg/mL) was added to each well and incubated for 4 h. The supernatants were discarded, and 150 µL DMSO was added to each well. Finally, the absorbance of each well was measured at 570 nm on a microplate reader after shaking rapidly in a horizontal vibrator[16].
1.3.6 Splenic lymphocyte proliferation assay
LPS and Con A were used as foreign antigens to induce T and B lymphocytes, respectively. The proliferation activity of splenic lymphocytes was measured by the MTT experiment[14]. In brief, the spleen obtained in section 1.3.1 was placed in cold PBS, gently pressed through a 200 mesh cell sieve and filtered through nylon mesh to obtain the cell suspension. The suspension was mixed with the red blood cell lysate and centrifuged at 1 000 ×gfor 5 min to remove red blood cells. Then, the cells were resuspended in RPMI-1640 medium and centrifuged. The supernatant fluid was discarded. The cells were transferred to a cell culture flask and incubated for 2 h. Finally, the adherent cells were removed, and the unattached cells suspension were collected as splenic lymphocytes. The cell concentration of splenocytes was adjusted to 5 × 107cells/mL in RPMI-1640 medium and plated in a 96-well plate (100 µL/well). The control group with Con A(5 µg mL) or LPS (5 µg/mL) and the control group without Con A and LPS were set for each group. They were incubated in a 5% CO2incubator for 48 h at 37 ℃. Then, 20 µL MTT(5 mg/mL) was added to each well and the plate was incubated for 4 h. The supernatant was discarded and 100 µL DMSO was added to each well and shaken for 10 min.Finally, the absorbance of each well was determined using a microplate reader at 570 nm.
1.3.7 Determination of secretory immunoglobulin A in the intestine
The intestine of sacrificed mice obtained in section 1.3.1 was homogenized in 1 mL PBS. The homogenized solution was centrifuged at 3 000 ×gfor 15 min at 4 ℃ to remove particulate matter. Subsequently, the supernatant was collected, and the level of sIgA was measured using an ELISA kit according to the manufacturer’s instructions.
1.3.8 Analysis of the levels of cytokines in serum
The levels of IL-1β, IL-6, IL-18 and TNF-α in serum obtained in section 1.3.1 were measured using commercial ELISA kits.
1.3.9 Western blot assay
The relative expression of immune proteins in spleen tissues was analyzed as described by Li Qian et al[14]. Briefly,the spleens of mice in each group were washed with cold PBS, cut into small pieces, and placed in homogenate tubes.Ten volumes of RIPA lysate were added to different tubes and homogenized for 2 min. Subsequently, tubes were placed in an ice bath for 30 min, and centrifuged at 12 000 ×gfor 10 min at 4 ℃. Proteins were detected by the BCA protein assay kits, separated by 12% (m/V) sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE), and transferred to polyvinylidene fluoride (PVDF) membranes.The membranes were blocked with 5% (m/V) skim milk(prepared in 0.5% TBST) at 37 ℃ for 1 h, then incubated with the primary antibody (1:1 000) for 12 h at 4 ℃.Primary antibodies includedβ-actin, NOD1, NOD2, AP-1,IKKα/β, NF-κB, p38, RIP2 and TAK1. After washing with TBST, the membranes were incubated with secondary antibodies (1:3 000) for 30 min at room temperature. The membranes were rewashed three times in TBS and were detected by an efficient chemiluminescence (ELC) kit. Bands were selected to perform densitometry quantification using Image J software.
1.4 Statistical analysis
All results were expressed as mean ± standard deviation (SD).The data were analyzed by the one-way analysis of variance(ANOVA) with Duncan’s multiple-range test using SPSS 24.0 software at the level of 0.05. Data graphs were drawn using Origin 7.0 software.
2 Results and Analysis
2.1 Effect of SCRSS on body mass and organ indices
As shown in Table 1, there was no obvious difference in the initial body mass of mice in each group. After treatment with CTX for 3 days, the body mass of mice in the MC group((23.71 ± 0.89) g) was remarkably decreased compared to the NC group ((18.74 ± 0.59) g) (P< 0.01). Notably, the final body mass of mice administrated SCRSS at 5.0 and 10.0 mg/(kgmb·d) had an increase by 15.3% and 14.4%relative to the MC group mice, respectively (P< 0.01).However, there was no significant difference in final body mass between the MC group and the low dose of the 2.5 mg/(kgmb·d) SCRSS group (P> 0.05). Comprehensively,these results indicated that SCRSS could effectively promote body mass gain in immunosuppressed mice, especially at the dose of 5.0 mg/(kgmb·d).
