Combination therapy of hydrogel and stem cells for diabetic wound healing
2022-11-24JiaNaHuangHaoCaoKaiYingLiangLiPingCuiYanLi
Jia-Na Huang,Hao Cao,Kai-Ying Liang,Li-Ping Cui,Yan Li
Jia-Na Huang,Hao Cao,Kai-Ying Liang,Yan Li,School of Biomedical Engineering,Shenzhen Campus of Sun Yat-sen University,Shenzhen 518107,Guangdong Province,China
Jia-Na Huang,Hao Cao,Kai-Ying Liang,Yan Li,Guangdong Provincial Key Laboratory of Sensor Technology and Biomedical Instrument,Sun Yat-sen University,Guangzhou 510006,Guangdong Province,China
Li-Ping Cui,Endocrinology Department,Panyu Central Hospital,Guangzhou 511400,Guangdong Province,China
Abstract Diabetic wounds (DWs) are a common complication of diabetes mellitus;DWs have a low cure rate and likely recurrence,thus affecting the quality of patients’ lives.As traditional therapy cannot effectively improve DW closure,DW has become a severe clinical medical problem worldwide.Unlike routine wound healing,DW is difficult to heal because of its chronically arrested inflammatory phase.Although mesenchymal stem cells and their secreted cytokines can alleviate oxidative stress and stimulate angiogenesis in wounds,thereby promoting wound healing,the biological activity of mesenchymal stem cells is compromised by direct injection,which hinders their therapeutic effect.Hydrogels form a three-dimensional network that mimics the extracellular matrix,which can provide shelter for stem cells in the inflammatory microenvironment with reactive oxygen species in DW,and maintains the survival and viability of stem cells.This review summarizes the mechanisms and applications of stem cells and hydrogels in treating DW;additionally,it focuses on the different applications of therapy combining hydrogel and stem cells for DW treatment.
Key Words: Combination therapy;Mesenchymal stem cells;Hydrogel;Diabetic wound;Cells delivery;Wound healing
INTRODUCTION
Diabetes mellitus (DM) is a significant global public health burden because of its high incidence and mortality rates[1].In 2019,1.5 million people died of DM[2].Diabetic wounds (DWs) are one of the most concerning complications of DM and affect up to 25% of diabetic patients[3].In addition to causing patient suffering,DW has a low cure rate and high amputation rate,and thus it places a long-term burden on society[4].
DW is difficult to heal because its healing process is unlike that of normal wounds.Normal wound healing typically includes three phases: Inflammation,proliferation,and remodeling.Various cells,growth factors,and cytokines play important roles in each phase to ensure a smooth wound healing progress[5].Owing to the elevated levels of reactive oxygen species (ROS),impaired immune function,and cellular dysfunction in the DW microenvironment,the healing stage stagnates in the inflammatory phase[6].In addition,the peripheral arterial disease leads to a lack of blood perfusion and hypoxia within wounds,thereby increasing ROS release[5].ROS also induces the expression of extracellular matrix (ECM) degradation enzymes that degrade ECM,thus precluding the normal matrix-cell interaction required for wound healing and prolonging the inflammation phase of DW healing[5].
DW healing remains a clinical challenge because of several complications in the DW microenvironment,including oxidative stress,chronic inflammation,and angiogenic dysfunction[7].Current clinical treatments (standard care) involve glycemic control,offloading,debridement,and infection management,which are painful and insufficient for curing DWs[8].Therefore,new approaches for improving DW healing must be developed.The application of functional hydrogel dressings or scaffolds is a promising advanced therapy[9].
Hydrogels are three-dimensional (3D) networks with a high water content and have been intensively studied because they can be functionalized and have good biocompatibility.Several studies have shown that hydrogels provide a moist environment,contribute to cell migration and tissue regeneration,and promote wound healing[10].Therefore,hydrogels are considered ideal dressings for DWs[11].Furthermore,hydrogels provide antioxidant,antibacterial,proangiogenic,and proliferative functions owing to the sustained release of bioactive agents encapsulated in hydrogels.Stem cells are bioactive agents that promote wound healing and are effective in skin regeneration[12].
Stem cells possess self-renewal and differentiation abilities and are essential for post-injury skin repair[13].Thus,stem cell therapy has become a promising new approach for treating DWs.Local injection of the cell suspension or stent implantation stimulates neovascularization,accelerates wound closure,prevents wound contracture and scar formation,and ultimately improves wound healing[14].However,the outcome of stem cell therapy is hindered by the poor bioactivity of stem cells and thus the low amounts of secreted cytokines in the hyperglycemic inflammatory microenvironment of DWs.Effective stem cell delivery remains a challenge[15].
To achieve better healing outcomes combining hydrogel and stem cell treatment is one of the most promising therapies for DWs[16].Although various reviews on stem cell therapy or hydrogel therapy for DWs have been reported,reviews on combined therapy are limited.Herein,we review the mechanisms of DW therapy combining hydrogel and stem cells and focus on preclinical studies of therapy combining hydrogel and stem cells for DWs.
FUNCTIONAL HYDROGELS FOR DW TREATMENT
Wound dressings play an essential role in DWs[2].Hydrogels have become appealing and promising among various wound dressings owing to their high moisture retention,biocompatibility,and similarities to living tissues[17].Hydrogels accelerate wound healing by maintaining gas exchange in the wound,reducing pain by absorbing exudates,preventing infection,and maintaining a moist environment for cell migration.In addition,hydrogels have been used as delivery systems to minimize drug toxicity and improve drug delivery efficiency[2].Functional hydrogels,such as antioxidant,immune regulation,and vascularization hydrogels,have been designed according to the wound microenvironment of DWs.
