Clinical diff erences among racially diverse children with celiac disease
2022-11-08aleyDesherMicheKleAlkalay
aley A. Desher · MicheKle J. Alkalay
Celiac disease (CD) is an immune-mediated disorder of the small intestine precipitated by the ingestion of gluten in genetically predisposed individuals. Though CD was formally considered a chronic enteropathy that mainly aff ects children of European origin, it is now known to aff ect individuals of various ages and racial backgrounds [ 1].
The National Health and Nutrition Examination Surveys from 1988 to 2012 revealed the prevalence of positive CD serology in Americans between 6 and 20 years old to be 0.7 overall, 1.2% of Caucasians, 0.2% of Hispanics, and 0.1%of Blacks [ 2]. Another study involved the examination of 454,885 duodenal biopsies from a racially diverse, primary adult patient population in the United States. Positive CD histology in Americans was reported to be highest in North Indians (1.51%), and lowest in South Indians (0%) and East Asians (0.15%) [ 3].
CD is a complex enteropathy with variable presentations.Case series in East Asia, South Asia, and Latin America have reported how the disease presents in diff erent racial groups [ 4— 7]. While these studies were notable for demonstrating the racial and geographic diversity of patients affl icted by CD, the absence of control groups precluded robust comparison. In terms of case—control studies conducted in pediatric populations, two Canadian studies compared predominantly South Asian minority populations with Caucasians. South Asian youth were reported to have better adherence to gluten-free diet (GFD), increased healthrelated quality of life, and lower body mass index (BMI),while their Caucasian counterparts had more gastrointestinal symptoms [ 8, 9].
Increasingly, more non-Caucasian children are diagnosed with CD each year, though there have been no studies to date that directly compare Hispanic, Black, or East Asian pediatric patients with Caucasian controls. This retrospective case—control study is the f irst to compare non-Caucasian pediatric CD patients with Caucasian patients, both overall and stratif ied by race.
This was a retrospective analysis of pediatric patients with CD between 6 months and 17 years of age followed at Children’s Medical Center Dallas between January 2012 and 2019. Diagnosis of CD was conf irmed by either duodenal biopsy showing positive histology, two tissue transglutaminase-IgA (tTG-IgA) values greater than ten times the upper limit of normal (ULN), or one tTG-IgA value greater than ten times the upper limit of normal in addition to positive endomysial antibodies (EMA-IgA) as per the 2020 European Society for Pediatric Gastroenterology, Hepatology and Nutrition guidelines [ 10]. Patients who did not meet the histological or serological requirements for diagnosis were excluded.
The patient cohort consisted of 89 non-Caucasians, which was def ined as non-Hispanic Black, non-Hispanic Asian,or Hispanic ethnicity, as well as 89 age/sex-matched non-Hispanic Caucasian pediatric patients with CD. Race and ethnicity information was extracted from the medical records of all 89 Caucasian controls and 81 non-Caucasians. The remaining 8 patients were coded as unknown in the electronic medical record. The name analysis software OnoMap,which has been validated previously for health-related datasets, was used to identify the racial backgrounds of these patients [ 11]. Following paired name taxonomy, two of the unknowns were classif ied as Asian and six were classif ied as Hispanic. Caucasian controls were assigned based on gender and chronological age.
International Classif ication of Diseases (ICD) codes for signs, symptoms, comorbidities, family history, and lab f indings were pulled from the electronic medical record of each patient. Redundant or synonymous codes were consolidated prior to analysis for improved sensitivity. Diagnosis of autoimmune thyroiditis (AT) was verif ied by meeting criteria for elevated thyroid peroxidase or thyroglobulin antibody levels.
BMI was calculated using height and weight values recorded on or within 2 weeks of diagnosis day. BMIpercentiles for children aged 2 years and older were then tabulated according to age and gender using Centers for Disease Control and Prevention growth curves [ 12]. Patients were placed into the following weight status categories:underweight (< 5th percentile), normal weight (5th to < 85th percentile), overweight (85th to < 95th percentile), and obese(95th percentile or greater). Failure to thrive was def ined as a weight for age below the 5th percentile on multiple occasions or weight deceleration crossing two major percentile lines on a growth chart.