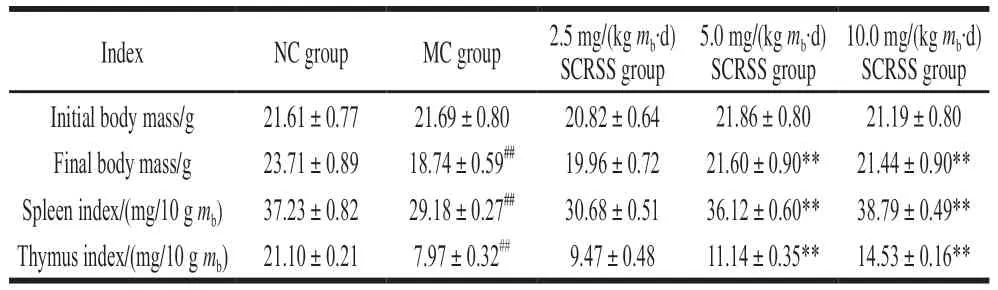
Table 1 Effect of SCRSS on body mass and visceral organ indices of CTX-induced immunosuppressed mice
The spleen and thymus are the main immune organs of the human body, so their indices are important indicators for evaluating the immune status of the human body[17].After giving CTX for 3 days, the spleen and thymus indices in CTX-treated mice were sharply decreased to(29.18 ± 0.27) mg/10 gmband (7.97 ± 0.32) mg/10 gmbfrom(37.23 ± 0.82) mg/10 gmband (21.10 ± 0.21) mg/10 gmbof the NC group mice, respectively (P< 0.01, Table 1),indicating that the immunosuppression model was successfully established[18]. Compared with the MC group,the spleen index of mice received SCRSS at 5.0 and 10.0 mg/(kgmb·d) was significantly increased by 23.8%and 32.9%, and the thymus index was increased by 39.8%and 82.3%, respectively (P< 0.01, Table 1). Although treated with SCRSS at the dose of 2.5 mg/(kgmb·d) led to a slight increase in organ indices, no statistical difference was observed.
2.2 Effect of SCRSS on phagocytic capacity of macrophages
The phagocytic capacity of mononuclear macrophages is the leading indicator for evaluating non-specific immunity in the body. In this study, we examined the phagocytic index to indirectly reflect the effect of SCRSS on the phagocytic capacity of macrophages[1]. As depicted in Table 2, a significant decrease in the phagocytic index of the MC group was observed compared with the NC group(P< 0.01). However, the middle and high dosage of SCRSS could effectively increase the phagocytic index compared with the MC group (P< 0.01), demonstrating SCRSS could improve the function of macrophages in immunosuppressed mice and promote non-specific immunity.
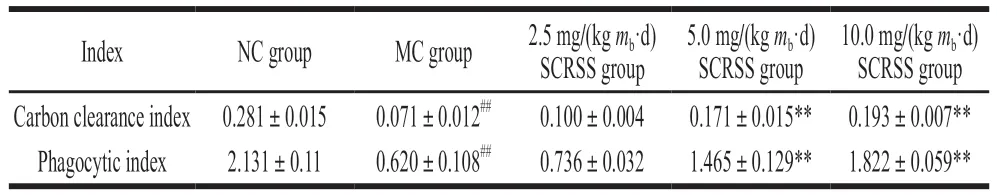
Table 2 Effect of SCRSS on carbon clearance index and phagocytic index of CTX-induced immunosuppressed mice
2.3 Histopathological analysis
Studies have shown that when the immune system is damaged, it often causes damage to related organs[19].To further evaluate the effect of SCRSS on the spleen ultrastructure, the histological morphology of the spleen stained with HE was studied. As shown in Fig. 1, in the NC group, the ultrastructure of spleen cells was dense and orderly arranged, and the interstitial space between them was small. In contrast, the spleen cells were scattered, and the intercellular space and necrotic areas were expanded in the MC group. Encouragingly, after SCRSS intervention,the spleen cells were compact and arranged well with clear nucleolus and less necrotic areas. In particular, the mice were treated with 5.0 mg/(kgmb·d) SCRSS showed similar spleen morphology as the NC group, which suggested that SCRSS have anobvious improvement effect on the damage of spleen in CTX-induced mice.