DWs are often accompanied by oxidative and antioxidant imbalancein vivo.Hydrogels are designed to alleviate excessive oxidative reactions.Self-antioxidant materials,such as 2-hydroxyethyl methacrylate[18] and polyvinyl alcohol,can directly act on wounds;additionally,gel-loaded antioxidant drugs,such as curcumin[19],or bioactive substances can be used to achieve antioxidant effects.These materials act as reducing agents.
Because the inflammatory phase has an active defense response to external stimuli,the inflammatory response aids in cleaning the wound during the healing process[20].However,in chronic wounds,such as DWs,owing to repeated tissue damage,cytokines continue to recruit immune cells to the wound,thereby resulting in an excessive inflammatory response and blocked healing[21].Therefore,the inhibition of excessive immune responses is also considered.Hydrogels,such as sodium alginate and zwitterionic hydrogels,can provide a protective microenvironment for wounds and regulate the transformation of macrophages between proinflammatory and anti-inflammatory[20].Meanwhile,antiinflammatory drug-loaded hydrogel dressings have a local sustained-release effect[22].Responsive hydrogels that can change their properties according to environmental clues to achieve sustained release of entrapped drugs are also desirable.
Angiogenesis is essential for tissue regeneration,whereas the formation of healthy blood vessels is hindered by various microenvironment conditions in DWs[23].Therefore,promoting blood vessel formation is conducive to DW healing.Studies have shown that some hydrogel materials,such as chitosan and hyaluronic acid,regulate the activity and distribution of cytokines or growth factors[24].These materials simulate the microenvironment of the ECM,thereby promoting tissue formation.Bioactive components,including epidermal growth factor and vascular endothelial growth factor,can also be encapsulated by hydrogels,which can promote the regeneration of blood vessels[25].
In general,the mechanism of hydrogels in DWs is relatively clear and positively affects DW healing.
CURRENT STUDIES OF MESENCHYMAL STEM CELLS FOR DW HEALING
In addition to selecting different hydrogel materials,drugs,and biological factors,using stem cells to treat DWs is desirable.Stem cells can asymmetrically replicate and differentiate into different cell types[26].With the unlimited replication capacity,they can provide numerous “sister” stem cells[15].Furthermore,because stem cells secrete pro-regenerative cytokines,stem cell therapy,which treats diseases or injuries by administering stem cells into damaged tissues,has been used as an intervention for DWs[27].Stem cells used for wound healing and tissue regeneration include embryonic stem cells,induced pluripotent stem cells,and mesenchymal stem cells (MSCs)[15].
Allogeneic,xenogeneic,and autologous MSCs have been widely used in skin regeneration and wound healing owing to their significant proliferation,migration ability,and long-term self-renewal potential[28].Considering the impaired function of MSCs derived from patients with diabetes and the risk of tissue rejection,allogeneic MSCs are more widely used[29].
MSCs that are locally injected into wounds are involved in various stages of wound healing.They reduce inflammatory responses through immunomodulation and growth factor production[15],accelerate neovascularization and epithelialization,and stimulate collagen synthesis[30],thereby accelerating wound healing[30].Additionally,clinical studies have demonstrated the efficacy of MSCs in treating diabetic ulcers[30].For example,injecting allogeneic MSCs into the dermis-epidermal junction[31] or subcutaneous and intramuscular tissue around wounds[32] facilitated DW healing in patients.
The potential benefits of MSC therapy have been demonstrated in several studies.Although simple transplantation methods,such as intravenous,subcutaneous,intramuscular,and local injection of MSCs,have achieved some preclinical and clinical success[5],MSC performance still has numerous limitations.Premature senescence and apoptosis of MSCs transplanted in DWs are some of the biggest limitations[33,34].Owing to hyperglycemia caused by DM,DWs generate a chronic inflammatory microenvironment and accumulate advanced glycation end products,which is not conducive to the survival of stem cells[35] and increases the degradation of growth factors secreted by the effector cells,thus compromising efficacy[36].Hence,the delivery strategy must be optimized to ensure cell viability,paracrine function,and differentiation function,which in turn ensures MSC therapy outcomes.
Abundant evidence has shown that using hydrogels to deliver MSCs improves DW healing.Hydrogels are ideal carrier systems for stem cells because they produce a relatively uniform distribution of transplanted cells and retain high water content,close to that of the native tissue,thus improving the retention and survival of stem cells at transplantation sites.Transplanted stem cells can exert their functions through paracrine signals and differentiate into the various cell types required in healthy tissues (Figure 1).