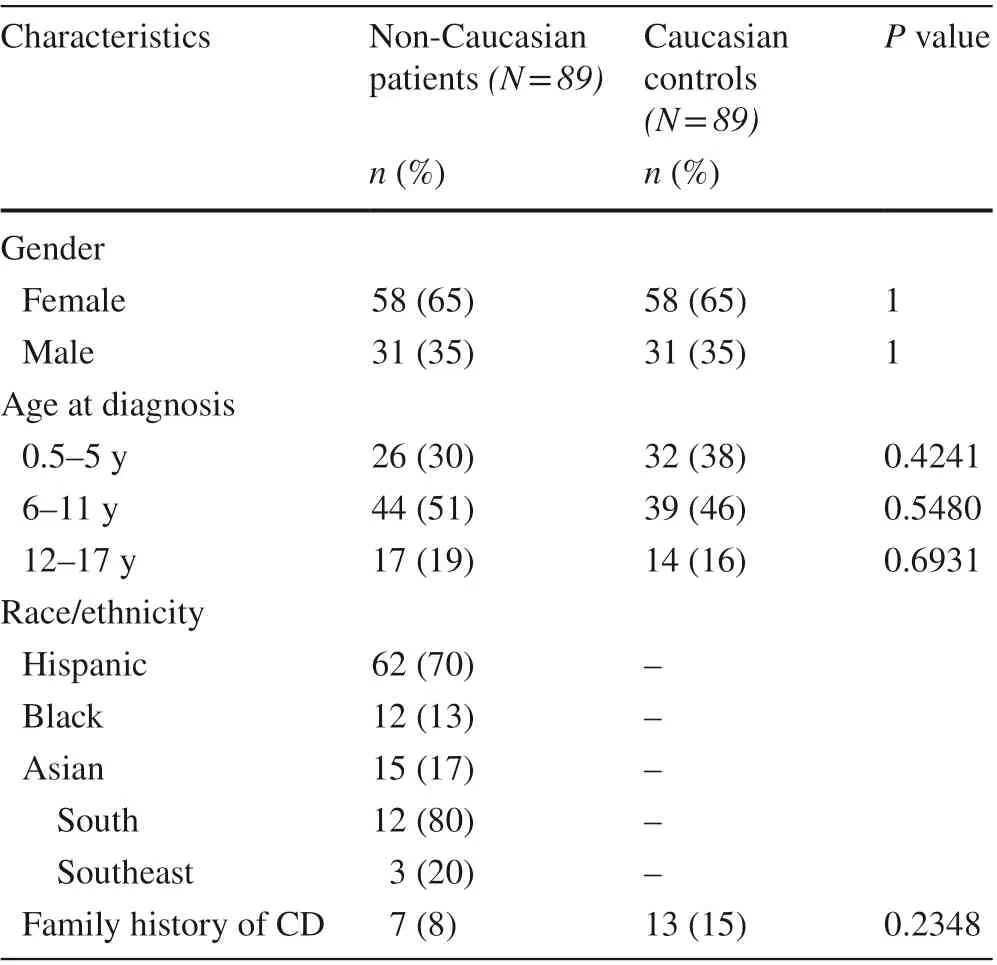
Table 1 Demographic characteristics of 89 non-Caucasian celiac disease patients and their age/sex-matched Caucasian controls
Pathology reports from diagnostic duodenal biopsy were collected for 72 non-Caucasians and 62 Caucasians. A total of 52 reports were available for Hispanics. The remaining subjects either did not undergo biopsy or had the procedure done elsewhere. Marsh type was abstracted from each report.Type 3a indicates mild villous atrophy, type 3b indicates moderate villous atrophy, and type 3c indicates complete villous atrophy. Dates of diagnosis and initial presentation were also manually abstracted.
Statistical analyses were performed using GraphPad Prism, version 9.0 (GraphPad Software Inc, San Diego, CA).All variables examined were binary categorical and summarized as percentages. Fisher’s exact test was used to assess the relationship between categorical variables. A two-sidedPvalue < 0.05 was considered statistically signif icant.
A total of 89 non-Caucasian pediatric CD patients were identif ied and compared to 89 age/sex-matched Caucasian controls. The two groups were similar for age at diagnosis and family history of CD (Table 1). Nearly half were diagnosed between the ages of 6 and 11 years. Hispanics composed most of the non-Caucasian group [62 (70%)]while there was a similar proportion of Blacks [12/89 (13%)]and Asians [159 (17%)]. Of the 15 Asian patients, 12 (80%)were South Asian and 3 (20%) were Southeast Asian. Of the 147 patients who were originally diagnosed at Children’s Medical Center Dallas, 137 (93%) received a diagnosis within 100 days from initial presentation. The remaining 31 patients were initially diagnosed at an outside hospital and subsequently received care at Children’s Dallas. One patient experienced a diagnosis delay longer than 365 days.A total of 169 (94%) patients were diagnosed by duodenal biopsy. The remaining 9 (6%) were diagnosed by antibody titers exclusively.