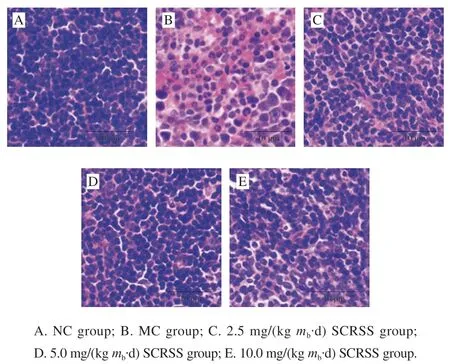
Fig. 1 Effect of SCRSS on morphological changes in the spleen of CTX-induced immunosuppressed mice (× 400)
2.4 Effect of SCRSS on the proliferation activity of macrophages
Macrophages are crucial objects for exploring cellular phagocytosis, cellular immunity, and molecular immunology.They are also essential bridges between innate immunity and adaptive immunity[20]. To further evaluate the immune function of SCRSS, the MTT method was used to detect the proliferation activity of peritoneal macrophages in mice. As illustrated in Fig. 2A, a significant decrease was observed in the proliferation activity of macrophages in the MC group(0.178), as compared to the NC group (0.679) (P< 0.05).Treatment with SCRSS at 5.0 and 10.0 mg/(kgmb·d)for 14 days, the proliferation activity of macrophages was significantly improved, especially at the dose of 5.0 mg/(kgmb·d) (P< 0.05). Similar to other indicators, there was no significant difference in the proliferation activity of macrophages between the MC group and the low dose of the SCRSS group (2.5 mg/(kgmb·d)).
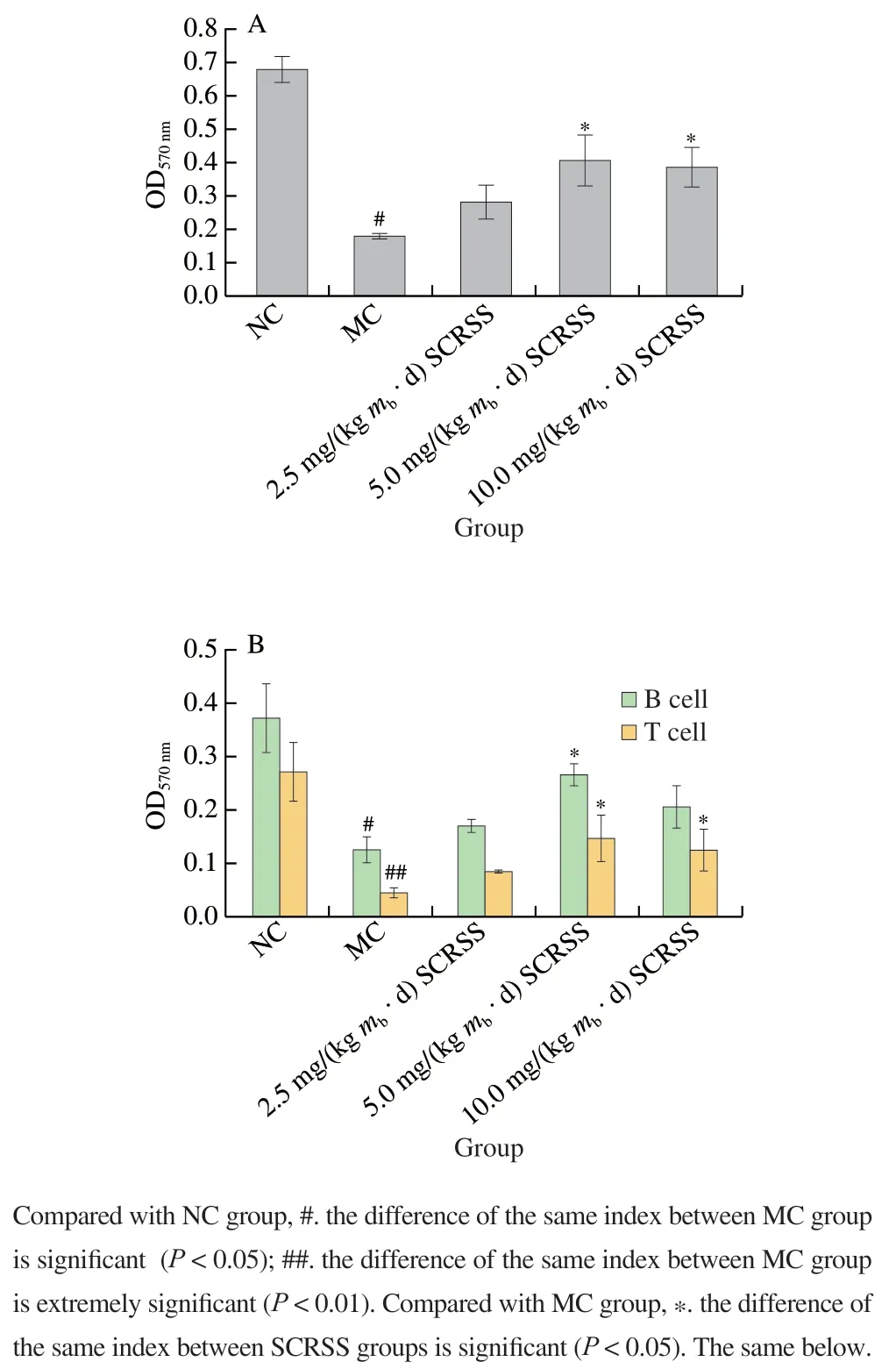
Fig. 2 SCRSS promoted the proliferation activity of macrophages (A)and splenic lymphocytes (B)
2.5 Effect of SCRSS on the proliferation of splenic lymphocytes
The spleen is a vital organ for T and B lymphocytes to generate immune responses. It activates lymphocytes to secrete various cytokines and is closely related to cellular immunity and humoral immunity[21]. In this study, Con A and LPS were used as foreign antigens to induce T and B lymphocytes, respectively.