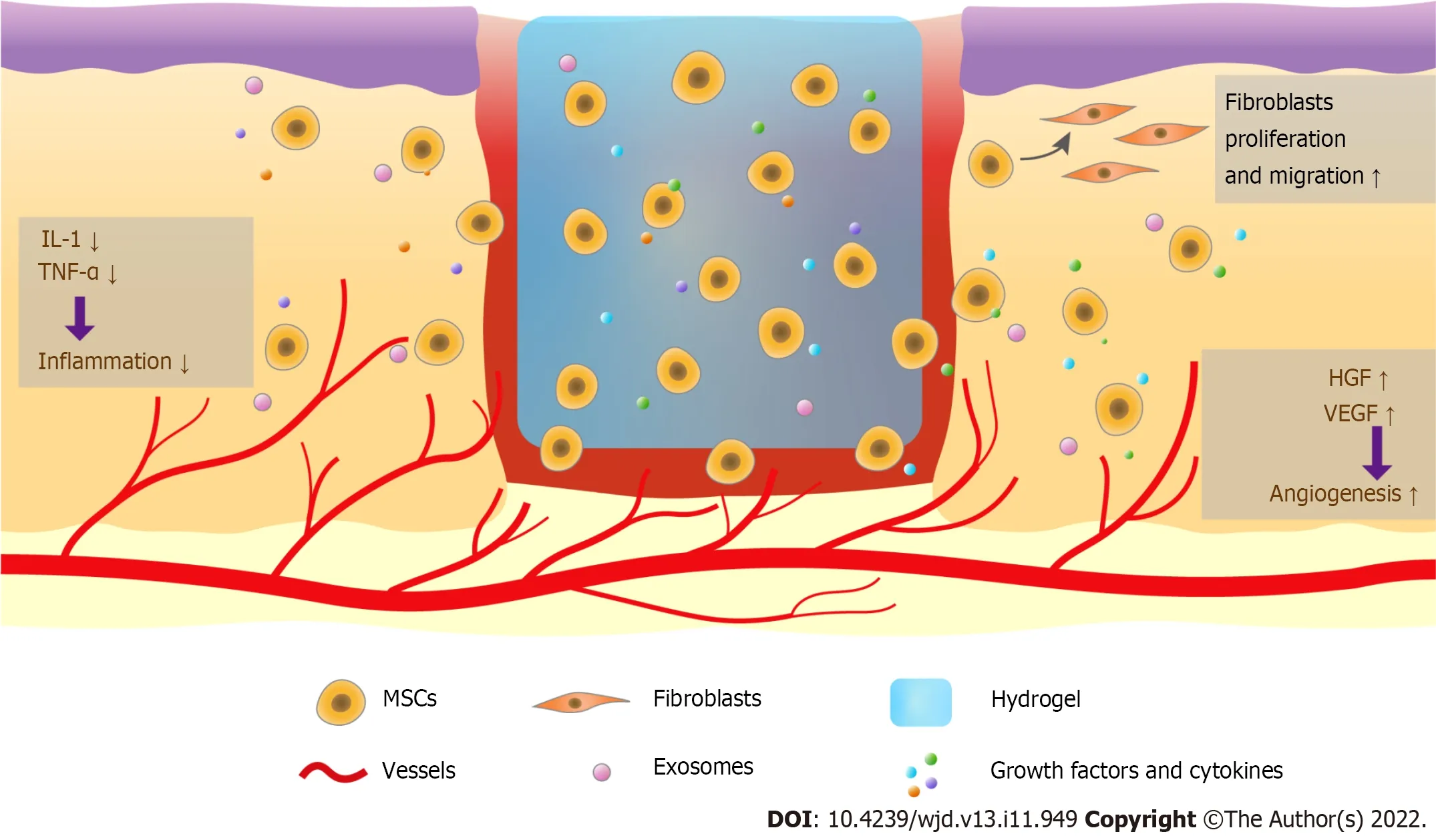
Figure 1 Therapy combining hydrogels and mesenchymal stem cells promotes diabetic wound healing.Mesenchymal stem cells (MSCs) in hydrogels are long-lasting in the wound and regulate wound healing.These cells release exosomes,growth factors,and cytokines,reduce the levels of interleukin-1,tumor necrosis factor-α,and other pro-inflammatory cytokines to modulate the inflammatory response,enhance angiogenesis via increasing vascular endothelial growth factor and hepatocyte growth factor,and promote fibroblast and keratinocyte migration.MSCs can also be transdifferentiated into other cell types to increase wound closure.MSCs: Mesenchymal stem cells;IL-1: Interleukin-1;TNF-α: Tumor necrosis factor-α;VEGF: Vascular endothelial growth factor;HGF: Hepatocyte growth factor.
APPLICATIONS OF COMBINATION THERAPY OF HYDROGEL AND STEM CELLS FOR DW HEALING
As previously discussed,although stem cell therapy has promising potentials for DW healing,the lack of an optimal delivery strategy is one of the biggest obstacles to its therapeutic efficacy.Traditional injection of MSCs always results in low cell viability and transient engraftment,whereas using advanced biomaterial scaffolds (such as films,nanofibers,and hydrogels)[13] to maintain cellular viability,proliferation,and differentiation has received considerable attention[37].Hydrogels have physical and biological characteristics similar to those of natural tissues[36];this renders them as ideal candidates for stem cell delivery.Inspired by the encouraging outcomes of hydrogels on DW healing and their function as a carrier system for drugs,the efficacy of MSCs has been improved with hydrogels[37].For a successful clinical application of the therapy,the optimal hydrogel composition for cell delivery must be considered,and appropriate application methods to ensure stem cell viability and promote DW healing must be designed.Currently,the most common application methods of hydrogels and stem cell combination therapy for DW healing are divided into hydrogel sheets,in situforming hydrogels,and hydrogel microspheres (MS) (Figure 2).
