Effi cacy of live attenuated vaccines after two doses of intravenous immunoglobulin for Kawasaki disease
2022-11-08oshihikoMorikawaHiYroshiSakakibaraMasaruMiura
oshihiko Morikawa · HiYroshi Sakakibara · Masaru Miura ,
Kawasaki disease (KD) is a vasculitis of unknown etiology occurring predominantly in children under f ive years old[ 1]. Coronary artery aneurysm, its most serious complication, occurs in about 25% of untreated patients [ 2]. Highdose intravenous immunoglobulin (IVIG) 2 g/kg is used to prevent coronary artery aneurysms [ 1, 3, 4], but about 20%of patients are nonresponsive and require a second dose for a total of 4 g/kg. The appropriate timing of live attenuated vaccine (LAV) administration in such cases is unclear. Based on the half-life of immunoglobulin G (IgG) [ 5], the onemonth interval after a single IVIG dose recommended in the current guidelines should be extended by another month to adjust for the doubled dose. A previous study demonstrated that measles antibodies became undetectable in patients with two doses of IVIG 2 g/kg (4 g/kg in total) nine months after IVIG administration [ 6]. However, it is unclear whether vaccinations on this schedule can elicit an immune response.Furthermore, most previous studies focused on the measles vaccine; the evidence for other vaccines, especially varicella,is still poor, and there are as of yet no studies comparing residual antibody titers after administration of IVIG 2 or 4 g/kg. We herein assessed the effi cacy of administering four types of LAV at nine months post-IVIG treatment, which previous studies have shown to be about the time when antibodies are degraded in children with KD who are unresponsive to their f irst IVIG treatment and have received IVIG 4 g/kg in total.
Fifty-one children with KD receiving IVIG were continuously assessed for eligibility, and 11 subjects with two IVIG doses received vaccinations. The three who received their initial vaccination at six months [178 days, standard deviation (SD) = 13 days], the recommended interval in Japan for LAV after a single dose of IVIG, were evaluated for effi -cacy at nine months, and the eight who received their initial vaccination at nine months (278 days, SD = 11 days) were evaluated for effi cacy at 12 months. These patients received a booster vaccination at 12 months in accordance with the guidelines [ 9] if the antibody titers were negative. The interval between IVIG treatment and the booster vaccination in subjects who received their initial vaccination at six months and nine months was on average 360 days (SD = 15 days)and 389 days (SD = 24 days), respectively (Supplemental Fig. 1).
Table 1 shows the patient characteristics. Table 2 shows the percentage of antibody positivity on enzyme immunoassay (EIA) for measles, mumps, rubella, and varicella after vaccination. Except for mumps, which was negative in all the subjects, the percentage of antibody positivity after vaccination was higher for those vaccinated at nine months.Regarding the initial vaccination against measles and rubella, the immune response was inadequate at six months post-IVIG, but seroconversion was observed in all the subjects for measles and rubella at nine months post-IVIG. The immune response to the mumps and varicella vaccines was insuffi cient at any vaccination timing. After the booster vaccine, all the subjects had seroconversion for measles and rubella, 82% had a positive titer for mumps and varicella,and 73% had a positive titer for all four vaccines.
Figure 1 shows the transition in the antibody titer for each vaccine on EIA before and after IVIG. The antibody titers were undetectable before, but increased markedly after IVIG treatment; they were very low at three months post-IVIG, and all the subjects were negative for the antibodies on EIA at six months post-IVIG, but unvaccinated subjects remained negative even after nine months. The antibodytiters increased after each vaccination. Comparison of antibody titers at three months after the initial vaccination with those at six months and nine months post-IVIG showed that the measles antibody titer was signif icantly higher in patients who were vaccinated at nine months post-IVIG (Supplementary Table 1).