The proliferation of T (0.089 versus 0.293) and B lymphocytes (0.162 versus 0.383) in the MC group was noticeably decreased compared to the NC group (P< 0.01,P< 0.05) (Fig. 2B). Treatment with different doses of SCRSS increased the proliferation activity of T and B lymphocytes in CTX-treated mice. In particular, the proliferation of T lymphocytes in mice administrated SCRSS at the doses of 5.0 and 10.0 mg/(kgmb·d) remarkably increased to 0.181 and 0.162 from 0.089 of the MC group mice,respectively (P< 0.05), whereas the proliferation of B lymphocytes was extremely improved in mice received SCRSS at 5.0 mg/(kgmb·d) relative to the MC group (0.289 versus 0.162) (P< 0.05). Results confirmed that SCRSS has a certain therapeutic effect on the proliferation of splenic lymphocytes in immunosuppressed mice, especially at the dose of 5.0 mg/(kgmb·d).
2.6 Effect of SCRSS on the content of sIgA in the intestine
As the main protective molecule of intestinal acquired immunity, sIgA plays a crucial role in the intestinal mucosal defense against the adhesion and colonization of pathogenic bacteria and enteric toxins[22-23]. To further investigate the effect of saponins on mucosal acquired immunity, the content of sIgA in the intestines of different groups was examined.As shown in Fig. 3A, the content of sIgA was 30.0% lower in mice of the MC group (0.247) than that in mice of the NC group (0.353) (P< 0.01). However, administration of SCRSS at 2.5, 5.0, and 10.0 mg/(kgmb·d) significantly increased the content of sIgA by 27.3%, 32.6%, and 18.4%, respectively(P< 0.01). These results indicated that SCRSS could improve the content of sIgA in the intestinal, thereby regulating intestinal mucosal immunity and reducing pathogen infection.
2.7 Effect of SCRSS on inflammation parameters in serum
Cytokines are crucial signal molecules for regulating the immune response through differentiation, maturation and activation[24]. The levels of four common serum cytokines were determined by ELISA kits to investigate the effect of saponins on humoral immunity. According to results (Figs. 3B–E),compared to the NC group, the levels of IL-1β,IL-6, IL-18, and TNF-α in serum of immunosuppressed mice were significantly decreased by 38.6%, 30.6%, 27.6%(P< 0.01), and 7.19% (P< 0.05), respectively. At the same time, treatment with different doses of SCRSS promoted the recovery of the levels of serum factors. Notably, at the tested dosages of 5.0 mg/(kgmb·d) SCRSS, the levels of IL-1β, IL-6 and IL-18 were remarkably increased by 55.4%, 46.2% and 44.8% (P< 0.01), respectively, whereas the level of TNF-α had an extremely increase by 46.2% in the 10.0 mg/(kgmb·d)SCRSS group (P< 0.01).