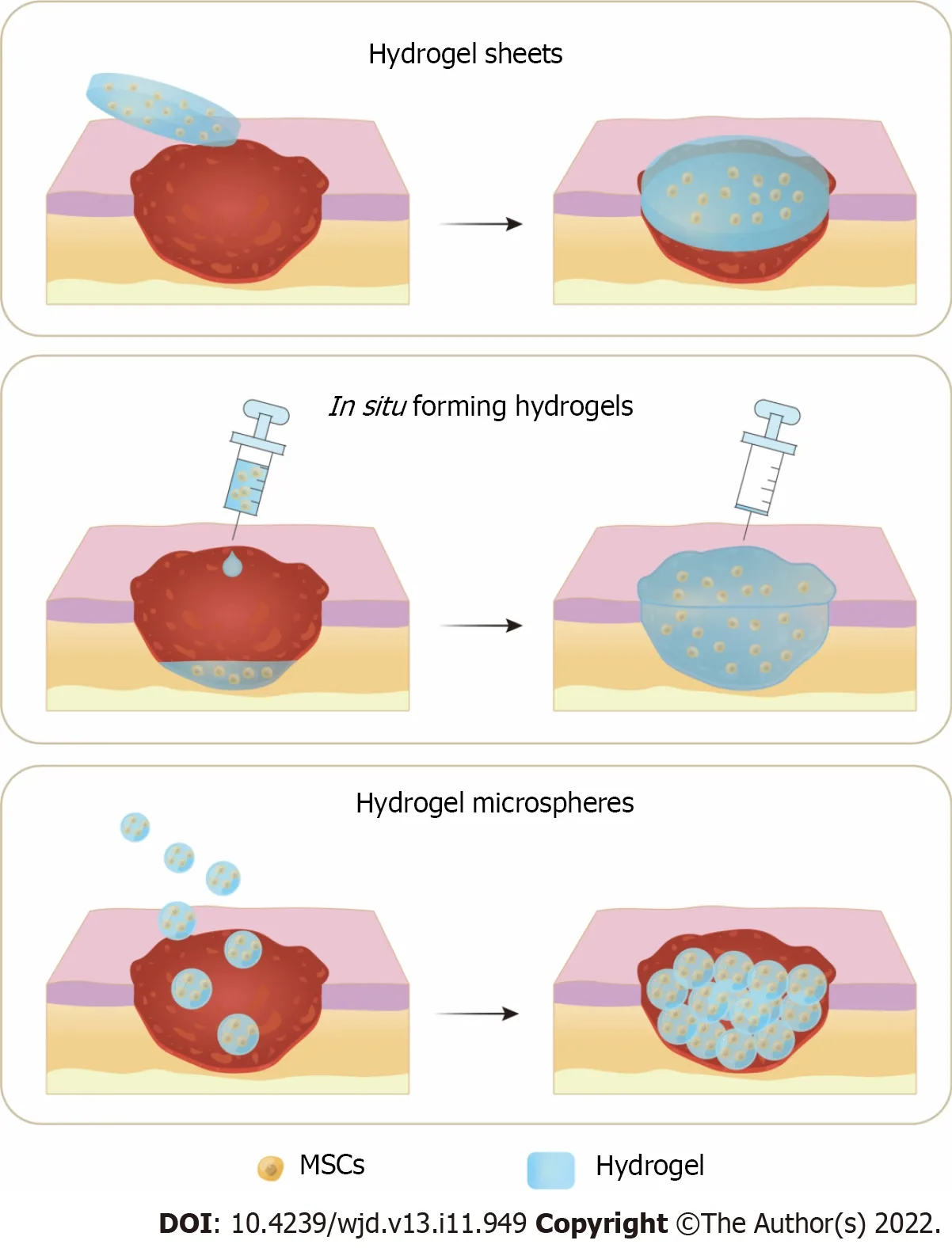
Figure 2 Three application methods of hydrogels and mesenchymal stem cells combination therapy for diabetic wound healing.A: Hydrogel sheets preformed before application;B: In situ forming hydrogels injected at the wound for sol-gel transition;C: Hydrogel microspheres applied onto the diabetic wound.MSCs: Mesenchymal stem cells.
HYDROGEL SHEETS
Applying hydrogel sheets on wounds is a convenient stem cell delivery method,wherein hydrogels are typically preformed in molds,with stem cells seeded onto or inside hydrogels.Rustadet al[38] seeded MSCs onto collagen-pullulan hydrogels and significantly accelerated wound healing and skin appendage recovery in mice within 11 d.The amount of microangiogenesis was approximately doubled in wounds treated with MSC-seeded hydrogel sheets compared with those treated with MSC injection.Given that the biomimetic hydrogel provides a functional niche to augment the regenerative potential of MSCs,the implanted MSCs differentiated into dermal fibroblasts,pericytes,and endothelial cells,which contribute to wound healing[38].In another study,Guoet al[39] demonstrated the improved retention and survival rate of MSCs in hydrogel sheets when transplanted into mouse hearts compared to cell suspension alone.Cells were observed inside the hydrogel sheets for over 9 d in ICR mice.
In vitroculturing of stem cells within hydrogels was found to promote cell adhesion and enhance stem cell functions by supporting normal phenotype maintenance and empowering the transdifferentiation capacity into specific skin lineages compared with the immediate transplantation of stem cellseeded hydrogels[40].Da Silvaet al[41] pre-cultured adipose-derived stem cells (ADSCs) in hyaluronic acid-based sponge hydrogel in neurogenic/standard media for 14 d before transplantation onto the DWs of mice.Wounds treated with pre-cultured ADSCs-loaded spongy hydrogels improved wound closure rates compared to the untreated control and acellular spongy hydrogel groups after healing for 4 wk.The hydrogel sheet promoted the polarization of M1-type macrophages to the M2 type (anti-inflammatory) and improved successful neoinnervation.
