Role of defensins in diabetic wound healing
2022-11-24ZhiXiangTanRuiTaoSiChengLiBingZhengShenLanXiaMengZhanYongZhu
Zhi-Xiang Tan,Rui Tao,Si-Cheng Li,Bing-Zheng Shen,Lan-Xia Meng,Zhan-Yong Zhu
Zhi-Xiang Tan,Rui Tao,Si-Cheng Li,Zhan-Yong Zhu,Department of Plastic Surgery,Renmin Hospital of Wuhan University,Wuhan 430060,Hubei Province,China
Bing-Zheng Shen,Department of Pharmacy,Renmin Hospital of Wuhan University,Wuhan 430060,Hubei Province,China
Lan-Xia Meng,Department of Neurology,Renmin Hospital of Wuhan University,Wuhan 430060,Hubei Province,China
Abstract The adverse consequences resulting from diabetes are often presented as severe complications.Diabetic wounds are one of the most commonly occurring complications in diabetes,and the control and treatment of this is costly.Due to a series of pathophysiological mechanisms,diabetic wounds remain in the inflammatory phase for a prolonged period of time,and face difficulty in entering the proliferative phase,thus leading to chronic non-healing wounds.The current consensus on the treatment of diabetic wounds is through multidisciplinary comprehensive management,however,standard wound treatment methods are still limited and therefore,more effective methods are required.In recent years,defensins have been found to play diverse roles in a variety of diseases;however,the molecular mechanisms underlying these activities are still largely unknown.Defensins can be constitutively or inductively produced in the skin,therefore,their local distribution is affected by the microenvironment of these diabetic wounds.Current evidence suggests that defensins are involved in the diabetic wound pathogenesis,and can potentially promote the early completion of each stage,thus making research on defensins a promising area for developing novel treatments for diabetic wounds.In this review,we describe the complex function of human defensins in the development of diabetic wounds,and suggest potential therapeutic benefits.
Key Words:Defensin;Diabetic wound;Wound healing;Inflammation;Re-epithelialization;Tissue regeneration
INTRODUCTION
Diabetes was estimated to affect over 536.6 million people worldwide in 2021,and an increase in prevalence occurs at a faster rate among middle-income countries[1].Diabetes mellitus gradually causes a series of complications,such as neuropathy,retinopathy,nephropathy,cardiovascular diseases,and diabetic wounds,as it develops.Due to lesions in the small blood vessels and peripheral nerves that are particularly prominent in the feet,diabetic wounds are usually presented as diabetic foot ulcers (DFUs),and are characterized by a delayed tissue growth and increased susceptibility to infection.Patients with DFUs have an increased risk of lower limb amputation,which has poor short-term prognosis associated with high mortality[2].Statistically,the five-year mortality and direct costs of care for patients with DFUs have been comparable to that of cancer[3].The large patient population and medical expenditure urgently requires an effective treatment method.
The mechanisms for diabetic wound development involve multifactorial etiologies,including a hyperglycemic microenvironment,abnormal host immune resistance,and neuropathy (Figure 1)[4].These mechanisms influence each other,instead of occurring independently,causing irreversible diabetic complications.The local damage to vessels and nerves,reduced growth factors expression,and lower collagen accumulation contribute to repeated outbreaks and the protracted course of diabetic wounds,leading to further infections.Although current treatments,including glycemic control,antiinfective treatment,and advanced dressing application,promote wound healing by regulating the local microenvironment,they also have disadvantages,such as protracted treatment periods,high costs,and occasionally inefficient results[5].Hence,studying methods that promote diabetic wound healing is ongoing.
With concern about antibiotic resistance growing more prominent,antimicrobial peptides (AMPs) have garnered attention as a new method of antibacterial therapy,including development of different formulation strategies for effective delivery to wounds,including AMPs loaded in nanoparticles,hydrogels,creams,gels,etc.As a representative AMP,the defensins properties are gradually being researched (Table 1)[6-23].Human defensins are divided into α-defensins and β-defensins[23].Human α-defensins mainly occur in neutrophils (human neutrophil peptide1-4,HNP1-4) or small intestinal Paneth cells (human defensin5-6,HD5-6).More extensively,31 human β-defensins (HBDs) have been described,and HBD1-4 is most widely-studied[24].Reportedly,the direct primary role of defensins is controlling microbial infections by killing bacteria and modulating the immune system.Moreover,defensins play different roles in different environments within the body,such as infected wounds,malignancy,atherosclerosis,pulmonary fibrosis,etc.In this review,we focused on investigating their mechanism of action in wound healing,especially chronic diabetic wounds.