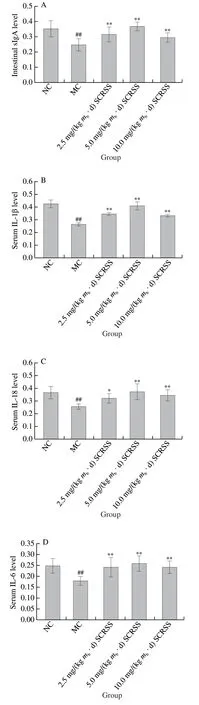
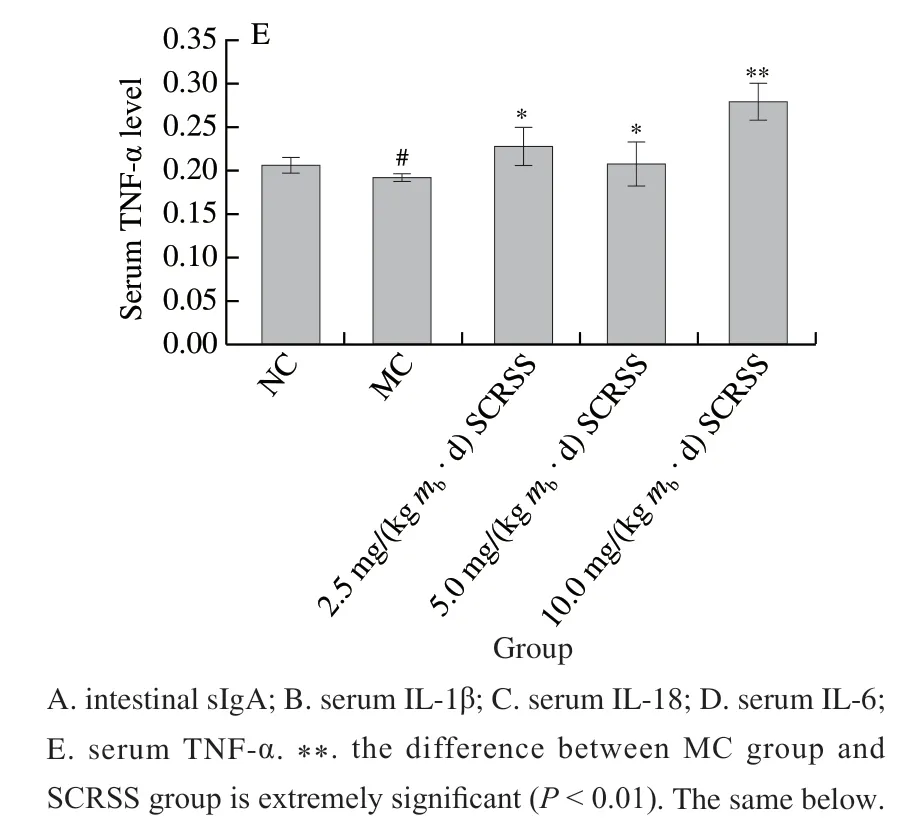
Fig. 3 SCRSS changed the levels of intestinal sIgA and serum cytokines
2.8 Effect of SCRSS on protein expression of spleen
To further investigate the possible protective mechanism of SCRSS in CTX-induced mice, we determined the protein expression of the spleen by Western bolt analysis. We combined the gray value to characterize each band. It can be seen from Fig. 4 that CTX significantly down-regulated the expression levels of NOD1 and NOD2, as compared to the NC group, respectively (0.16 versus 0.56, 0.15 versus 0.55)(P< 0.01), whereas SCRSS at 2.5 and 5.0 mg/(kgmb·d)effectively almost recovered them to the normal levels.
According to Fig. 5, the expression levels of NF-κB,AP-1, p38, RIP2,TAK1, and IKKα/β in the MC group were remarkably down-regulatedcompared with those in NC group(P< 0.01). However, the expression levels of the above proteins obviously increased in mice treated with different doses of saponins, especially at the dose of 5.0 mg/(kgmb·d),indicating that SCRSS can regulate immunity through the NOD-like receptor signaling pathway. Significantly,compared with MC group, SCRSS activated the downstream signaling cascade by up-regulating the expression level of NOD1 and NOD2 (P< 0.01), resulting in the up-regulation of RIP2, IKKα/β, TAK1, NF-κB, p38, and AP-1 nucleation(P< 0.01), thereby enhancing immunity.