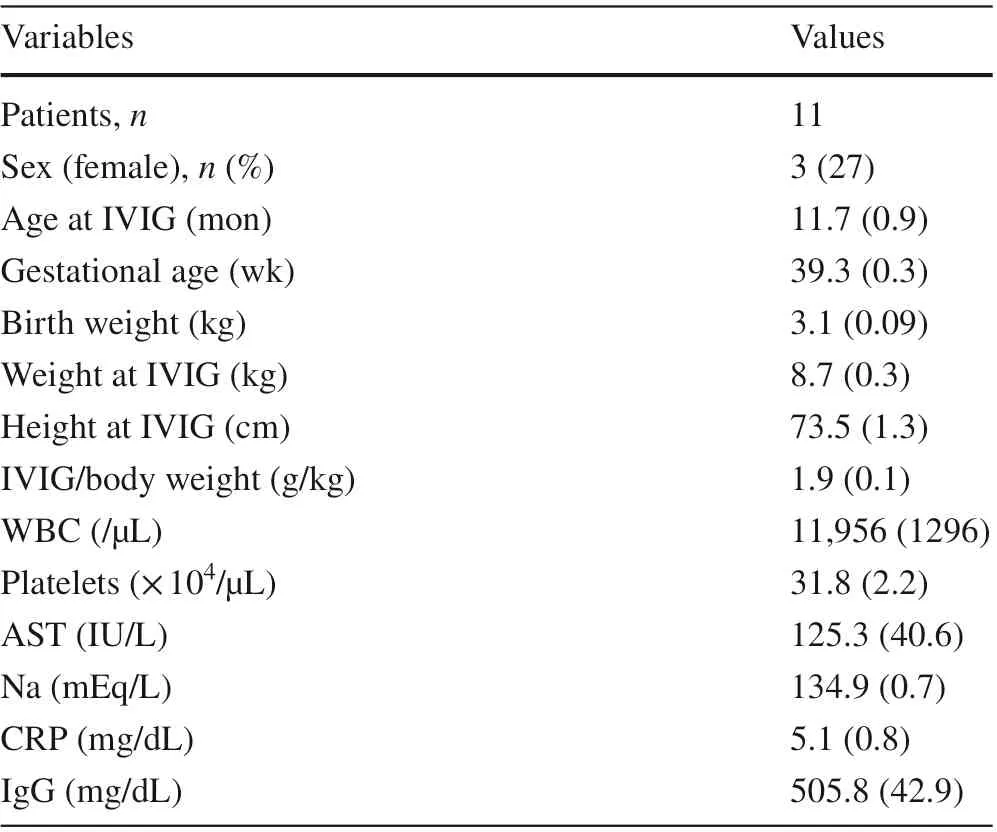
Table 1 Patient characteristics
The present study found the optimal timing for the measles and rubella vaccines to be nine months post-IVIG;however, the mumps and varicella vaccines were ineffective at either timing. A previous study assessing the effi cacy of LAV in patients with KD with only a single IVIG dose (2 g/kg) reported a suboptimal eff ect of vaccination at six months post-IVIG [ 7]. In addition, a retrospective study concluded that vaccination at nine months post-IVIG was preferable [ 8]. In terms of the timing of LAV administration post-IVIG, the Advisory Committee on Immunization Practices recommends an interval of 11 months for IVIG 2 g/kg [ 9] and 12 months for 4 g/kg based on evidence of the inhibition of the response to the measles vaccine for f ive months after administration of an 80 mg/kg intramuscular dose to healthy children[ 10] and on the estimated half-life of immunoglobulin of one month. However, the positive results in all the subjects who received the vaccines at nine months suggested that where the measles and rubella vaccines are concerned, a shorter interval post-IVIG may be better than the guideline recommendations, which extrapolate the intervals based on the half-life of immunoglobulin. In the present study, vaccination at six months post-IVIG led to a signif icantly lower measles antibody titer than vaccination at nine months, suggesting that a six-month interval between the IVIG treatments and vaccination is too short.
On the other hand, the poor effi cacy of the mumps and varicella vaccines may have been caused by the quantity of specif ic antibodies in the immunoglobulin used to measure the antibody titers and by the vaccine strain. Because the immunoglobulin was obtained from blood donors, their immune characteristics could have aff ected the results [ 7].Furthermore, some concerns have been raised about the effi cacy of the Torii strain of the mumps vaccine [ 11].