Although little is known about defensins involvement in diabetic wounds,existing studies indicate that they play potential roles in complex pathophysiological changes of diabetic wounds[25,26].This review summarizes and analyzes known experimental data about the role of defensins in diabetic wound healing,particularly for inflammation,cell proliferation and migration,regeneration of blood vessels and nerves,and antibacterial activities.Research articles on the role of defensins in diabetic wounds,published between inception and September 10,2022,were collected from various search engines,such as PubMed,Google Scholar,Web of Science,and Science Direct using the following keywords: AMPs,defensins,host defense peptides,diabetic,refractory,and chronic wounds,wound healing,etc.Identified studies and relevant citations within these studies were reviewed.
MULTIFACTORIAL MECHANISM OF DEFENSINS DURING WOUND HEALING
The response to tissue injury involves multiple cellular and extracellular events,including inflammation,re-epithelialization,and angiogenesis,followed by fibroplasia with collagen synthesis,and tissue remodeling.Defensins may be a multifactorial modulator in the management of this process,which interferes in diabetic wounds (Table 2,Figure 2).
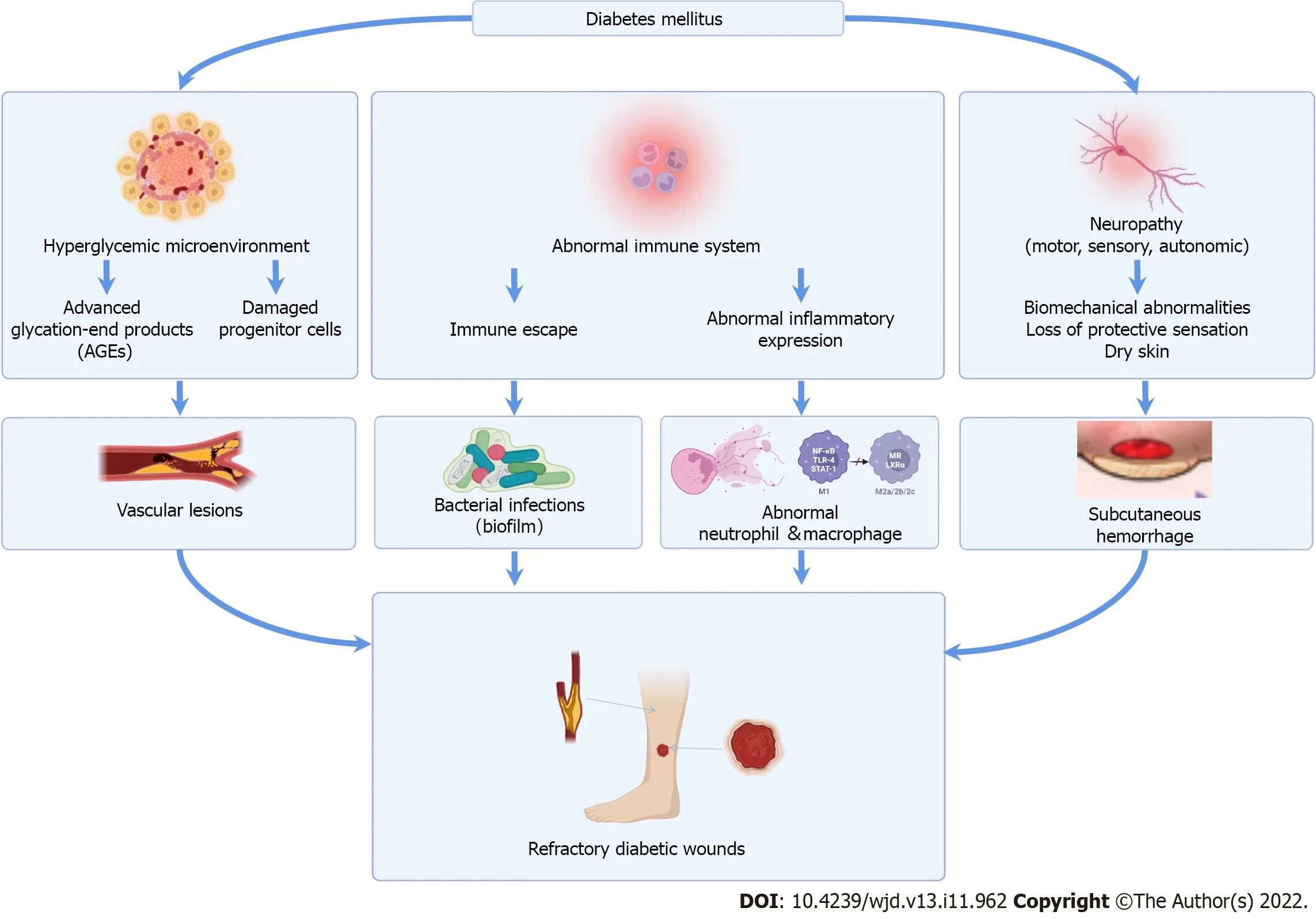