Statistically significant differences were detected for four clinical parameters (Fig. 1). CD antibody positivity,def ined as two tTG-IgA values > 10 × ULN or one tTGIgA value > 10 × ULN with positive EMA-IgA, was more prevalent among non-Caucasians than their Caucasian controls (37% vs. 20%,P= 0.0198). Autoimmune thyroiditis was also more prevalent among non-Caucasians than their Caucasian controls (13% vs. 2%,P= 0.0098). Vitamin D def iciency was more prevalent among non-Caucasians than their Caucasian controls (16% vs. 4%,P= 0.0230) as well as among Blacks than their Caucasian controls (42% vs. 0%,P= 0.0373). Failure to thrive was more prevalent among the Hispanic subgroup than their Caucasian controls (16% vs.3%,P= 0.0298). Clinical characteristics were not found to be statistically diff erent between non-Caucasians and Caucasian controls (Table 2).
Of the 72 available pathology reports from diagnostic duodenal biopsy for the non-Caucasian group, 36 (50%)were Marsh type 3a, 20 (28%) were 3b, and 16 (22%) were 3c. Of the 62 available reports for the Caucasian group, 31(50%) were 3a, 18 (29%) were 3b, and 13 (21%) were 3c.Antibody positivity varied by villous atrophy in the combined cohort of 134 available reports, present in 9 (9/67,13%) subjects with Marsh type 3a, 20 (20/38, 53%) subjects with 3b, and 11 (11/29, 38%) subjects with 3c. Failure to thrive correlated with villous atrophy in the Hispanic cohort of 52 available reports, present in 3 (3/27, 11%) subjects with Marsh type 3a, 3 (3/15, 20%) subjects with 3b, and 4(4/10, 40%) subjects with 3c. Chi-square analysis revealed a statistically signif icant relationship (P= 0.03).
The demographics of the study population had similarities to and diff erences from the greater Dallas population.The study group had a higher proportion of girls, consistent with the established increased prevalence of CD among females [ 13]. There was a similar proportion of Hispanics within the non-Caucasian study group compared to the city’s non-Caucasian population (70%vs.61%) [ 13]. However,the proportion of Blacks was lower (13%) and the proportion of Asians was higher (17%) compared to the Dallas non-Caucasian population (4%) [ 13]. The higher observed prevalence of South Asian compared to Black CD patients is consistent with population-level data [ 2, 3].
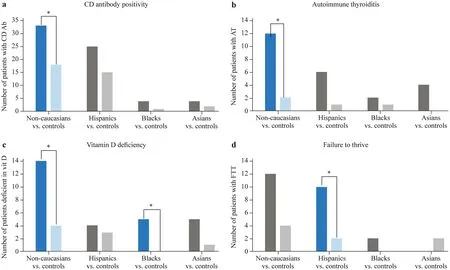
Fig. 1 Clinical diff erences among non-Caucasian celiac disease patients as a whole and stratif ied by race. The left column of each cluster represents non-Caucasian patients. The right column of each cluster represents their corresponding age/sex-matched (1:1) Caucasian controls. Celiac disease (CD)antibody positivity, autoimmune thyroiditis, vitamin D def iciency and failure to thrive are shown (a, b, c & d). Blue clusters indicate a statistically signif icant ( P < 0.05) diff erence The control bar for Hispanics vs. controls in graph D should be light blue
CD antibody positivity was found to be more prevalent among the non-Caucasian group compared to Caucasian controls. The 2020 European Society for Pediatric Gastroenterology, Hepatology and Nutrition guidelines established the positive predictive value of tTG-IgA > 10 × ULN for a diagnosis of CD to be > 95% in 28 of 30 evaluated data sets[ 10]. The strong correlation between tTG-IgA titers and degree of intestinal villous atrophy has been well documented [ 15— 17]. In one study, strongly positive antibody titers were > 98% specif ic for Marsh 3a or greater lesions[ 15]. The present study supported these f indings with highest prevalence of antibody positivity among Marsh 3b, followed by 3c, and then 3a. Though abdominal distention and diarrhea have previously been correlated with high CD antibody titers [ 15, 16], the non-Caucasian group was not observed to have a greater burden of these symptoms.