Subjects who were negative for the antibodies after their initial vaccination all became positive for measles and rubella antibodies after a booster vaccination, and 80% became positive for mumps and varicella antibodies.These results were in line with those of previous studies evaluating the effi cacy of booster vaccinations in subjects who were unresponsive to the initial measles, mumps, and rubella vaccination after a single-dose IVIG treatment [ 7,12]. This similarity may be explained by the degradation of the residual antibodies, the eff ectiveness of the booster vaccination, or both [ 7]. Vaccine effi cacy may be inhibited for a certain period even after the antibody titer of each virus falls below detectable levels. Maternal antibodies are undetectable in most infants during the f irst six months oflife [ 13], and the response to LAV is suboptimal until after age one year [ 14]. The varicella and mumps vaccines were ineff ective at nine months post-IVIG, and seroconversion occurred mainly after a booster vaccination, emphasizing the importance of booster vaccinations in KD patients as in generally healthy children [ 15].
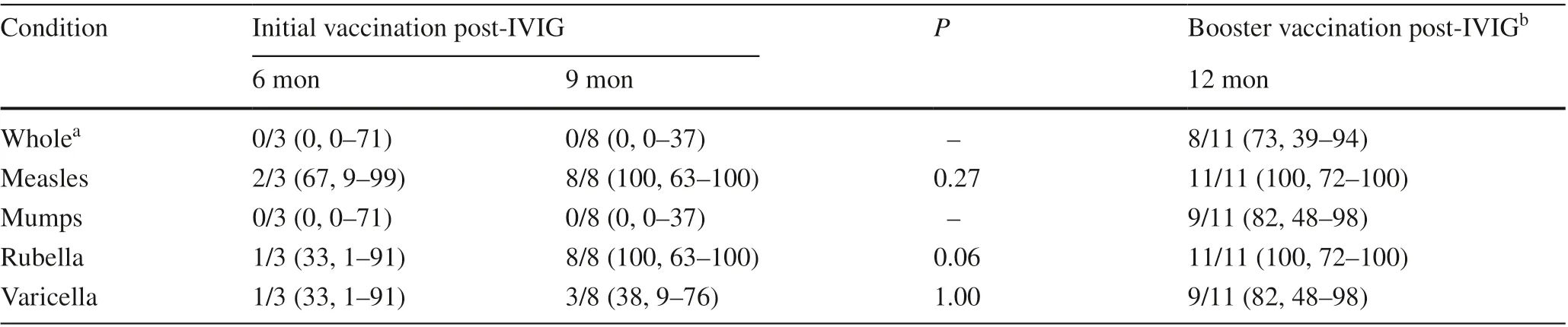
Table 2 Seroconversion after initial and booster vaccinations per vaccine
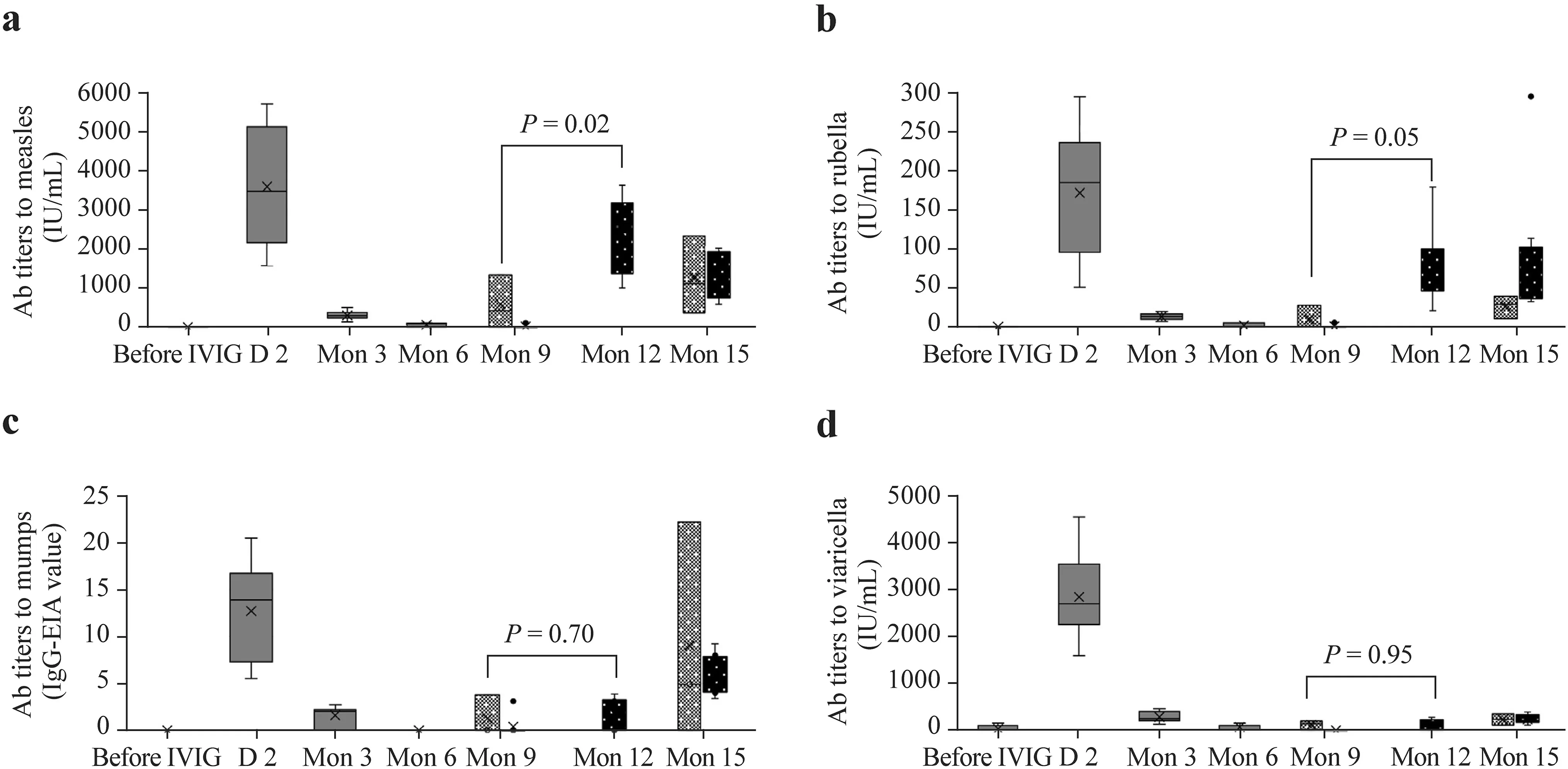
Fig. 1 Box diagram of antibody titers to measles ( a), rubella ( b), mumps ( c), and varicella ( d) on enzyme immunoassay before and at 2 days, 3,6, 9, and 15 months post-IVIG. Pre-IVIG, day 2, month 3, and month 6 show changes in the antibody titers before and after IVIG. The left side shows the antibody titers at month 9 in subjects vaccinated at month 6; the right side shows the titers in those vaccinated at month 9. Subjects vaccinated at month 6 were not tested for antibody titers at month 12. The bars above the column at month 9 and month 12 demonstrate a comparison of post-vaccination antibody titers between vaccination at months 6 and month 9 post-IVIG