Figure 1 Mechanism of refractory diabetic wounds.The mainstream views include: Hyperglycemic microenvironment,abnormal immune system and neuropathy.Hyperglycemic microenvironment results in the complex formation of advanced glycation end products (AGEs) and cytokines,as well as circulating progenitor cell dysfunction.AGEs can significantly inhibit the proliferation of endothelial cells and alter the structure of collagen and elastin in the vascular wall,causing microvascular injury in the wound.The hallmarks of abnormal immune system are polymorphonuclear cell dysfunction,late neutrophil infiltration and suppressed macrophage polarization.As a result,diabetic wound healing is delayed and susceptible to bacterial infections and even biofilm formation.Neuropathy is occasion of subcutaneous hemorrhage,ultimately leads to skin breakdown.
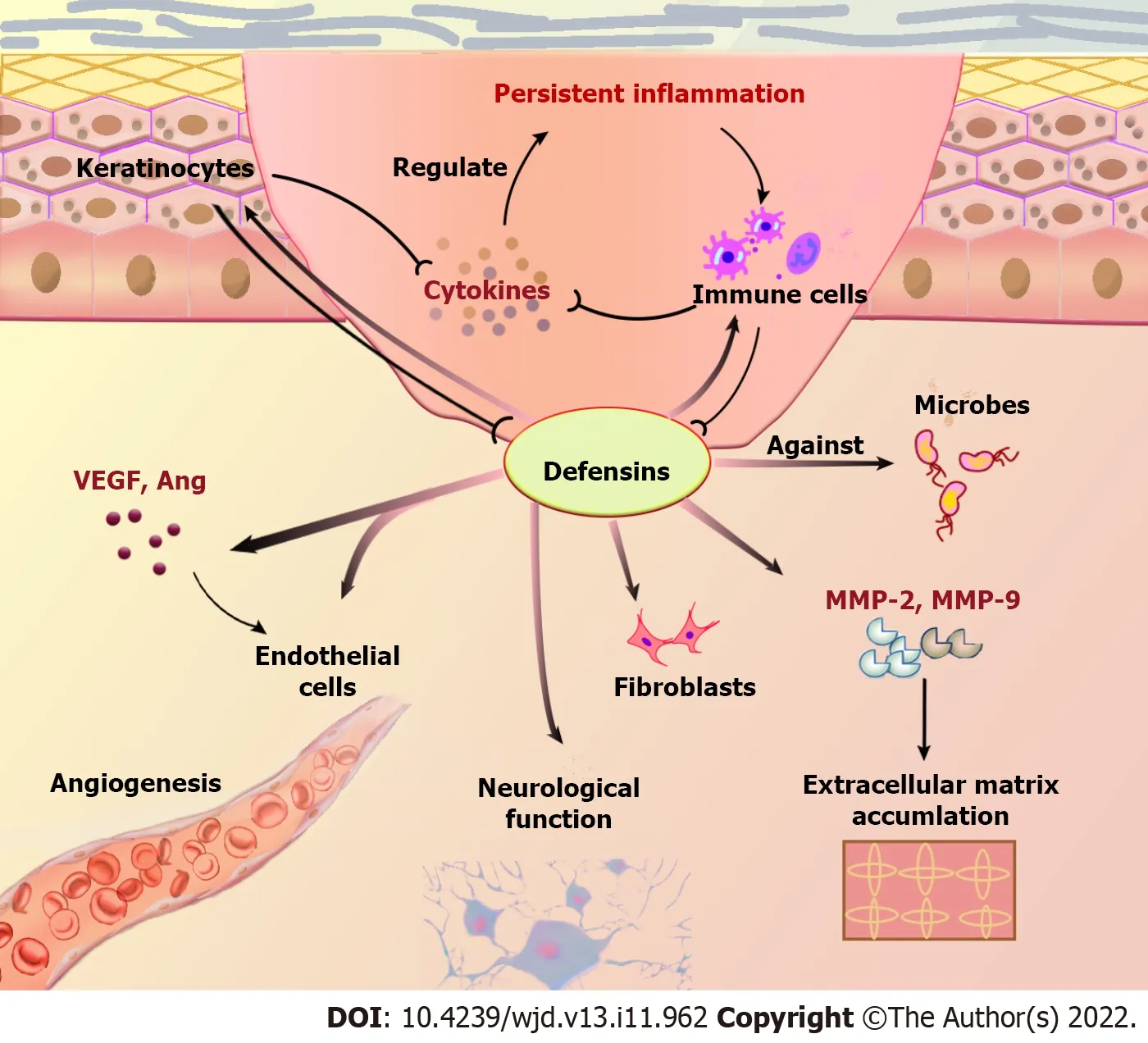
Figure 2 Role of defensins in diabetic wound healing.VEGF: Vascular endothelial growth factor;Ang: Angiogenin.
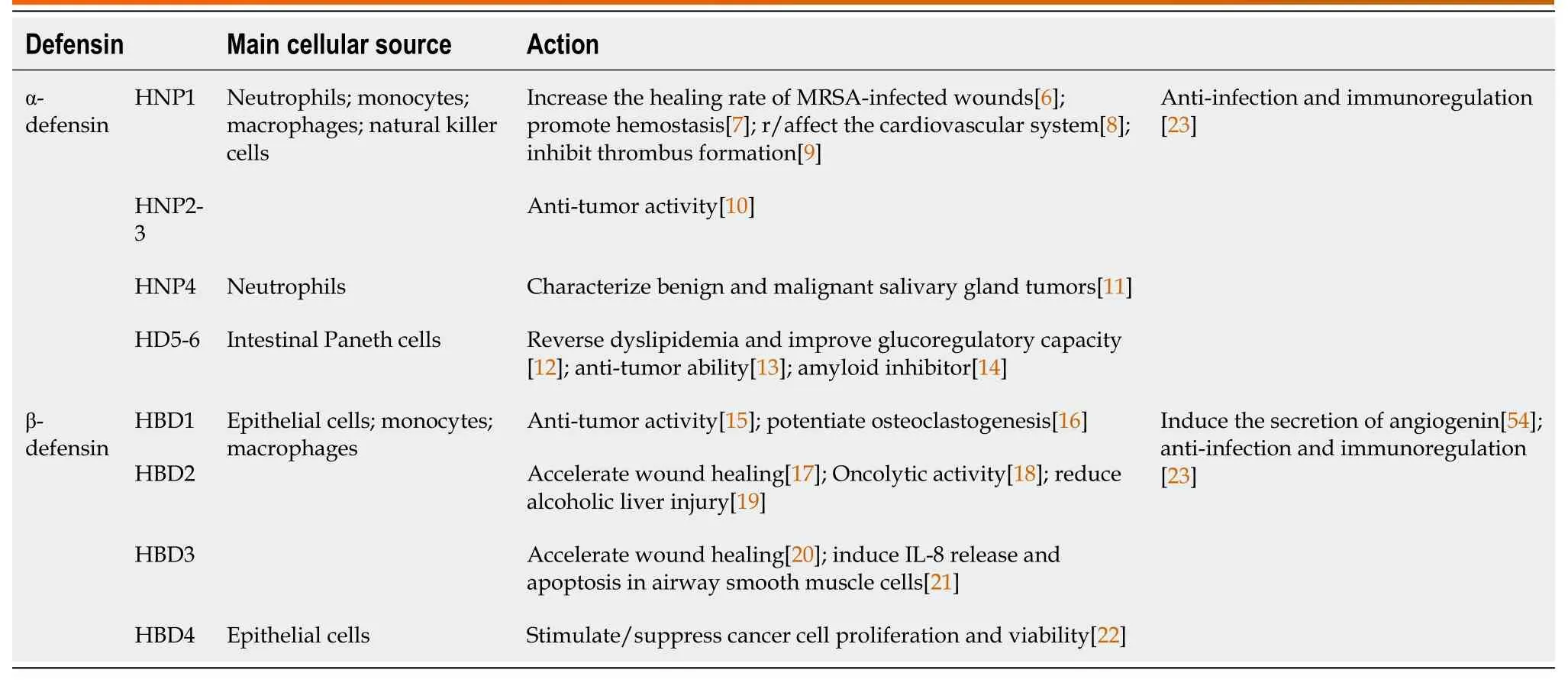
Table 1 Defensins play multiple roles in different diseases
Defensins triggered by inflammation
The first phase of wound healing is the inflammatory phase,characterized by platelet aggregation and leukocytes migration,including neutrophils and macrophages that secrete defensins and consequently clear the wound area[27].Studies suggest defensins promote recruitment and accumulation of leukocytes at inflammatory sites,and simultaneously release a series of chemokines[28,29].In diabetic wounds,the number of neutrophils increases abnormally and macrophage polarization is suppressed,leading to an excessive inflammatory