The present study also revealed a higher prevalence of autoimmune thyroiditis among non-Caucasian CD patients than in Caucasian controls. The increased rate of antithyroid peroxidase positivity among Hispanic adolescents compared to Caucasians supports this f inding [ 18]. Although this is the f irst report of racial diff erences in AT among CD patients,high comorbidity of CD with AT has been widely observed[ 19— 22]. A Swedish study reported the hazard ratio of AT among children with CD compared to healthy controls to be 4.7 (95% CI = 2.1—10.5) [ 19]. The coexistence of these diseases has been largely attributed to common genetic determinants, specif ically human leukocyte antigen (HLA)-DQ2/DQ8 and cytotoxic T-lymphocyte-associated antigen-4(CTLA-4) [ 23]. Thus, it is feasible that the increased prevalence of AT among non-Caucasian children with CD may be attributed, in part, to distinct haplotypes.
Vitamin D def iciency was found to be more prevalent among the non-Caucasian group compared to Caucasian controls, as well as among the Black subgroup compared to Caucasian controls. These f indings are consistent with population-level data, showing that Black children are most aff ected, followed by Hispanic children, and then Caucasian children [ 24]. Symptomatic vitamin D def iciency, def ined as either rickets or hypocalcemic convulsions, has also been reported to be more prevalent in Black children [ 25].Increased vitamin D def iciency among the non-Caucasian and Black subjects may be attributed to diagnostic delay,malabsorption in the setting of villous atrophy, or etiologies separate from CD.
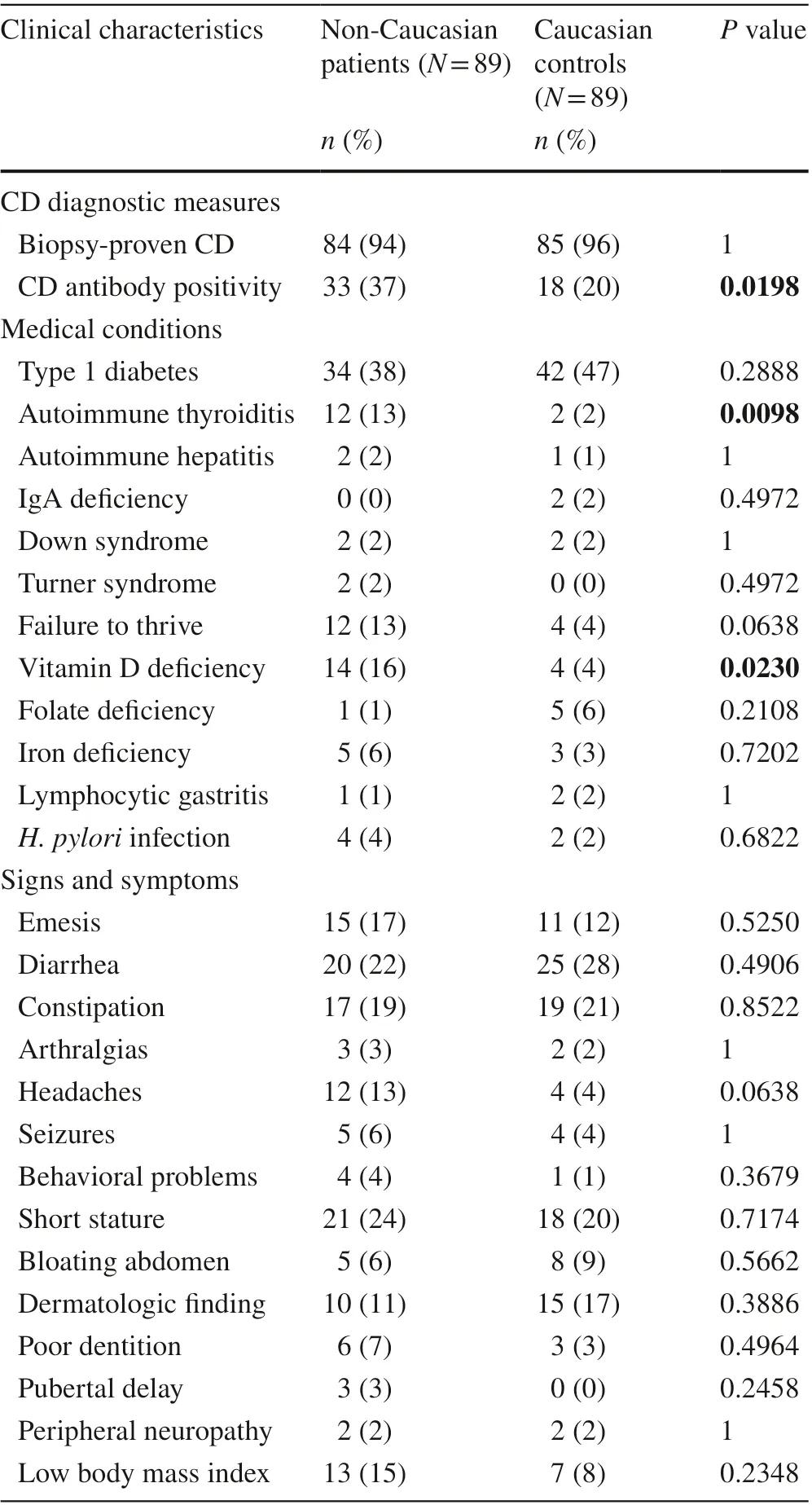
Table 2 Clinical characteristics of 89 non-Caucasian celiac disease(CD) patients and their age/sex-matched Caucasian controls. P values that meet signif icance level < 0.05 are bolded
Severe growth failure among children with CD has been noted to be more common in developing regions, such as North Africa, the Middle East, and the Indian continent,than in developed countries [ 26]. This discrepancy has been attributed to challenges in attaining nutrition goals in resource limited environments. Along similar lines, a potential explanation for increased failure to thrive among the Hispanic subgroup might be the higher rate of food insecurity among Hispanic families (39.1%) compared to Caucasian families (15.1%) in the United States [ 27]. Pediatric CD patients with growth failure have previously been shown to have higher CD antibody, alanine aminotransferase, and thyroid-stimulating hormone levels than those with normal growth [ 28 ]. The Hispanic subgroup in the present study likewise had increased CD antibody positivity and autoimmune thyroiditis compared to their Caucasian controls,though these diff erences were not statistically signif icant.