treatment. The cross in the box plot indicates the mean value. The small circles indicate values falling outside the 1.5 × interquartile range and below the f irst quartile or above the third quartile. The broken line in each graph indicates the cutoff level for the antibody titers. IVIG intravenous immunoglobulin, Ab antibody, IgG immunoglobulin G, EIA enzyme immunoassay
In the present study, the antibodies for each virus,which increased after immunoglobulin administration,were undetectable on EIA at six months post-IVIG. A previous report of changes in the measles antibody titers on EIA in KD patients after two IVIG treatments demonstrated that a residual titer was detectable at six months but was undetectable at nine months [ 6]. The results of the present study were in line with these f indings. In previous reports investigating changes in antibody titers after a single IVIG dose, antibodies were detectable at f ive months in patients receiving on average IVIG 1.3 g/kg [ 16], and the measles antibody titer at six months in patients receiving on average 2 g/kg was 33% on EIA; 48% for rubella on hemagglutination inhibition assay; and 6% for varicella on EIA [ 7]. Comparison of the results of the present study with those of a previous study evaluating changes in the titer of antibodies against the four viruses on EIA after a single IVIG dose [ 7] demonstrated a signif icantly higher residual antibody titer in the two-IVIG-dose group for all the viruses on day 2. However, after three months, the titers did not diff er signif icantly between the groups (Supplementary Table 2). In KD patients, increased vascular permeability causes protein leakage and decreased serum protein [ 17], and the serum IgG in these patients correlated inversely with fever duration [ 18]. Residual antibodies became undetectable more quickly in severe KD patients who received two IVIG doses [ 6]. The f indings of the present study support these observations.