expression[30,31].As defensins are released in response to inflammation from neutrophils and macrophages,which act as a signal to instigate recruitment of immune cells,a positive-feedback loop is created.HBDs can reportedly participate in degranulation of mast cells and induce secretion of proinflammatory factors by keratinocytesviathe p38 and ERK1/2 MAPK pathways activation[32,33].Through the same action sites,HNPs produce vasoactive by-products in endothelial cellsviaROS-dependent mechanisms,and stimulate the increased expression of IL-6 and IL-8 by activating p42/44 MAPK pathways[34,35].
In contrast,studies investigating associations between defensins and inflammatory mediators exhibited controversial results.HBDs have demonstrated an immunosuppressive effect by downregulating the TIR,TRAF-6,and NF-κB of TLR signaling pathways[36].Moreover,HBDs contribute to their anti-inflammatory ability by inducing M2 macrophage differentiation[37].Previous experiments established that HBDs can be beneficial in inflammatory diseases,such as periodontitis,considering its anti-inflammatory properties[38].A study on HNPs from dying neutrophils exhibited an immunosuppressive effect of the α-defensins that inhibited macrophage stimulation[39].The HNP1 “bipolar effect” represents the reduction of inflammatory responses with a physiological dose,enhanced expression of inflammatory factors with a high dose,and significant reductions in cell viability and interleukin-10 expression with increased concentration levels[40].Overall,defensins perform different functions under different conditions,including concentration levels[40].Defensins are often used as disease-related markers as dysregulation of their levels is caused by immune system disorders and effectors produced themselves or through associated cells[41].However,the causal relationship and sequence of cascades remain unclear.Several studies emphasized the relationship between delayed wound healing and uncontrolled inflammatory responses,and defensins as efficient adjustors playing a regulatory role in the process.
Defensins promote skin reconstruction
Failure to re-epithelialize is one of the most significant indicators for chronic wounds.Re-epithelialization is achieved through keratinocyte migration,proliferation,and differentiation.The HNP1,HBD2,HBD3,and HBD4 can reportedly induce proliferation and migration of keratinocytes,which can consequently secrete HBDs,thereby promoting reconstruction of the cellular barrier to accelerate wound healing[42,43].Subsequent studies reported that HBD3 enhances phosphorylation of the FGFR1/JAK2/STAT3 pathways to promote keratinocyte proliferation and migration[20].HBD1 potentially acts as a relevant transcription factor by protecting keratinocytes from apoptosis during epithelial reorganization[44].In other words,defensins have properties that promote wound epithelialization by affecting keratinocyte activity,and thus facilitating early wound closure.