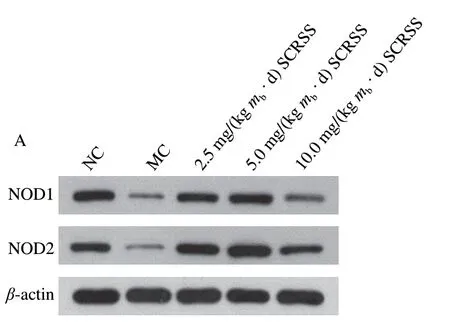
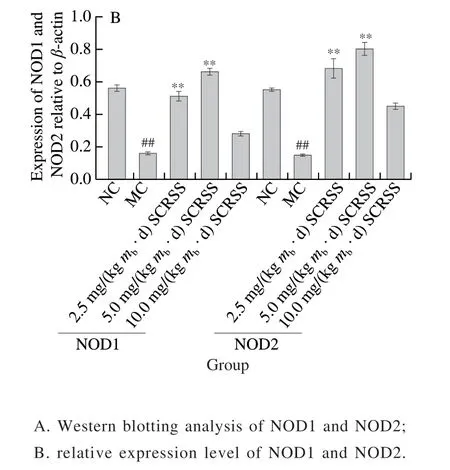
Fig. 4 SCRSS up-regulated the expression of NOD1 and NOD2 in spleen
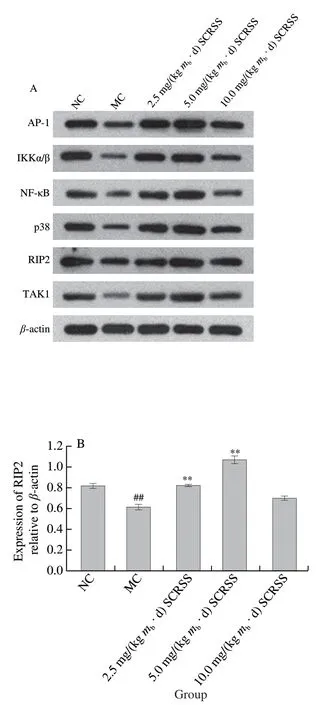
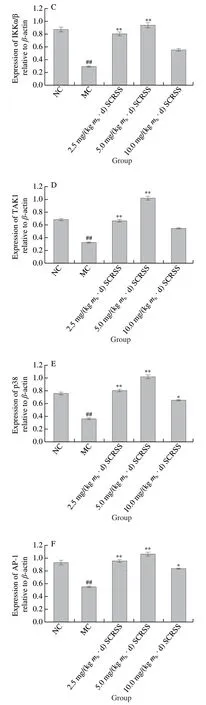
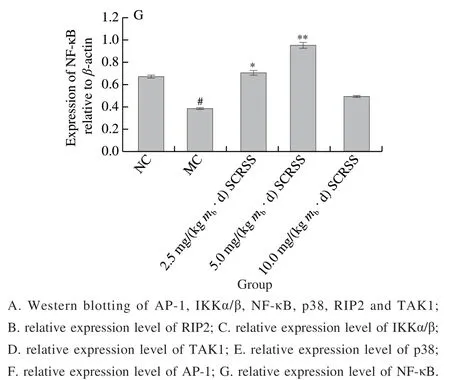
Fig. 5 SCRSS up-regulated the relative expression levels of RIP2,IKKα/β, TAK1, p38, AP-1 and NF-κB in spleen cells
3 Discussion
CTX is a chemotherapeutic agent widely used in treating organ transplantation, cancer and autoimmune diseases, but it can cause a series of side effects such as immunosuppression,oxidative stress, myelosuppression and cytotoxicity[13,25-26].CTX can inhibit hematological parameters by interfering with DNA synthesis and affecting immune cells and hematopoietic cells[27]. It can also lead to overall immunological dysfunction by affecting the intestinal mucosal barrier and inhibiting the production of splenocyte cytokines[28-29]. Due to the toxicity of CTX, the immunosuppressive model induced by CTX has been widely used to explore the regulatory effects of natural products on immune responses[29]. Therefore, in this study,we evaluated the immunomodulatory effects of SCRSS by establishing CTX-induced immunosuppressive mice model.
The spleen and thymus are the primary immune organs of mammals, and they participate in the process of humoral immunity and cellular immunity[19]. The change in the immune organ indices is closely related to the levels of immunity moderation[24]. In this study, the organ indices of the MC group were remarkably lower than the NC group.However, SCRSS at different concentrations exhibited improvement of spleen and thymus indices compared to the MC group. Liu Xiao et al.[30]demonstrated that ginsenoside Rg3 could increase the weight of the spleen and thymus to resist immunosuppression. Based on the above results,SCRSS may restore immune function by regulating the development of immune organs and repairing the damage of immune organs.