The most important limitations of the present study were that it was observational and too small for any def initive conclusions to be drawn.
In conclusion, the LAV seroconversion rate was low for measles and rubella at six months after two IVIG doses for KD but was adequate at nine months. The mumps and varicella vaccines were ineff ective at either timing, but a seroresponse was achieved by a booster vaccination. While the measles and rubella vaccines should be administered at least 9 months post-IVIG therapy, the present study indicated that only one vaccination may be insuffi cient, especially for mumps and varicella. The timing of vaccination should be adjusted according to the prevalence of the disease in each region. In areas where the frequency of epidemics is high,early vaccination may be considered, but if the vaccine is administered early, its effi cacy should be evaluated serologically later. If the sero-response is found to be inadequate, a booster vaccination should be administered.
Supplementary InformationThe online version contains supplementary material available at https:// doi. org/ 10. 1007/ s12519- 022- 00594-6.
AcknowledgementsThe authors would like to thank Dr. Yuho Horikoshi of the Division of Infectious Diseases, Department of Pediatrics at Tokyo Metropolitan Children's Medical Center for his support;Ms. Makiko Yoshida, Ms. Yuriko Ozaki, and the staff at the Clinical Research Support Center of Tokyo Metropolitan Children’s Medical Center for their help with data management; Mr. Takashi Nakamoto and Ms. Masako Tomotsune for their assistance with data collection;and Mr. James Robert Valera for his assistance in reviewing and editing this manuscript. The authors would like to express their deep appreciation to all the participants and their parents and all the staff at the participating institutions.
Author contributionsYM conceptualized and designed the study, performed the statistical analysis, and drafted and revised the manuscript.HS coordinated the study, supervised the data collection, and revised the manuscript. MM helped to conceptualize and design the study,interpret the data, and review and revise the manuscript. All the authors approved the f inal version of the manuscript as submitted and agree to be accountable for all aspects of the work.
FundingThis work was supported by a grant from the Tokyo Metropolitan Government and the Japan Kawasaki Disease Research Center.
Data availabilityThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approvalThe ethics committee at each institute approved this study. The study is registered in UMIN Clinical Trials Registry (No.UMIN000007174, registration date: January 31, 2012; https:// cente r6.umin. ac. jp/ cgi- open- bin/ ctr/ ctr. cgi? funct ion= brows& action= brows&type= summa ry& recpt no= R0000 08452).
Conflict of interestMM received honoraria from the Japan Blood Products Organization, Teijin Pharma Limited, Nihon Pharmaceutical,and Mitsubishi Tanabe Pharma Corporation which were unrelated to the submitted work. The other authors have no conf licts of interest to disclose.
杂志排行
World Journal of Pediatrics的其它文章
- Single ventricle: amphibians and human beings
- Clinical diff erences among racially diverse children with celiac disease
- Associations between body mass index in diff erent childhood age periods and hyperuricemia in young adulthood: the China Health and Nutrition Survey cohort study
- Frequency and safety of COVID-19 vaccination in children with multisystem inf lammatory syndrome: a telephonic interview-based analysis
- Music therapy and pediatric palliative care: songwriting with children in the end-of-life
- Secondary genomic f indings in the 2020 China Neonatal Genomes Project participants