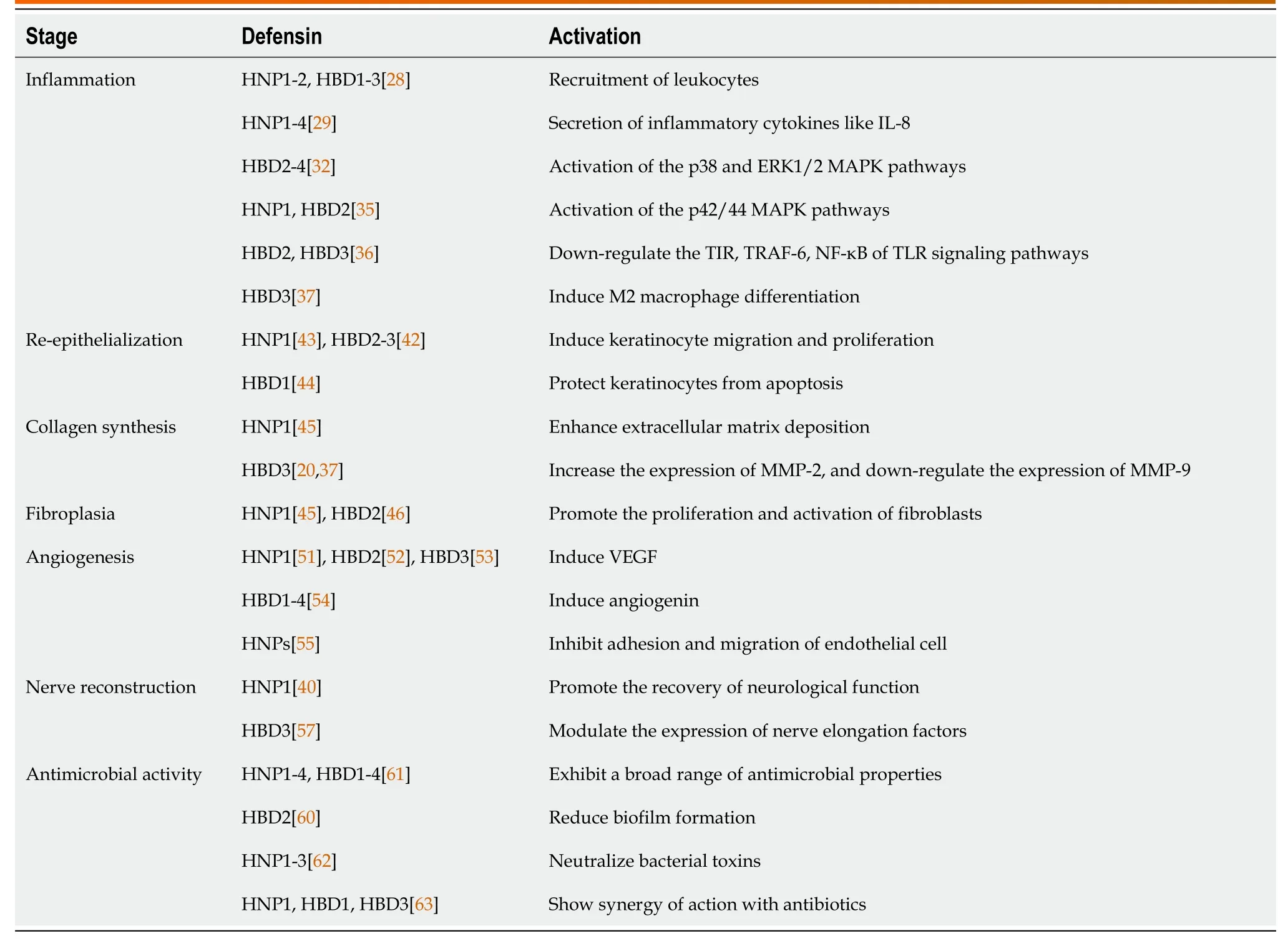
HNP: Human neutrophil peptide;HBD: Human β-defensins;MMP: Matrix metalloproteinase.