Macrophages are immune effector cells derived from monocytes and spread throughout bodily tissues[15].Outstanding phagocytic activity promotes macrophages to become a crucial line of defense for the body to eliminate pathogens and resist the invasion of foreign microorganisms[31]. In addition, macrophages are involved in the secretion of cytokines and T cell activation[19]. A previous study has reported thatPanax ginsengenhances the phagocytosis of macrophages by increasing the phagocytic index and rate[32]. Consistent with the above results, this study showed that SCRSS could promote the proliferation of macrophages and enhance their phagocytic ability. These results indicated that SCRSS could enhance cellular immunity by regulating the function of macrophages.
Lymphocytes can be divided into T lymphocytes and B lymphocytes[33]. T lymphocytes induced by Con A can directly participate in the cellular immune process and play a crucial role in anti-cancer, anti-tumor, and delayed hypersensitivity reactions. In contrast, B lymphocytes induced by LPS can differentiate into plasma cells after being stimulated by antigens. Plasma cells further secrete or synthesize antibodies, and finally perform the function of humoral immunity[33]. Therefore, the proliferation activity of splenic lymphocytes is widely used to assess immunity. At the dose of 5.0 and 10.0 mg/(kgmb· d), SCRSS remarkably promoted the proliferation activity of splenic lymphocytes.Similarly, Bhardwaj et al.[34]found that tea saponin regulates immune response by inducing the proliferation of T lymphocytes, increasing the levels of IL-1 and TNF-α, and downregulating the level of IL-10. In addition, it was reported that some interleukins (such as IL-6 and IL-1β) could mediate cellular immune function and promote the proliferation and differentiation of B/T cells[35]. In agreement with these results, SCRSS also increased the levels of IL-6 and IL-1β.Therefore, SCRSS may enhance the immune activity of immunosuppressive mice by activating lymphocytes affected by increased cytokines.
Immunoglobulin A can be divided into serotypes and secretory immunoglobulins. The former can mediate antibody dependent cell-mediated cytotoxicity (ADCC), while the latter was the main component of the mucosal defense system and the first line of defense to prevent pathogens from invading[23]. sIgA is secreted by intestinal plasma cells and transported to the mucosa through polymeric immunoglobulin receptor (pIgR)[36]. Cytokines can affect the transcription of pIgR[37]. For example, TNF-α and IL-1β can upregulate the transcription and expression of pIgR by activating the NF-κB pathway[37-38]. In the present study, CTX decreased the levels of sIgA and cytokines such as TNF-α and IL-1β in mice.However, SCRSS treatment reversed the immunosuppression induced by CTX. Therefore, SCRSS may indirectly regulate the secretion of sIgA by increasing the levels of cytokines.Further research is needed to explain the mechanism of cytokines promoting sIgA secretion.
Cytokines are essential targets of immune regulation and maintain the body’s immune homeostasis by cooperating with immune cells[33]. For example, TNF-α, produced by activated monocytes and macrophages, participates in the regulation of immune response and inflammatory[19]. IL-18,a pro-inflammatory cytokine, can promote the proliferation and activation of natural killer cells and T lymphocytes[39].Similarly, IL-6 and IL-1β play an important role in inducing the proliferation of T cells and defending against viruses and microbial infections[35,40]. In the current work, the intervention of different doses of SCRSS increased the levels of IL-1β,IL-6, IL-18, and TNF-α in serum. This is consistent with the research results of Bhardwaj et al.[34]and Top et al.[41].These results suggested that the improving effect of SCRSS on immunosuppression may be partly due to enhanced cytokine secretion.
NOD1 and NOD2 are the prominent members of the nucleotide-binding and oligomerization domain-like receptor (NLR) family, and are also intracellular pattern recognition receptors (PRRs)[42-43]. They play a vital role in innate immunity through synergy. They respectively recognize theγ-D-glutamyl-meso-diaminopimelic acid (iEDAP) and muramyl dipeptide (MDP) of the invading bacteria and are activated[42]. RIP2, ubiquitinated by the E3 ligase, is activated and binds to NOD1 or NOD2 through the caspase recruitment domain (CARD)-CARD interactions[44-45].Subsequently, TAK1 was recruited and the IKK complex was activated, which triggers the activation of NF-κB pathway and MAPK pathway. This promoted immune cells to secrete pro-inflammatory cytokines[42,44-46]. In this study, SCRSS activated the downstream signaling cascade by upregulating the expression level of NOD1 and NOD2. RIP2 was recruited through the homotypic interactions between CARD of NOD and RIP2, which resulted in the upregulation of the levels of IKKα/β, TAK1, NF-κB, p38, and AP-1, thereby promoting the release of IL-6, IL-18, IL-1β, and TNF-α (Fig. 6). These findings suggested that SCRSS enhanced the immune function of mice by regulating the NOD-like receptor signaling pathway.