Because of the high concentration of inflammatory cytokines in the DW microenvironment,which impairs the activity of MSCs and degrades growth factors secreted by stem cells,single functional hydrogel sheets may not be sufficient for DW healing[42].To be more suitable for DW treatment,hydrogel sheets that inhibit inflammatory responses or protease activity are more effective[43].Ahmedet al[44] studied the wound healing efficacy of bone marrow-derived mesenchymal stem cells (BMSCs) delivered by nitric oxide (NO)-releasing hydrogels on diabetic rabbits.As an endogenous molecule,NO increased angiogenesis and improved immune responses during acute infections.NO-releasing hydrogels increased the viability and proliferation of BMSCs under oxidative stress.In addition to improving collagen deposition and promoting re-epithelialization and angiogenic activity,the NOreleasing hydrogel with BMSC treatment upregulated the expression of growth and cytoactive factors for DW healing within 16 d[44].
In addition to traditional manufacturing technology,3D bioprinting builds special structures layerby-layer according to a predetermined computer model that better fits the skin’s architecture and geometry,providing hydrogel sheets with more complex structures[45].Xiaet al[46] developed curcumin-incorporated 3D bioprinting gelatin methacryloyl (GelMA) to seed ADSCs and promote DW healing within 21 d.Curcumin encapsulation in 10% GelMA hydrogel exhibited inhibitory effects on ROS generation and ADSC apoptosis,and living cells were detected after scaffolds embedded with ADSCs were implanted into the backs of nude mice for 21 d.Further,the scaffold increased the amount of collagen deposition and induced angiogenesis in DWs[46].In addition to 3D bioprinting,multifunctional hydrogel sheets with complex 3D structures can be produced by folding or weaving microfibershaped hydrogels[47].Hydrogel sheets can also be easily functionalized,such as the thermally responsive release of stem cells or drugs[48] for oxidative stress resistance,antibacterial activity,and other functions.
Because stem cells can be cultured separately and the hydrogel sheet is easy to handle,combination therapy with hydrogel sheets and MSCs is easily translated into a clinical setting[49].According to a clinical report,Ravariet al[50] applied BMSCs along with platelets,fibrin glue,and bone marrowimpregnated collagen matrix onto wounds,which resulted in the complete wound closure in 3 of 8 patients with aggressive,refractory DWs within 4 wk of treatment.Additionally,topical administration of placenta-derived mesenchymal stem cells in a sodium alginate hydrogel completely healed diabetic foot ulcers[51].However,this clinical case report must be evaluated further because of the limited sample size of the report.Although functionalizing or changing shapes is very convenient,hydrogel sheets must be pre-formed before application.Because hydrogel sheets are not conducive to long-term storage and the bonding between the sheets and wound surface is limited,in situforming hydrogels have attracted attention.
IN SITU FORMING HYDROGELS
In situforming hydrogels are another mainstream application of combination therapy,with stem cells suspended in the precursor solution before application[52].After the mixed precursor solution is injected into the wound site,the hydrogel containing stem cells is formedin situon wound bedsviachemical bonds[53].Compared with hydrogel sheets,injectable hydrogels are more flexible in their application;this flexibility allows them to adapt to complex-shaped wounds and fit closely[54].Ekeet al[55] designed a precursor solution composed of GelMA and methacrylated hyaluronic acid containing ADSCs,which can be crosslinked within 40 s of ultraviolet irradiation to form hydrogelsin situ.Reportedly,the hydrogel promoted cell proliferation,andin vivostudies revealed a three-fold increase in vascularization for the ADSC-loaded hydrogel group compared to the hydrogels without cells.
However,because ultraviolet irradiation may induce chromosomal and genetic instability[56],ultraviolet-crosslinked hydrogels on exposed wounds negatively affect cell viability and differentiation[57],which is detrimental to wound healing.Owing to its high biocompatibility and specificity[58],enzymatic crosslinking has received considerable attention[59].Yaoet al[52] developed a gelatinhydroxyphenyl hydrogel with the dual enzyme crosslinking of horseradish peroxidase and galactose oxidase,and the hydrogel encapsulated with BMSCs achieved gelation within 5 min at the wound site.The gelatin-hydroxyphenyl hydrogel provides a friendly 3D microenvironment for BMSCs,thereby improving the transplanted cells’ survival and accelerating wound closure[52].
Given that frequently studied natural hydrogels,such as gelatin,collagen,or hyaluronic acid,contain a single component of ECM,their potential to provide the optimum microenvironment for stem cell proliferation and differentiation is limited[60].ECM maintains the original components of the native tissue and is considered an ideal scaffold for tissue regeneration[61].Chenet al[62] developed an ECMderived hydrogel from human decellularized adipose tissue matrix to deliver ADSCs to DWs.The hydrogel was preparedviapepsin digestion and pH neutralization.The paracrine activity of ADSCs encapsulated in the hydrogel was enhanced,whereas the secretion of hepatocyte growth factor increased,thus promoting neovascularization during wound healing[62].Compared with the untreated control,local ADSC injection,and acellular hydrogel groups,treatment with ADSC-hydrogel composites accelerated wound closure in diabetic mice and restored cutaneous appendages within 14 d[62].