The results herein should be considered in the context of some limitations. While abstracting ICD codes allowed systematic collection of a large amount of data, the timeline of when diff erent clinical characteristics were present in the disease course was unable to be digitally abstracted. The study was further limited by small subgroup sample sizes.Because several diff erent clinical characteristics were evaluated, there also exists a risk for inf lation of Type I error.Most of the subjects diagnosed exclusively by antibody titers were identif ied in 2015 or later, which suggests that the 2012 guidelines were not adopted immediately. Some CD patients in the early study period could have been missed which limits the sample size and underestimates the proportion of patients with exclusive antibody diagnosis.
Nevertheless, this study is the f irst to compare clinical features between non-Caucasian and Caucasian pediatric CD patients. Clinicians should consider the results of this study when diagnosing and treating CD in diverse patient populations.
AcknowledgementsThe authors would like to acknowledge Dr. Edaire Cheng for her study design recommendations and Dr. Bradley Barth for his guidance during the manuscript submission process.
Author contribution KD: methodology, investigation, formal analysis,writing—original draft, and writing—review and editing; MA: conceptualization, methodology, formal analysis, writing—review and editing,and supervision.
FundingThe authors do not report any grant support.
Data availabilityAll data generated or analyzed during this study are included in this published article. The authors report that some of the data herein have been shared previously in a virtual poster presentation at the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition 2021 meeting.
Declarations
Conflict of interestThe authors have no conf licts of interest to disclose related to the research or assistance with manuscript preparation. No f inancial or non-f inancial benef its have been received or will be received from any party related directly or indirectly to the subject of this article.
Ethical approvalThe University of Texas Southwestern Institutional Review Board deemed the present study (IRB #STU 2019—1357)acceptable.
杂志排行
World Journal of Pediatrics的其它文章
- Single ventricle: amphibians and human beings
- Effi cacy of live attenuated vaccines after two doses of intravenous immunoglobulin for Kawasaki disease
- Associations between body mass index in diff erent childhood age periods and hyperuricemia in young adulthood: the China Health and Nutrition Survey cohort study
- Frequency and safety of COVID-19 vaccination in children with multisystem inf lammatory syndrome: a telephonic interview-based analysis
- Music therapy and pediatric palliative care: songwriting with children in the end-of-life
- Secondary genomic f indings in the 2020 China Neonatal Genomes Project participants