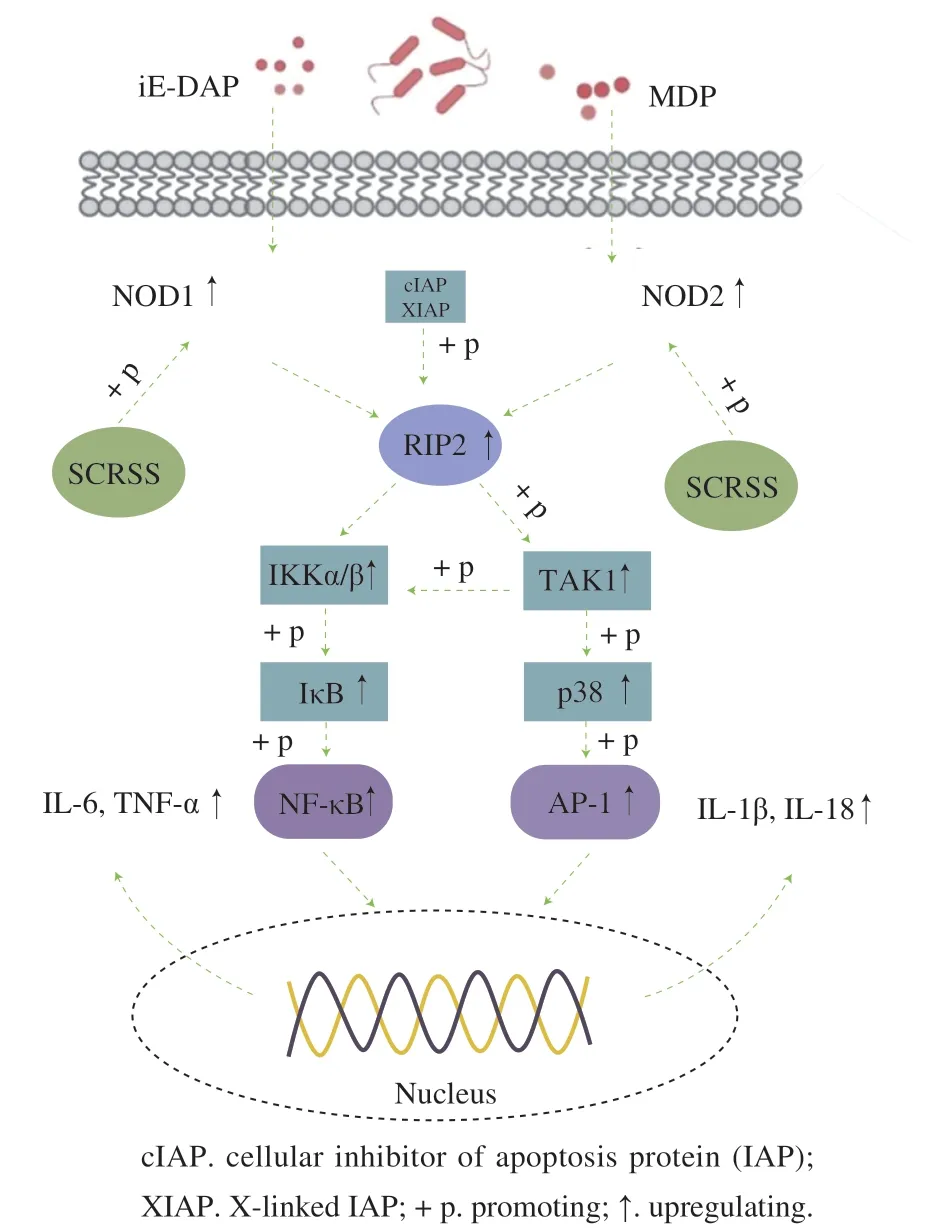
Fig. 6 SCRSS regulated immune activity through the NOD-like receptor signaling pathway
4 Conclusion
In summary, SCRSS could reverse immunosuppression by promoting the development of immune organs, activating the immune response of splenic lymphocytes, enhancing the function of macrophages, and regulating the levels of intestinal sIgA and cytokines. Meanwhile, SCRSS could regulate the expression of levels of NOD1, NOD2, RIP2,IKKα/β, TAK1, NF-κB, p38, and AP-1, thereby promoting the secretion of IL-1β, IL-6, IL-18, and TNF-α. Therefore,SCRSS has potential to be a novel immunomodulatory candidate, and may provide considerable evidence for finding nutritional targets based on immunosuppression.