For better DW healing outcomes,specific materials are co-entrapped inside the hydrogel for hemostasis and anti-inflammatory properties,and the stem cell viability in the hydrogel can reach an ideal state by optimizing its mechanical strength.Xuet al[63] encapsulated MSCs in an injectable hydrogel system of GelMA and chitosan-catechol cross-linked with dithiothreitol to repair full-thickness DWs.Chitosan-catechol has a good hemostatic effect,and zinc ions were introduced into the hydrogel to enhance angiogenesis.The cell adhesion,proliferation,and differentiation potency of umbilical cordderived mesenchymal stem cellsin vitrowere well maintained in GelMA with optimal stiffness.At the same time,the hydrogel-umbilical cord-derived mesenchymal stem cells combined treatment promoted DW healing by inhibiting the inflammatory factors TNF-α and IL-1βin vivo,with a wound closure rate of 92.2% within 14 d.Compared with the untreated control,local umbilical cord-derived mesenchymal stem cell injection,and acellular hydrogel groups,collagen deposition was significantly abundant on day 7,whereas the most vascular regeneration with the earliest hair follicle formation was found on day 14[63].
Dispersive MSCs are usually loaded inside hydrogels.Recently,3D MSC spheroids were found to possess better differentiation potential than dispersive MSCs[64],which exhibited enhanced vascularization and anti-inflammatory effects[65],thereby promoting wound closure[66].Yanget al[67] combined injectable thermosensitive chitosan/collagen/β-glycerophosphate hydrogels with 3D MSC spheroids,rapidly converted to a gel by physical cross-linking at body temperature,and then completely covered the wound surface and fitted to any shape of the wound bed.Compared with the local 2D monolayer MSC injection and 2D monolayer MSC-encapsulated hydrogel groups,angiogenic factors were much higher for wounds treated with 3D MSC spheroid-encapsulated hydrogel (almost 3-fold),and neovascularization was enhanced,thereby achieving complete re-epithelialization within 3 wk of implantation[67].
Althoughin situforming hydrogels adapt to complex-shaped wounds and fit tightly,thus enabling flexible use at the wound bed,the bulk hydrogel formed at the wound site produces poor tissue infiltration and thus low stem cell survival.Compared within situforming hydrogels,hydrogel MSs have a larger specific surface area and more specific functions,thus playing an essential role in the medical field.
HYDROGEL MS
Hydrogel MSs exhibit good dispersion and stability in physiological environments with a high drugloading capacity[68].Their drug-carrying[69] and bioactive factors[70] are highly effective in wound healing.We previously demonstrated that antibiotic and growth factor separately loaded alginate/CaCO3MSs prepared using microfluidic technology sustainably released drugs and exhibited pH sensitivity.These MSs were embedded in the regenerated tissue and functioned as scaffold materials.They improved wound healing with thicker granulation tissue and stimulated angiogenesis,ideally meeting the requirements of different stages of wound healing[71].Leiet al[70] developed biohybrid agarose MSs conjugated with basic fibroblast growth factor,which achieved local growth factor delivery,stimulated angiogenesis,and enhanced wound healing in diabetic mice.
The special geometry of hydrogel MSs is conducive to the diffusion of nutrients and wastes[72].MSs that deliver stem cells can release stem cells,thereby promoting proliferation and differentiation of surrounding cells and enhancing the formation of integrated functional tissues[73].Stem cell-loaded MSs have been applied in various tissue systems,including cartilage[74],bone[75],bone marrow[72],and brain[76].Intracerebral implantation of stem cells using MSs in the rat brain improved stroke treatment[76].Maoet al[72] demonstrated that microgel encapsulation sustained MSC survival after intravenous injection in mice and enhanced the immunoregulatory capacity of MSCs in a bone marrow transplantation model.
Considering that our previous study demonstrated that hydrogel MSs act as scaffolds and gradually integrate into regenerated skin tissue,we designed gelatin MSs encapsulated with ADSCs from rats (rADSC/MS) with an ideal mechanical strength and degradation rate that matched tissue regeneration to improve DW healing[77].Gelatin MSs promoted the adhesion and proliferation of fibroblast cells and maintained the viability of encapsulated rADSCs.Slowly released exosomes from rADSCs were eventually internalized by HUVECs,which suggested a potential exosome mechanism for improving wound healing.The implanted rADSC/MS gradually integrated into the regenerated skin tissue,thus facilitating the arrangement of neat collagen fibers.Compared with the untreated group and the MS group,rADSCs embedded in rADSC/MS promoted M2 macrophage polarization and recovery of peripheral nerves,formed larger blood vessels,and eventually generated a dermis close to normal tissue within 14 d[77].
Previous studies have demonstrated that hydrogels provide a functional niche for MSCs,which enhances MSC regeneration potential and promotes wound healing.Preclinical studies on the combined treatment of DWs with hydrogels and stem cells are summarized in Table 1.