Furthermore,defensins seemingly play an important role in fibroblasts and collagen matrix accumulation,which is essential for dermal reconstitution.HNP1 can reportedly promote proliferation and activation of fibroblasts more effectively than HBD2 at the same concentration,and the increased collagen gene expression can only be observed by its stimulation[45].A study also proved that HBDs indirectly stimulate fibroblast migration by activating protein kinase C[46].High levels of pro-inflammatory cytokines and inflammatory chemokines in diabetic wounds lead to an increased production of matrix metalloproteinases (MMPs),especially MMP-2 and MMP-9,thereby inhibiting extracellular matrix formation and dermis reconstruction[47,48].Studies have suggested that the use of an inhibitor for MMP-2 and MMP-9 accelerates wound healing in diabetic mice by maintaining the balance between systematic inflammation and cytokine biosynthesis[49].HBD3 may potentially reverse the pathological condition as they have shown an inhibitory effect on MMP-9,which may result from cytotoxicity for dendritic cells in high concentrations[50].Instead,HBD3 reportedly increases the expression of MMP-2,which is essential for angiogenesis and prolonged matrix remodeling[20].To explain these contradictory findings,further clarification and a comprehensive analysis on the mechanism of wound healing is necessary,as well as verification through specific experiments.
Defensins involved in regeneration of blood vessels and nerves
Angiogenesis is a vital physiological process in wound healing and largely regulated by growth factors,specifically vascular endothelial growth factor (VEGF) and angiogenin.HNP1,HBD2,and HBD3 was proven to bind to cell surface receptor proteins,thus promoting VEGF expression and improvement of vascularization[51-53].The novel role of HBDs in angiogenesis was also identified,revealing that HBD1-4 increases secretion of angiogenin dose-dependently[54].However,the opposing actions can be described as a consequence of on-site recruitment of distinct subpopulations from circulation.HNPs can reportedly inhibit adhesion and migration of endothelial cells,and block VEGF-induced endothelial cell proliferation and capillary formation upon inflammatory stimulation[55].These studies have shed a light on the mechanistic complexity of HNPs angiogenesis.
Neuropathy caused by diabetes is the influencing factor for subcutaneous hemorrhage underneath the callus formation,ultimately leading to skin breakdown[56].Studies have proven that HNP1 administration can promote recovery of neurological function following sciatic nerve injury[40].Additionally,HBD3 modulates the expression of nerve elongation factors that are involved in epidermal hyperinnervation and hypersensitivity to warm sensations[57].As a result,application of defensins can help prevent delayed treatment due to peripheral neuropathy and difficulty in mastering wound conditions in patients with diabetic wounds.
Defensins exhibit antimicrobial activity
Healing of refractory diabetic wounds is often associated with susceptibility to bacterial infections and formation of biofilms[58].As a class of small cationic molecule peptides with broad-spectrum antimicrobial activity,defensins are produced to eliminate invading pathogens during the initial stages of wound formation[59].While the important role of the pore-formation mechanism has been recognized in many studies,other mechanisms,such as disruption of cell wall synthesis,metabolic activity,ATP and nucleic acid synthesis,and amino acid uptake,have also been proposed in recent years[60].HNPs and HBDs both exhibit a strong tendency to eliminate various pathogens,includingStaphylococcus aureusandEscherichia coli,which often invades chronic wounds[61].Specifically,HBD2 exhibits biofilm inhibitory activity by inducing structural changes that interfere with the biofilm precursor’s transport into the extracellular space[60].Additionally,HNPs were proven to protect leukocytes from neutralization by gram-positive pathogenic bacterial toxins[62].Furthermore,they can potentially avoid the emergence of resistance when implemented with other antimicrobial therapies[63].Defensins are not only more effective against drug-resistant bacteria,as compared to antibiotics,but can also preserve the resident bacteria,despite the lack of target specificity as an intractable problem preventing their use as a therapeutic drug[64].