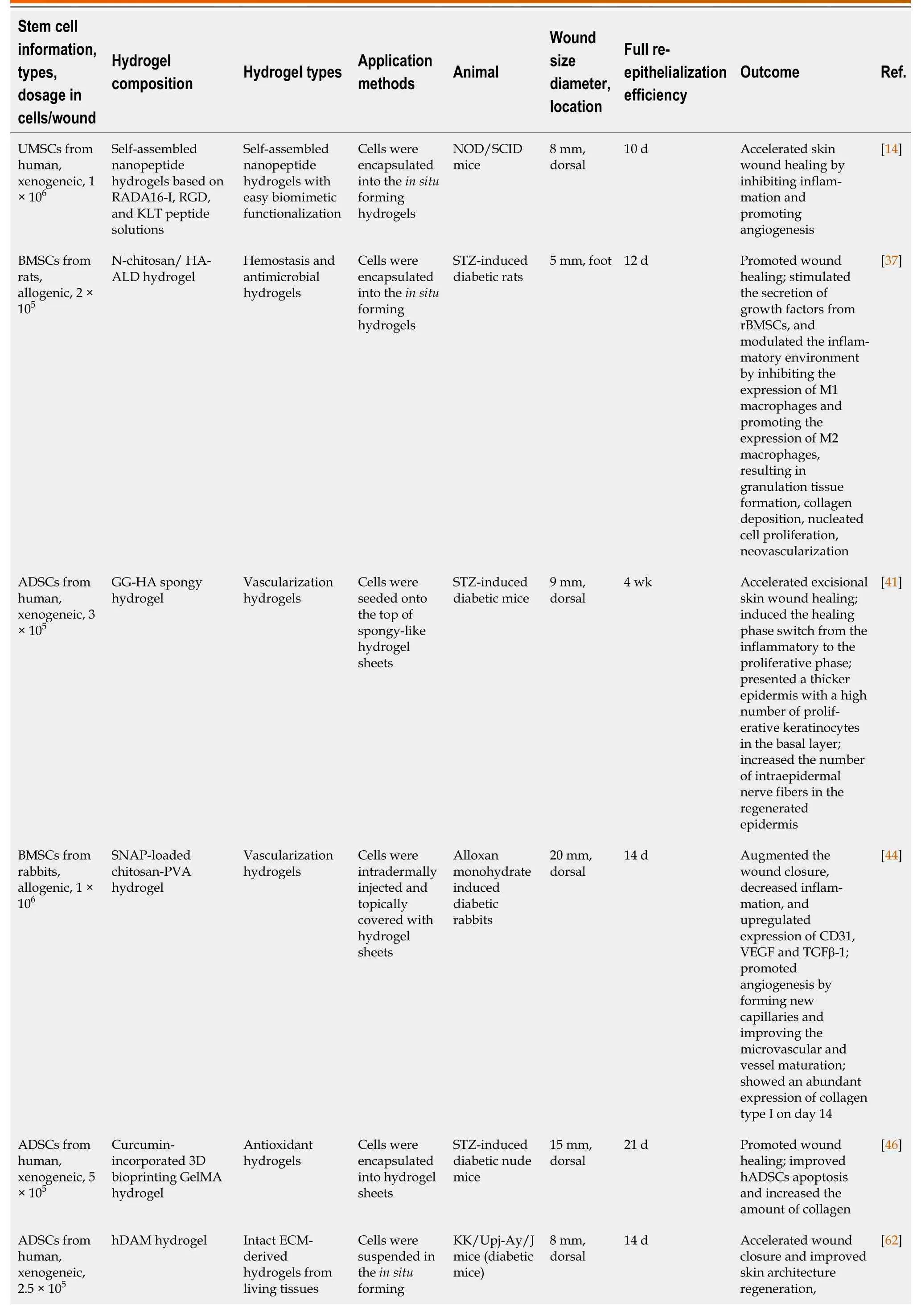
Table 1 Summary of studies regarding therapy combining hydrogels and stem cells for diabetic wound healing
CONCLUSION
This review discussed the benefits associated with therapy combining hydrogels and MSCs for DW healing.Researchers have explored different application methods for stem cell delivery with hydrogels,including hydrogel sheets,in situforming hydrogels,and hydrogel MSs.In addition to providing a friendly microenvironment for stem cells,this strategy enhances the adhesion between the dressing and wound and facilitates the function of stem cells,ultimately benefiting vascular and neural regeneration in DWs.Among these application methods,hydrogel MSs have the advantages of a larger specific surface area,more uniform dispersibility,and more specific functions;additionally,they can effectively deliver various types and functions of cells into the wound.Therefore,hydrogel MSs loaded with stem cells are expected to play an important role in clinical practice.
Therapy combining hydrogels and MSCs has shown great potential for DW healing.However,the plasticity of MSCs has led to their double-sidedness for clinical applications.Although the multi-differentiation ability provides them with good application prospects,it increases the risk of tumorigenicity[78].As a solution,cell-free treatments,such as exosomes and artificial cell products derived from the MSCs secretome have attracted recent interest.Exosomes and secretomes retain the paracrine factors of stem cells[7].Although extensive studies have explored the combination therapies of hydrogels and MSCs for DW healing,additional work is required to optimize parameters,such as the storage and transport stability of cells,and avoid their tumorigenic and immunogenic risks.Further improvement and testing of this technologyin vivowill also contribute to the clinical transformation of combination therapy.
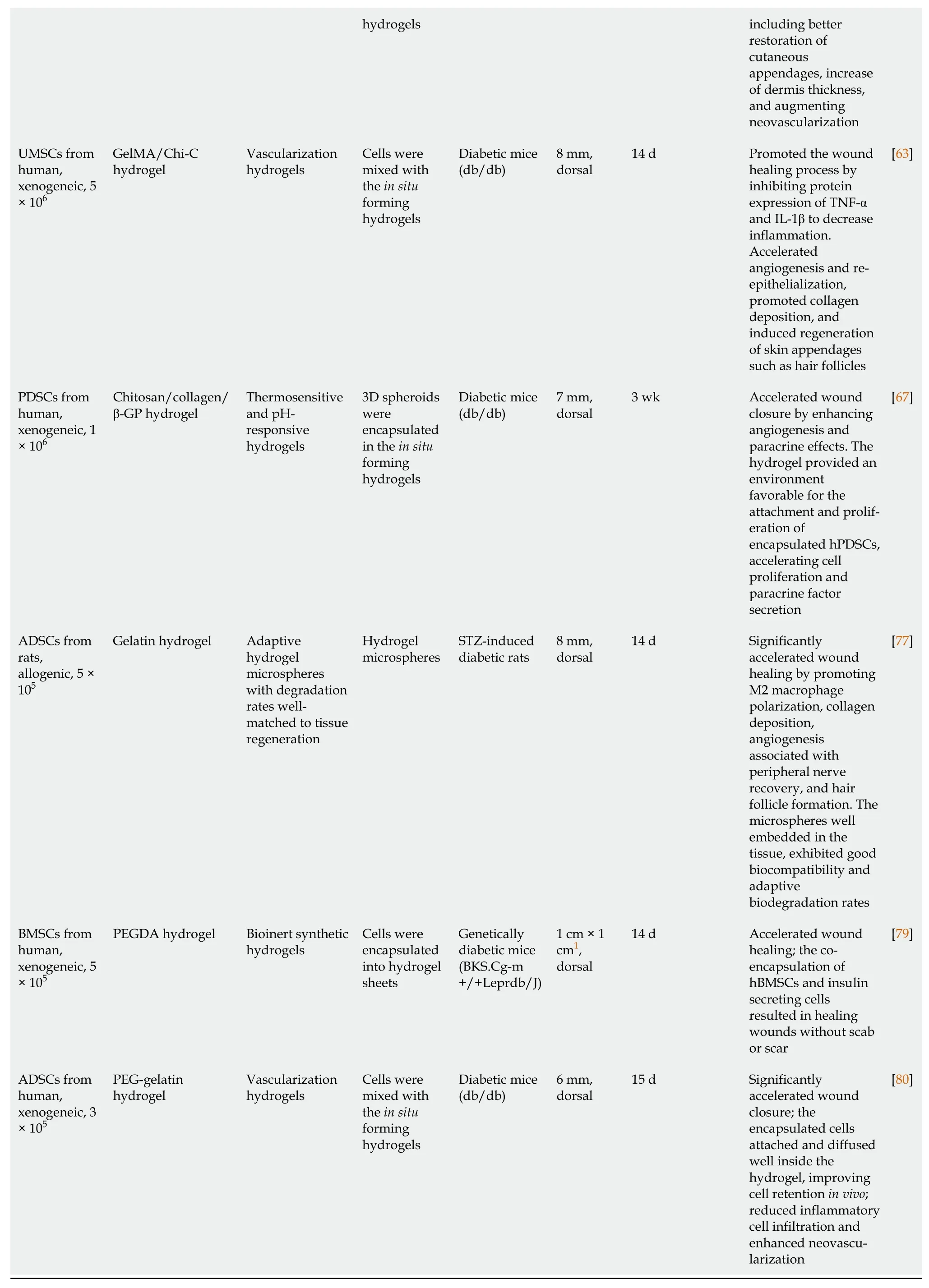
1Wound size (side length × side length).3D: Three dimensional;ADSCs: Adipose-derived stem cells;β-GP: β-glycerophosphate;BMSCs: Bone marrow-derived mesenchymal stem cells;Chi-C: Chitosan-catechol;ECM: Extracellular matrix;GelMA: Gelatin methacryloyl;GG-HA: Gellan gum-hyaluronic acid;HA-ALD: Hyaluronic acid-aldehyde;hADSCs: Human adipose-derived stem cells;hBMSCs: Human bone marrow-derived mesenchymal stem cells;hDAM: Human decellularized adipose tissue matrix;hPDSCs: Human placenta-derived mesenchymal stem cells;N-chitosan: N-carboxyethyl chitosan;PDSCs: Placenta-derived mesenchymal stem cells;PEG: Poly(ethylene glycol);PEGDA: Polyethylene glycol diacrylate;PVA: Polyvinyl alcohol;rBMSCs: Rat bone marrow-derived mesenchymal stem cells;SNAP: S-nitroso-N-acetyl-penicillamine;STZ: Streptozotocin;UMSCs: Umbilical cord-derived mesenchymal stem cells;VEGF: Vascular endothelial growth factor.
FOOTNOTES
Author contributions:Li Y designed the research study;Huang JN and Cao H searched the literature and drafted the initial manuscript;Liang KY and Cui LP provided further revision;all authors have read and approved the final version of the manuscript.
Supported bythe Shenzhen Basic Research Project,No.JCYJ20190807155 805818;and the Foundation of Guangdong Provincial Key Laboratory of Sensor Technology and Biomedical Instrument,No.2020B1212060077.
Conflict-of-interest statement:There are no conflicts of interest to report.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Yan Li 0000-0003-3115-8380.
S-Editor:Chen YL
L-Editor:Filipodia
P-Editor:Chen YL
杂志排行
World Journal of Diabetes的其它文章
- Risk factor analysis and clinical decision tree model construction for diabetic retinopathy in Western China
- Dietary Nε-(carboxymethyl) lysine affects cardiac glucose metabolism and myocardial remodeling in mice
- Role of defensins in diabetic wound healing
- Nutritional supplementation on wound healing in diabetic foot: What is known and what is new?
- Advances in neovascularization after diabetic ischemia
- Orthotic approach to prevention and management of diabetic foot: A narrative review