CONCLUSION
Although there is ambiguity regarding its exact role,refractory healing of diabetic wounds is speculated result from interactions between multiple pathophysiological changes in the microenvironment of hyperglycemic and persistent inflammation.This affects immune cell function and composition of defensins at the wound site.In human skin,HBD1 is constitutively expressed in epithelial cells,while inducible HNP1-4 by neutrophils and HBD2-3 by keratinocytes mainly[65].It was obtained through a biopsy that HBD2-4 were overexpressed in the border area of DFUs[25].Studies generally agree that inadequate HBD expression is associated with poor wound healing,and many methods that promote diabetic wound healing are seemingly carried out by promoting defensins expression[66,67].In diabetic wounds,higher HNP1,HNP3,and HNP4 expressions are more common in the central part than in the marginal areas,thus causing a significant increase in IL-8 expression under the influence of advanced glycation end products (AGEs)[29].
Defensins affect the expression and secretion of cytokines,cell proliferation,migration,and apoptosis,and are also involved in all stages of wound healing.Contrary to its proven activity in fighting pathogens and promoting tissue reconstruction,the role of defensins in inflammation and vascularization remains unclear.This discrepancy could be due to pro-inflammatory and anti-inflammatory properties being attributed to HBDs at lower concentrations[28,29],compared to antibacterial effects at higher concentrations exhibited in different experiments[48].Thus,effects of defensins may vary depending on concentration.Furthermore,HNP1 and HBD3 exhibit increased cytotoxic effects with the increased concentration,which can also be related to a greater hydrophobicity[43,68].Therefore,changing the local distribution or structure of defensins can have beneficial effects and prevent toxic side effects.
Studying every type of defensins within a single experiment is difficult.Additionally,certain defensins can exhibit different or contradictory effects within the same environment due to differences in experimental complexity and aims of the experiment.These factors create a huge obstacle in horizontal comparison among similar experiments.Cytotoxicity caused by defensins is difficult to assess,which indicates that topical application may be more appropriate than the systemic administration.Considering the unstable biochemical properties of defensins,topical application alone may be insufficient.To overcome this limitation,researchers are studying biological dressings as an alternative;however,formulation of an ideal material has not yet been achieved.However,animal studies on defensins exhibit improved healing outcomes,and display stable effects through application of new materials or genetic engineering methods[17,20,69].These findings present defensins as a promising therapeutic approach owing to modern techniques,such as development of new materials to efficiently load active factors and novel protein sequences to highlight their beneficial effects.
Defensins regulates chronic inflammation,tissue regeneration,angiogenesis,and nerve recovery,as well as antimicrobial properties;therefore,they are a promising treatment for diabetic wounds.There is an urgent need to find the appropriate dosing regimens and develop new biological dressing alternatives to incorporate active factors.Hence,further preclinical investigations are necessary to understand extensive molecular mechanisms of defensins in the treatment of diabetic wounds,and consequently determine suitable therapeutic strategies.
FOOTNOTES
Author contributions:Tan ZX and Tao R wrote the manuscript and proposed research subtopics;Shen BZ was responsible for navigating the literature,sharing the relevant studies,and drawing the tables included in this review;Meng LX and Li SC drew the figures in the manuscript,formatted citations and compiled references,verified spelling,punctuation,and grammatical errors;Zhu ZY revised and formatted the body of the manuscript,and coordinated the whole work.
Supported bythe Scientific Research Project of Hubei Health Committee,No.WJ2021F106.
Conflict-of-interest statement:Authors declare no conflicts of interests for this article.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Zhi-Xiang Tan 0000-0002-1614-133X;Rui Tao 0000-0001-8782-4421;Si-Cheng Li 0000-0003-4354-0288;Bing-Zheng Shen 0000-0003-1205-0139;Lan-Xia Meng 0000-0001-5307-671X;Zhan-Yong Zhu 0000-0003-4323-4862.
S-Editor:Chen YL
L-Editor:A
P-Editor:Chen YL
杂志排行
World Journal of Diabetes的其它文章
- Risk factor analysis and clinical decision tree model construction for diabetic retinopathy in Western China
- Dietary Nε-(carboxymethyl) lysine affects cardiac glucose metabolism and myocardial remodeling in mice
- Combination therapy of hydrogel and stem cells for diabetic wound healing
- Nutritional supplementation on wound healing in diabetic foot: What is known and what is new?
- Advances in neovascularization after diabetic ischemia
- Orthotic approach to prevention and management of diabetic foot: A narrative review
